Abstract
The morphologic criteria for tall cell variant (TCV) of papillary thyroid carcinoma (PTC) were modified in the 2017 WHO Classification of Tumors of Endocrine Organs, with a decrease in the requirements for both the height of cells and in the percentage of tumor demonstrating a tall cell morphology. The aim of this study was to determine if the change in criteria would result in a significant increase in the percentage of tumors that meet criteria for TCV. In addition, we evaluated the correlation between morphology, molecular alterations, and clinical behavior of TCV. We studied three cohorts to evaluate the above stated questions. The first cohort was comprised of 97 PTC consecutively resected over a 12-month period that were originally diagnosed as classic PTC, PTC with tall cell features, or TCV. Tumor slides of each case were reviewed to determine the percentage of the tall cell component (< 30%, 30–49%, and > 50%) and the height of the cells in this component. This cohort was evaluated to determine if the change in WHO criteria would result in a significant increase in the percentage of tumors that meet criteria for TCV. Our second cohort consisted of nine consecutively resected PTC with a tall cell component > 30% (with tall cells defined as at least 2–3× as tall as wide) that had molecular characterization through a targeted, next-generation sequencing (NGS) assay. The molecular characteristics were correlated with the percentage of the tall cell component. Finally, a third cohort comprised of seven clinically aggressive TCV (defined as those with T4 disease, disease recurrence, or subsequent tumor dedifferentiation) was evaluated to determine histologic and molecular characteristics. In cohort 1, the number of cases classified as TCV increased significantly with the change in definition of TCV: 8 (8%) cases met the previous criteria for TCV (cells 3× as tall as wide in > 50% of the tumor), whereas 24 (25%) cases met the new 2017 WHO criteria (cells 2–3× as tall as wide in > 30% of the tumor) (p = 0.0020). Molecular analysis of cohort 2 revealed that all 9 cases harbored a BRAF V600E mutation. Pathogenic secondary mutations were absent in cases with < 50% tall cells, but they were detected in 2 (33%) of 6 cases with > 50% tall cells (2 cases with TERT promoter mutations, including 1 that also had an AKT2 mutation). Histologic and molecular analysis of the clinically aggressive cohort (cohort 3), revealed that all cases had > 50% tall cells and 3 (43%) had secondary oncogenic mutations (all TERT promoter mutations). We found that the modified morphologic criteria put forth in the 2017 WHO tripled the number of cases that would be classified as TCV. Moreover, clinically aggressive tumors and those harboring secondary oncogenic mutations all had a tall cell component > 50%. Additional large multi-institutional studies incorporating clinical outcome and molecular data would be valuable to determine the best histologic definition of TCV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tall cell variant (TCV) of papillary thyroid carcinoma (PTC) accounts for approximately 6% of PTC and is considered an aggressive PTC subtype [1, 2]. It has double the rate of extrathyroidal extension and nearly triple the rate of distant metastases compared to classic PTC; moreover, the recurrence rate and cause-specific death rate of TCV are 22% and 8% compared to 7% and < 1% for classic PTC [2]. There are studies demonstrating that the more aggressive clinical behavior of TCV is not simply secondary to the higher rates of extrathyroidal extension and distant metastases of TCV [3,4,5]. For example, two SEER studies showed that the aggressive behavior of TCV was independent of factors such as patient age, extrathyroidal extension, or metastases [4, 5]. Additionally, in a study by Ito and colleagues evaluating 1700 PTC patients with slides re-reviewed to identify cases of TCV, the authors found a significantly worse disease-free survival and cause-specific survival for TCV compared to classic PTC in multivariate analysis [3]. The morphologic criteria for TCV were recently modified in the 2017 WHO Classification of Tumors of Endocrine Organs, with a decrease in both the required height of cells (3× as tall as wide modified to 2–3× as tall as wide) and in the percentage of tumor demonstrating a tall cell morphology (a “predominance of tall cells” suggesting a cutoff of > 50% modified to > 30%) [6, 7]. This change was based on studies which demonstrated that tumors possessing a tall cell component comprising less than 50% of the tumor behave more aggressively than classic PTC [8,9,10,11]. In addition to the height of the tumor cells, TCV also demonstrates additional cytologic characteristics including eosinophilic cytoplasm and nuclei with pronounced nuclear features of PTC [7]. Although the molecular profile of TCV overlaps with that of classic PTC, TCV has been shown to have a higher mutation density than classic PTC, consistent with its more aggressive clinical behavior [12]. The BRAF V600E mutation, present in approximately 70% of classic PTC, is present in > 90% of TCV [12]. TERT promoter mutations have been linked to aggressive clinical behavior in thyroid carcinomas, especially when found together with a BRAF V600E mutation [13]. TERT promoter mutations are present in approximately 30% of TCV compared to 12% of classic PTC [14]. The aim of this study was to determine if the change in criteria put forth in the 2017 WHO would result in a significant increase in the percentage of tumors that meet criteria for TCV. In addition, we evaluated the correlation between morphology, molecular alterations, and clinical behavior of TCV.
