Abstract
Lately, vitamin D has been linked with metabolic and immunological processes, which established its role as an essential component of human health preservation. Vitamin D has been defined as natural immune modulators, and upon activation of its receptors (VDRs), it regulates calcium metabolism, cellular growth, proliferation and apoptosis, and other immunological functions. Epidemiological data underline a strong correlation between poor vitamin D status and higher risk for chronic inflammatory illnesses of various etiologies, including autoimmune diseases. Epidemiological, genetic, and basic studies indicated a potential role of vitamin D in the pathogenesis of certain systemic and organ-specific autoimmune diseases. These studies demonstrate correlation between low vitamin D and prevalence of diseases. In addition, VDRs’ polymorphisms observed in some of these autoimmune diseases may further support a plausible pathogenic link. Notably, for some autoimmune disease, no correlation with vitamin D levels could be confirmed. Thus, in the current review we present the body of evidence regarding the plausible roles of vitamin D and VDR’s polymorphism in the pathogenesis of autoimmunity. We summarize the data regarding systemic (i.e., systemic lupus erythematosus, rheumatoid arthritis, etc.) and organ-specific (i.e., multiple sclerosis, diabetes mellitus, primary biliary cirrhosis, etc.) autoimmune diseases, in which low level of vitamin D was found comparing to healthy subjects. In addition, we discuss the correlations between vitamin D levels and clinical manifestations and/or activity of diseases. In this context, we address the rational for vitamin D supplementation in patients suffering from autoimmune diseases. Further studies addressing the mechanisms by which vitamin D affects autoimmunity and the proper supplementation required are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
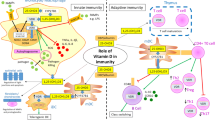
The mosaic of autoimmunity is comprised of a complicated interplay between endogenous and exogenous factors such as genetic, hormonal, and environmental ones, of which vitamin D has been recognized as both an exogenous and endogenous player [1]. Vitamin D is a fat-soluble pro-hormone found in significant amounts in certain fish and in small amounts in other ingredients of the Western diet. It is synthesized in large quantities in skin exposed to UV rays of sunlight. Following the syntheses of vitamin D, it is converted in vivo into biologically active metabolites namely 25(OH)D and 1,25(OH)D [2]. The latter regulate numerous functions in various cell types, through binding to vitamin D receptors (VDR) on both calcemic and noncalcemic tissues [2]. Thus, upon activation of VDRs, they not only control calcium metabolism but also elicit a wide variety of biological responses, which influence cellular growth, proliferation, apoptosis, and immune modulation [3, 4]. Vitamin D binds to VDRs on various cells participating in immune responses, thereby modulating both the activation and deactivation of the innate and adaptive responses [4, 5]. For instance, vitamin D may induce innate tolerance by promoting tolerogenic dendritic cells on the one hand while on the other induce a robust macrophage response to infections (i.e., mycobacterium tuberculosis) [2, 6]. Additionally, both humoral and cellular adaptive responses are affected by vitamin D. Decreased proliferation and antibody production by B cells have been documented following exposure to vitamin D [7]. While the later effect on the cellular response is comprised of a switch from Th1 to Th2 cytokine profile, ameliorating Th17 pathway via transcriptional modulation of interleukin-17A, as well as induction of T regulatory cells and immune tolerance [3, 8, 9]. In this context, seasonal variation in vitamin D levels was reported to parallel changes in peripheral blood human T cell compartment [10]
Thus, vitamin D has been accepted as one of the natural immune modulators and regulator of various immune-mediated processes [11]. In the current review, we aim to present the accumulated data on the plausible roles of vitamin D in the pathogenesis of autoimmune diseases as well as the beneficial effects of vitamin D supplementation in this context.