Materials and Methods
Study Population and Data Acquisition
Approval from the Brigham and Women’s Hospital (BWH) institutional review board was obtained. Three different cohorts were investigated to evaluate our study questions. For each cohort, all available tumor slides for cases were reviewed to determine the percentage of the tall cell component (< 30%, 30–49%, and > 50%) and the height of the cells in this component (2–3× as tall as wide versus 3× as tall as wide) (Fig. 1). No tumors in any cohort had an anaplastic or poorly differentiated component (as defined by the 2017 WHO). Additionally, for each cohort, patient demographics and histopathologic features were recorded, including patient age, sex, tumor size, presence of extrathyroidal extension, lymph node status, and margin status.
Cohort 1 was comprised of 97 PTC consecutively resected within a 12-month period at BWH that were initially diagnosed as classic PTC, PTC with tall cell features, or TCV. This cohort was evaluated to determine if the change in WHO criteria would result in a significant increase in the percentage of tumors that meet criteria for TCV. Cohort 2 consisted of nine consecutively resected PTC with a tall cell component > 30% (with tall cells defined as at least 2–3× as tall as wide) that had molecular characterization through a targeted, next-generation sequencing (NGS) assay (see below). Cohort 3 was comprised of seven clinically aggressive TCV (defined as those with T4 disease, disease recurrence, or subsequent tumor dedifferentiation). Cohorts 2 and 3 were evaluated to examine the correlation between morphology, molecular alterations, and clinical behavior of TCV.
Molecular Characterization by Targeted Next-Generation Sequencing
A subset of cases had molecular characterization via the OncoPanel assay developed at Brigham and Women’s Hospital [15, 16]. OncoPanel molecular testing was performed using formalin-fixed paraffin-embedded tissue or freshly frozen tissue. Tumor DNA was isolated per standard methods (Qiagen, Valencia, CA) from macrodissected regions of tumor. Libraries were prepared from 50 ng of DNA using a customized solution-phase hybrid capture approach (Agilent Technologies, Santa Clara, CA). Bait sets covered 447 genes. Next-generation sequencing was performed using an Illumina HiSeq 2500 (Illumina, San Diego, CA). Sequencing was analyzed by a variety of internally developed and publicly available tools, as previously described [16].
Statistical Analysis
A t test or Fisher exact test was used to compare differences in continuous and categorical variables, respectively. For all statistical methods, p values < 0.05 were considered significant.
Results
The clinicopathologic characteristics of cohort 1 are summarized in Table 1. A total of 97 consecutively resected PTC were identified from 71 (73%) women and 26 (27%) men, with a mean age of 48 years (range 21–81). The mean tumor size was 1.5 cm. Extrathyroidal extension, lymph node metastases, and positive surgical resection margins were present in 28 (29%), 46 (59%), and 16 (16%) cases, respectively. With tall cells defined as at least 3× as tall as wide, 1 (1%) had 30–49% tall cells, and 8 (8%) had > 50% tall cells. With tall cells defined as at least 2–3× as tall as wide, 9 (9%) had 30–49% tall cells, and 15 (16%) had > 50% tall cells. Overall, 8 (8%) cases met the prior WHO criteria for TCV (tall cells at least 3× as tall as wide in > 50% of cells), whereas 24 (25%) cases fulfilled the current WHO criteria for TCV (tall cells at least 2–3× as tall as wide in > 30% of cells) (p = 0.0020). There was no difference in tumor size or rates of extrathyroidal extension, lymph node metastases, or margin status between subgroups (Table 1).