Geo-epidemiology, Sunlight Exposure, and Vitamin D
Determination of vitamin D status is preformed via measurements of 25(OH)D serum levels [2]. In the last decade, the recommended levels of vitamin D (25OH) have been an issue of great debate. Currently, it is accepted that maintaining serum 25-OH vitamin D concentration at a level of 30 ng/ml (80 nmol/l) or more is beneficial for maintaining bone health and calcium homeostasis [2, 12], whereas the levels required to maintain noncalcemic functions of vitamin D are yet unknown [13]. Low levels of vitamin D (below 20 ng/ml) have been documented in healthy and diseased population worldwide mainly in Northern climate [14]. However in the last decade, probably due to changes in life habits, even in sunny climates healthy subjects present with lower levels of vitamin D [15]. Epidemiological data underline a strong correlation between poor vitamin D status and higher risk for chronic illnesses of various etiologies, including cancers, autoimmune diseases, cardiovascular morbidity, diabetes, and infectious diseases [12]. A case in point is infection with mycobacterium tuberculosis (TB). Decades ago, patients with tuberculosis were sent to convalesce in sanatoriums being exposed to sunlight, and the mechanisms by which vitamin D affects the immune responses to TB have been elucidated [6]. Intriguingly, infection with TB has been linked with the presence of autoantibodies and autoimmune diseases both of which may be affected by vitamin D status [6].
In parallel increase in the incidence of autoimmunity has been documented in the last decades, and an association between low sun exposure inducing lower vitamin D and higher prevalence of certain autoimmune diseases was observed [13, 16, 17]. Furthermore, several autoimmune diseases, including inflammatory bowel disease, multiple sclerosis, type I diabetes, and rheumatoid arthritis (RA), have been documented to be more prevalent in Northern latitudes where sun exposure is reduced [18, 19].
Vitamin D and Autoimmunity
The mechanisms underlying the assumption that vitamin D is linked with autoimmunity are its anti-inflammatory and immunomodulatory functions, as well as the presence of VDRs on most immune cells. In addition, several epidemiological observations support this notion.
Perhaps the most established link between environmental factors and autoimmunity lies with the interactions between infections and autoimmunity [20]. Vitamin D triggers the innate response to infection including the activation of Toll-like receptors, antibacterial peptides (i.e., cathelicidin), and cells of the innate response [6, 21]. Hence, one may suggest that by suppressing infections, vitamin D modulates autoimmunity. Another example of this triangular link is the connection between Epstein–Barr virus (EBV), autoimmunity, and vitamin D. EBV is considered one of the most notorious infectious agents while considering induction of autoimmunity [22]. Additionally, EBV was allied with downregulation of VDR expression and vitamin D beneficial effects [23].
Other epidemiological supports to this supposition are the numerous studies on vitamin D levels comparing populations with and without an existent autoimmune disease. We and others have reported a linkage between low levels of vitamin D and the presence of some autoimmune diseases. Of note, not all autoimmune diseases are linked with low vitamin D, and its levels were comparable to healthy population in certain diseases. Additionally, in our studies, which include all together more than 3,000 patients with various autoimmune diseases, low vitamin D levels were associated with specific clinical manifestations of certain autoimmune diseases (Table 1), further supporting a possible pathogenic connection.
Vitamin D and Systemic Autoimmune Diseases
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disease predominantly affecting women. The disease literally involves any body system or organ, while skin manifestations are among the most common. SLE patients are sensitive to sunlight exposure/ultraviolet radiation (i.e., photosensitivity) that may cause exacerbation of the disease. For this reason, patients are routinely instructed to avoid the sun and to use sunblocks extensively. These preventive methods result also in blocking UVB-induced synthesis of vitamin D3 (cholecalciferol) in the skin, and thus could be one of the reasons for vitamin D deficiency among SLE patients [24]. Apparently, many case–control studies have demonstrated a significantly lower level of serum vitamin D in SLE patients compared with matched healthy controls [25–27]. Being crucial to calcium–phosphorus homeostasis, vitamin D deficiency may contribute to osteopenia, osteoporosis, and renal disease in SLE patients similarly to healthy subjects [28]. However, the importance of vitamin D as an immune modulator raises the possibility of specific roles in SLE pathogenesis. In certain studies, low serum concentrations of 25-OH vitamin D correlated with SLE disease activity, further supporting a pathogenic association between the two. For instance, Amital et al. [29] recently studied vitamin D levels in a cohort of 378 patients with SLE originating from Israel and Europe. In this study, a significant inverse correlation between serum vitamin D levels and disease activity scores as measured by the SLEDAI-2K and ECLAM scales was reported. Of note, inconsistencies regarding such association have been reported by other researches [27, 30], which may depend on factors such as different method of evaluation of SLE activity, time of evaluation of vitamin D level as well as genetic and geo-epidemiological ones. Another role for vitamin D in SLE relates to SLE-associated cardiovascular disease. The excess cardiovascular risk in SLE cannot be explained entirely by traditional cardiovascular risk factors [31]. In a recent study, low vitamin D levels were significantly associated with fatigue and cardiovascular risk among SLE patients, although most of these associations can be explained by BMI [32]. Therefore, whether the low levels of vitamin D are the result or the cause of advanced SLE disease is yet to be determined. Nonetheless, the numerous beneficial effects of vitamin D and the high prevalence of vitamin D deficiency in SLE patients support vitamin D supplementation in this group of patients.
An association between vitamin D receptor (VDR) gene BsmI polymorphisms and SLE has been reported in certain populations. A possible role of the VDR B allele in influencing disease susceptibility and the development of nephritis was documented in Han Chinese and Japanese ones [33, 34]. Intriguingly, in a relatively smaller study of Thai SLE patients, no correlation between VDR gene BsmI polymorphism and SLE disease could be reported [35].
Antiphospholipid Syndrome
Antiphospholipid syndrome (APS) is an autoimmune hypercoagulable state caused by antibodies against cell membrane phospholipids (aPL) which provokes blood clots (thrombosis) in both arteries and veins as well as obstetric morbidity and different systemic manifestations. Thrombosis in APS is mediated by antiphospholipid antibodies as anti-β2GPI ones. The latter induce endothelial dysfunction, monocyte and platelet activation, as well as overexpression of tissue factor, adhesion molecules, and cytokines, of which the role of tissue factor is considered to be pivotal [36]. From a clinical point of view, the role of vitamin D in the antiphospholipid syndrome has been rarely described. We have documented low vitamin D levels among 160 APS patients [37]. In a second study, we were able to confirm a higher prevalence of vitamin D deficiency ( ≤15 ng/ml) among APS patients compared to matched healthy controls (49.5 vs. 30 %, respectively, p < 0.001). Notably, we observed a significant inverse correlation between vitamin D levels and thrombotic manifestations (p < 0.05). Additionally, in an in vitro model of anti-β2GPI-induced endothelial cell activation, we were able to document decrease expression of tissue factor following the addition of the active form of vitamin D, thus suggesting a role for vitamin D supplementation in preventing APS-mediated thrombosis [38]. In support of this notion, a recent Swedish study reported of an association between sun exposure and thromboses among more than 29,000 subjects. A 50 % increased risk of venous and arterial thrombosis was observed during winter compared with other seasons, whereas a significantly lower risk of thrombosis was reported in women who were more sun exposed [39]. Nowadays, no data are available regarding VDR polymorphisms and APS.
Rheumatoid Arthritis
RA is the most common autoimmune arthritis. The interactions between environment and genes are crucial in all stages of this autoimmune disease [40], of which vitamin D deficiency was found to be common in RA patients affecting up to 65 % of them [41]. VDR polymorphism was found to be associated with RA onset and activity [42], and experimental data showed that VDRs are present on macrophages, chondrocytes, and synovial cells from RA affected joints.
Several studies addressed the issue of association between low vitamin D levels and RA disease. In certain studies, RA activity, disability scores, and clinical manifestations were related to vitamin D status [43, 44]. Patel and colleagues [45] found a strong inverse association between baseline levels of serum 25(OH)D in patients with newly diagnosed inflammatory polyarthritis, 45 % of whom were classified as having RA at 1 year. In addition, vitamin D levels were related to baseline disease activity, RA disease activity scores (DAS28), and health assessment questionnaires scores. In another study, an inverse association between vitamin D status and pain was documented [46]. Last but not least, a recent discovery suggests that the reduced serum levels of vitamin D commonly seen in RA may increase fibroblast-like synoviocyte mediated cartilage and bone invasion and erosions, thus supporting a plausible role for vitamin D supplementation to prevent or reduce bone and joint destruction [47]. On the other hand, in a study of 499 active RA patients, no correlation between serum 25(OH) vitamin D levels and disease activity was observed nor with response to therapy or radiographic progression [48]. Additional study did not find any link between vitamin D intake and the incidence of RA [49].