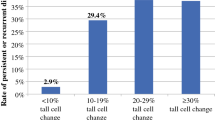
Pathologic characteristics of cohort 2, i.e., PTC with a tall cell component > 30% (with tall cells defined as at least 2–3× as tall as wide) that had molecular characterization, are shown in Table 2. All cases had a BRAF V600E mutation. Two (22%) cases had secondary mutations involving the TERT promoter, with one of these tumors harboring an additional AKT2 mutation (another known oncogenic mutation in thyroid carcinomas). Both cases with secondary mutations were in tumors with > 50% tall cells and a height to width ratio of at least 3. In cohort 3 (the cohort comprised of clinically aggressive TCV), 3 (43%) cases had pathogenic secondary mutations in addition to a BRAF V600E mutation (Table 3). All 3 of these cases had TERT mutations. Two of the cases with TERT mutations also demonstrated loss of function mutations in a second gene (COL7A1 and EWSR1). Excluding 1 case where all slides were not available for review, all clinically aggressive cases had > 50% tall cells, with a height to width ratio of at least 3 in 2 (33%) cases and 2–3 in 4 (67%) cases.
Discussion
The aim of this study was to determine if the change in criteria put forth in the 2017 WHO Classification of Tumors of Endocrine Organs would result in a significant increase in the percentage of tumors that meet criteria for TCV. In addition, we evaluated the correlation between morphology, molecular alterations, and clinical behavior of TCV. We found that the number of cases classified as TCV increased significantly with the modification in criteria put forth in the 2017 WHO: 8% of cases met the old criteria for TCV (cells 3× as tall as wide in > 50% of the tumor), whereas 25% met the new criteria for TCV (cells 2–3× as tall as wide in > 30% of the tumor). In other words, the change in the WHO definition tripled the number of cases that would be classified as TCV. Additionally, we found that all clinically aggressive tumors (defined as those with T4 disease, disease recurrence, or subsequent tumor dedifferentiation) as well as those with secondary oncogenic mutations all had a tall cell component of > 50%.
The increase in the number of tumors diagnosed as TCV could potentially have significant clinical implications. Based on the classification of TCV as an aggressive PTC variant, the American Thyroid Association (ATA) characterizes it as an intermediate risk tumor in the absence of other high-risk features [1]. ATA guidelines indicate total thyroidectomy or lobectomy can be considered for low-risk tumors under 4 cm without extrathyroidal extension or clinical evidence of lymph node metastases; however, the guidelines indicate that intermediate-risk tumors should undergo total thyroidectomy and radioactive iodine treatment should be considered [1]. Compared to lobectomy, total thyroidectomy increases the risk of recurrent laryngeal nerve damage and permanent hypoparathyroidism and necessitates lifelong thyroid hormone replacement [17]. Additionally, radioactive iodine treatment can result in salivary gland dysfunction and has been linked to an increased risk of developing second malignancies [18,19,20,21,22]. Hence, increasing the number of patients diagnosed with TCV could put more patients at risk for complications. For biologically aggressive tumors, the clinical benefit of aggressive treatment outweighs potential complications; however, it is unclear if all tumors that would be included in the 2017 WHO definition of TCV warrant more aggressive management. For patients who underwent an initial lobectomy, a TCV diagnosis would potentially trigger a completion thyroidectomy; thus, exposing the patient to a second surgery and driving up healthcare costs [23]. Finally, a diagnosis of TCV also affects patient counseling given the increased risk of recurrence and mortality associated with TCV. Clearly, it is important to be as accurate as possible when counseling patients regarding these risks.