Nonetheless, it is widely accepted that treatment of vitamin D deficiency in patients with RA is relevant as deficiency is common in this group of relatively older patients. Moreover, vitamin D therapy may reduce the increased risk of falls and fracture in this group. Interestingly, climatotherapy at the Dead Sea induced significant increase in 25-OH-D serum levels and reduced musculoskeletal pain and disease severity [50]. While in murine models of human arthritis, 1,25-dihydroxycholecalciferol inhibits the progression of arthritis [51]. Taking it all together, although there is clinical uncertainty, one may suggests that in patients with RA, especially older ones, and post-menopausal women, vitamin D supplementation may be beneficial [52].
Systemic Sclerosis
Systemic sclerosis (SSc) is a chronic progressive systemic autoimmune disease of unknown etiology. The disease is characterized by excess synthesis and deposition of collagen and other extracellular matrix components in a variety of tissues and organs. This leads to vasomotor disturbances, fibrosis, atrophy of the skin and subcutaneous tissue, and multi-organ involvement. Vitamin D deficiency in SSc may be related to several factors, including insufficient sun exposure due to disability, reduced vitamin D production in the skin due to fibrosis and thickening of the skin as well as insufficient intake because of gut involvement and malabsorption. On the other hand, low levels of vitamin D may play a role in the occurrence of the disease itself.
Vitamin D deficiency was reported in several studies regarding patients with SSc from different areas around the globe including patients from sun-exposed area as the south of Spain and Morocco [53–56]. A plausible association with exposure to the sun was also suggested by Seriolo et al. [57] who reported on seasonal variations in serum levels of 25-hydroxyvitamin-D in these patients. Age and vitamin D deficiency were identified as risk factors of osteoporosis and fractures in SSc patients. In addition, vitamin D status has been linked with specific manifestations of SSc. We have recently found a correlation between vitamin D deficiency and skin thickening (measured by Rodnan’s Score) in SSc patients [58].Others reported a link between vitamin D levels and severity of joint pain, immunological status [53] or longer disease duration, lower DCLO, higher pulmonary arterial pressure, and higher inflammatory markers [59]. Hence, vitamin D deficiency seems to be very common among SSc populations independent of their geographic origin. This deficiency is related to osteoporosis as well as SSc disease manifestations. Thus, vitamin D supplementation may be recommended to SSc patients to normalize its status. The beneficial effects of higher doses of vitamin D, especially in patients with inflammatory activity or severe disease, require further studies [56].
Sjogern's Syndrome
Primary Sjogren's syndrome (SS) is a slowly progressive autoimmune disease of the exocrine glands. In up to 50 % of patients, extraglandular manifestations (i.e., lungs, kidney, liver, skin, musculoskeletal, and nervous system) may be exhibited. However, unlike most systemic autoimmune diseases, vitamin D level in SS was found to be similar to healthy subjected, and its association with certain systemic manifestations of disease is still an issue of debate. In a small Danish study performed in 1990, serum levels of 1, 25-OH D3 were equivalent, whereas 25-OH-D3 levels were lower in 41 SS patients compared to normal controls [60]. More recent data from another small case control study from Hungary found no significant differences in vitamin D levels in 25 SS patients with and without extraglandular manifestations [61]. Lately, we evaluated vitamin D levels among 176 primary SS patients from several European countries and 163 matched healthy controls. Although vitamin D levels were comparable between SS patients and controls, lower levels of vitamin D correlated with the presence of peripheral neuropathy and lymphoma [62]. Both of which were linked to vitamin D deficiency in other conditions further supporting the plausible role of vitamin D in the pathogenesis of these expressions.