The change in WHO criteria for TCV was based on studies which demonstrated that tumors possessing a tall cell component comprising less than 50% of the tumor behave more aggressively than classic PTC [8,9,10,11]. In a study by Ganly and colleagues, they reported a decreased 10-year disease-specific survival for patients with tumors with tall cell features (defined as tumors with 30–49% tall cells, with tall cells 2–3× as tall as wide) compared to those with classic PTC [10]. In addition, they described subsequent dedifferentiation of three tumors with tall cell features. Beninato and colleagues reported higher rates of extrathyroidal extension, positive margins, and lymph node metastases in tumors with > 10% talls compared with classic PTC, though the recurrence rate was not significantly different between the two groups [8]. Oh and colleagues reported that tumors with tall cell features (defined as those with 10–49% tall cells, with the tall cells at least 3× as tall as wide) had similar T stage, extrathyroidal extension, N stage, lateral lymph node metastases, and BRAF status compared to tumors with a tall cell component > 50% [11]. Dettmer and colleagues showed that a 10% cutoff for tall cells was significantly associated with advanced tumor stage and lymph node metastases [9]. Additionally, they found that a tall cell component > 10% was the only significant factor for overall survival, tumor-specific, and relapse-free survival (and was maintained in multivariate analysis). Although the study by Dettmer and colleagues showed significance in multivariate analysis, their cohort was enriched for patients with an adverse event, raising the question of whether this could potentially influence results. The two SEER studies showing that the aggressive behavior of TCV was independent of factors such as patient age, extrathyroidal extension, or metastases used an undefined definition for TCV (since slides are not re-reviewed centrally), but it is worth noting that TCV comprised < 2% of PTC in the study by Kazaure and colleagues [4, 5]. In the study by Ito and colleagues showing significantly worse disease-free survival and cause-specific survival for TCV compared to classical PTC in multivariate analysis, TCV was defined according to the former WHO criteria (> 50% of the tumor composed of tall cells with cells 3× as tall as wide) and TCV accounted for less than 4% of their cohort [3]. It is unclear whether the results from all of these studies taken together warrant the change of criteria put forth in the 2017 WHO given the number of patients potentially affected.
Our study was not designed to evaluate the prognostic significance of the percentage of the tall cell component because we did not have clinical outcome data for our main cohort. However, we did correlate histologic findings and aggressive clinical behavior with molecular characteristics in tumors from a small number of patients. Consistent with the results reported by Dettmer and colleagues [9], we found that although TERT promoter mutations were present in some of the clinically aggressive TCV, not all clinically aggressive tumors harbored TERT promoter mutations (or secondary oncogenic mutations). However, it is possible that for tumors with tall cells, parameters such as presence of secondary oncogenic mutations, including but not limited to TERT promoter mutations, or gross extrathyroidal extension might be a better way to identify patients that would benefit from more aggressive treatment than the characteristics of the tall cell component. We also recognize that there is considerable interobserver variability in the diagnosis of TCV [24]. It may be that image analysis could help pathologists determine height of cells, though this ultimately would not provide insight into the biologic implications of the findings.
In conclusion, we found that the number of cases diagnosed as TCV would triple using the 2017 WHO criteria. Although this is a small, single institution, retrospective study, this finding is concerning given the potential clinical ramifications. In the last decade, there has been a call for efforts both to decrease overtreatment of thyroid carcinomas and to find ways to better predict the natural history of disease [25]. Additional large multi-institutional studies incorporating clinical outcome and molecular data would be valuable to determine the best histologic definition of TCV so that patients are risk stratified and treated appropriately.
References
Haugen BR, Alexander EK, Bible KC et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. ed.^, eds.: Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA, 2016; 1–133.
Wang X, Cheng W, Liu C, Li J Tall cell variant of papillary thyroid carcinoma: current evidence on clinicopathologic features and molecular biology. Oncotarget 7: 40792–40799, 2016.
Ito Y, Hirokawa M, Fukushima M, Inoue H., Yabuta T., Uruno T., Kihara M., Higashiyama T., Takamura Y., Miya A., Kobayashi K., Matsuzuka F., Miyauchi A. Prevalence and prognostic significance of poor differentiation and tall cell variant in papillary carcinoma in Japan. World journal of surgery 32: 1535–1543- discussion 1544-1535, 2008.
Kazaure HS, Roman SA, Sosa JA Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Annals of surgical oncology 19: 1874–1880, 2012.