Vitamin D and Undifferentiated Connective Tissue Disease
Undifferentiated connective tissue disease (UCTD) may be considered as an early stage of all systemic autoimmune diseases and refers to a situation in which certain clinical manifestations and immunological abnormalities are suggestive of an autoimmune disease but do not fulfill any disease classification. According to the literature, eventually 30-40 % of the UCTD patients will be diagnosed as having a defined autoimmune disease as SLE, RA, mixed connective tissue disease (MCTD), SS, etc. Patients with undifferentiated connective tissue disease show vitamin D deficiency and, interestingly, patients who progress into a full blown defined connective tissue disease have lower vitamin D levels than those who remain in the undifferentiated connective tissue disease stage [63, 64]. These data were supported by the finding that low vitamin D levels are related to CD4+Th17/nTreg imbalance in UCTD patients, which was suggested as having a potential role in the progression from UCTD towards a well-established connective tissue disease [42]. Thus, it seems that vitamin D substitution therapy can improve the fine balance of pro- and anti-inflammatory processes in the disease.
Vitamin D and Mixed Connective Tissue Disease
MCTD is a disorder characterized by overlapping features of systemic lupus erythematosus, systemic sclerosis, and polymyositis. Serologically, MCTD is distinguished by the presence of high titers of anti-U1-ribonucleoprotein RNP antibody. A high prevalence of vitamin D insufficiency in MCTD patients was reported. In addition, it has been shown that the low vitamin D levels in MCTD patients are inversely related to the rise of inflammatory cytokines [65].
Inflammatory Myopathies
The inflammatory myopathies are rare disorders unified by feature of muscle weakness and inflammation. These diseases include polymyositis, dermatomyositis, and inclusion body myositis as the most common. Lately, an association between statin-related muscle disease and vitamin D was suggested, but such a link with inflammatory myopathies was rarely reported.
In a small study of 21 patients with juvenile dermatomyositis, serum 25(OH)D levels were inversely associated with disease activity [66]. We have recently evaluated a large cohort of adult patients with inflammatory myopathies and found their levels of vitamin D to be comparable to healthy subjects; moreover, no association was observed between clinical manifestations of disease and vitamin D levels (unpublished data).
Vitamin D and Organ-Specific Autoimmune Diseases
Multiple Sclerosis
Multiple sclerosis (MS) is an autoimmune demyelinating disease characterized by neurological and cognitive manifestations. MS is more common in women, in certain ethnic populations, and in subjects living in high altitudes with low sun exposure [16] The etiology of MS is yet unknown, although several genetic and environmental factors have been implicated in its development. Among the dietary components related to MS, vitamin D status has been studied extensively [67, 68]. Low levels of vitamin D are common in patients with MS, and alongside, limited sun exposure increases the likelihood of MS appearance, especially in young age [69, 70]. Moreover, low levels of vitamin D were associated with increased relapses of MS and vice versa [71].
Vitamin D was reported to induce changes in the gene expression of immune cells derived from patients with MS [72]. Furthermore, a complex process including autonomic nervous system dysfunction (i.e., reduction in arterial blood pressure and alterations in cerebral autoregulation), combined with inflammation, was suggested as crucial in the development of MS. These autonomic dysfunctions were found to be altered by environmental factors such as the Epstein–Barr virus and vitamin D, and possibly by the combined effect of both [73].
The substantial evidence on association between low levels of vitamin D and MS prompted the performance of therapeutic trials. In a small study, the administration of high dose (6,000 I.U.) of vitamin D compared to low dose (1,000 I.U.) for 6 months to patients with relapsing remitting MS did not show any significant clinical or radiological (MRI) effect [74]. However, administration of much higher doses of vitamin D [i.e., 40,000 IU/day over 28 weeks, followed by 10,000 IU/day (12 weeks)] appeared to be safe and reduce the rate of relapses, although this phase I/II study lacked statistical precision and the design requirements to adequately assess the beneficial effects of this therapy [75]. In another study, treatment with vitamin D decreased inflammatory activity in MS as revealed by an anti-inflammatory cytokine profile [76].