Morris LGT, Shaha AR, Tuttle RM, Sikora AG, Ganly I Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid : official journal of the American Thyroid Association 20: 153–158, 2010.
DeLellis RA, Lloyd R, Heitz PU, Eng C World Health Organization Classification of Tumours: Pathology and Genetics Tumours of Endocrine Organs, 2004.
Lloyd RV, Osamura R, Kloppel G, Rosai J WHO Classification of Tumours of Endocrine Organs, 2017.
Beninato T, Scognamiglio T, Kleiman DA, Uccelli A, Vaca D, Fahey III TJ, Zarnegar R Ten percent tall cells confer the aggressive features of the tall cell variant of papillary thyroid carcinoma. Surgery 154: 1331–1336- discussion 1336, 2013.
Dettmer MS, Schmitt A, Steinert H et al. Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocrine-related cancer 22: 419–429, 2015.
Ganly I, Ibrahimpasic T, Rivera M, Nixon I, Palmer F, Patel SG, Tuttle RM, Shah JP, Ghossein R Prognostic implications of papillary thyroid carcinoma with tall-cell features. Thyroid : official journal of the American Thyroid Association 24: 662–670, 2014.
Oh WJ, Lee YS, Cho U, Bae JS, Lee S, Kim MH, Lim DJ, Park GS, Lee YS, Jung CK Classic papillary thyroid carcinoma with tall cell features and tall cell variant have similar clinicopathologic features. Korean journal of pathology 48: 201–208, 2014.
Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell 159: 676–690, 2014.
Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA oncology 3: 202–208, 2016.
Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, Sun H, el-Naggar AK, Xing M Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocrine-related cancer 20: 603–610, 2013.
Sholl LM, Do K, Shivdasani P et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI insight 1: e87062, 2016.
Garcia EP, Minkovsky A, Jia Y, Ducar MD, Shivdasani P, Gong X, Ligon AH, Sholl LM, Kuo FC, MacConaill LE, Lindeman NI, Dong F Validation of OncoPanel: A Targeted Next-Generation Sequencing Assay for the Detection of Somatic Variants in Cancer. Archives of pathology & laboratory medicine 141: 751–758, 2017.
Kandil E, Krishnan B, Noureldine SI, Yao L, Tufano RP Hemithyroidectomy: a meta-analysis of postoperative need for hormone replacement and complications. ORL; journal for oto-rhino-laryngology and its related specialties 75: 6–17, 2013.
Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med 39: 1551–1554, 1998.
Almeida JP, Sanabria AE, Lima EN, Kowalski LP Late side effects of radioactive iodine on salivary gland function in patients with thyroid cancer. Head & neck 33: 686–690, 2011.
Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab 93: 504–515, 2008.
Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer 117: 4439–4446, 2011.
Sandeep TC, Strachan MW, Reynolds RM et al. Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab 91: 1819–1825, 2006.
Agrawal N, Abbott CE, Liu C, Kang S, Tipton L, Patel K, Persky M, King L, Deng FM, Bannan M, Ogilvie JB, Heller K, Hodak SP Noninvasive Follicular Tumor with Papillary-Like Nuclear Features: Not a Tempest in a Teapot. Endocr Pract 23: 451–457, 2017.
Hernandez-Prera JC, Machado RA, Asa SL, Baloch Z, Faquin WC, Ghossein R, LiVolsi VA, Lloyd RV, Mete O, Nikiforov YE, Seethala RR, Suster S, Thompson LD, Turk AT, Sadow PM, Urken ML, Wenig BM Pathologic Reporting of Tall-Cell Variant of Papillary Thyroid Cancer: Have We Reached a Consensus? Thyroid 27: 1498–1504, 2017.
Welch HG, Doherty GM Saving Thyroids - Overtreatment of Small Papillary Cancers. The New England journal of medicine 379: 310–312, 2018.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, K.S., Higgins, S.E., Marqusee, E. et al. Tall Cell Variant of Papillary Thyroid Carcinoma: Impact of Change in WHO Definition and Molecular Analysis. Endocr Pathol 30, 43–48 (2019). https://doi.org/10.1007/s12022-018-9561-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-018-9561-4