Last but not least, the extent to which potential genetic determinants of vitamin D levels may be related to multiple sclerosis (MS) risk has recently been explored. A nested case–control study included 214 MS cases and 428 age-matched controls which were assessed for gene–environment interactions. No associations were observed for any single-nucleotide polymorphisms (SNPs) in VDR in this cohort; however, an interaction (p = 0.04) between dietary intake of vitamin D and the vitamin D receptor FokI polymorphism on MS risk was observed. In other words, a protective effect of increasing vitamin D levels using 400 IU/day was evident in individuals with the 'ff ' genotype (RR = 0.2, 95 % CI, 0.06, 0.78; p = 0.02) [77]. In another study, the interactions between HLA-DRB1*15 allele, the main genetic risk factor for MS in Caucasians, and vitamin D/vitamin D receptor (VDR) complex were analyzed. The results of this study support a protective effect of the rs731236 TT VDR genotype that modulates VDR expression and confers protection against MS in HLA-Dn RB1*15-positive individuals [78].
Autoimmune Thyroid Disease
Autoimmune thyroid diseases (AITDs), namely Hashimoto's thyroiditis (HT) and Graves' disease, are the most prevalent autoimmune disorders. Recently, we have found [79] a statistical significant deficiency of vitamin D (less than 10 ng/ml) in European patients with AITDs compared to healthy individuals (72 versus 30.6 %; P < 0.001). Of note, patients with Hashimoto's thyroiditis were most severely affected. Low levels of vitamin D were related to the presence of antithyroid antibodies and abnormal thyroid function tests [79], suggesting the involvement of vitamin D in the pathogenesis of AITDs and the advisability of supplementation. Thyroid autoimmunity is linked with infertility and adverse pregnancy outcomes, including miscarriage or preterm deliveries, even in euthyroid women. Lack of vitamin D was suggested as a predisposing factor to infertility and pregnancy loss, suggesting a potential interplay with thyroid autoimmunity in the context of infertility [80].
Other reports have yielded conflicting results. In a study from India, 87 % of the cohort exhibit low levels of vitamin D (less than 25 nmol/l). However, these values showed only weak inverse correlation with anti-thyroid antibody titers. The authors speculated that this weak association is due to the narrow range of serum 25(OH) D values in this study [81]. In another study, vitamin D levels in patients with early autoimmune thyroid diseases did not differ from those of matched healthy population [82].
Several studies of different populations have documented links between VDR polymorphism and autoimmune thyroid diseases. For instance, in Chinese patients in Taiwan, a significant difference between HT patients and normal controls in VDR SNP was observed. In this cohort, patients who carry the C/C homozygote of the VDR-FokI gene polymorphism in exon 2 were found to have a higher risk of developing HT [83]. Other studies evaluating Croatian population confirmed this concept. In the latter population, an association between HT and haplotypic variants within the VDR gene 3′-region previously linked to VDR mRNA expression was reported [84], while an association between Graves' disease and VDR gene BsmI/ApaI/TaqI polymorphisms was reported in patients from Eastern Croatia. The ApaI and BsmI "AA" and "BB" genotypes, respectively, as well as combined "BBAAtt" genotype, appeared to confer protection against Graves' disease, whereas ApaI "aa" and TaqI "TT" genotypes were associated with an increased risk for Graves' disease in this population [85].
Celiac Disease
Celiac disease (CD) is a common immune-mediated disorder that occurs in genetically predisposed individuals (carriers of HLA-DQ2 and DQ8 haplotypes) upon consumption of wheat (gluten). A well-established relationship between low bone mineral density and CD was reported, mainly during active disease, in which nutritional and vitamin D status may be decreased [86]. However, we have recently analyzed the levels of vitamin D in a large cohort of Spanish and Israeli patients with celiac disease. In our cohort, most patients were on gluten-free diet with no evidence of active disease, and their vitamin D status was comparable to healthy matched controls [87]. Moreover, we could not find any report on vitamin D levels or VDR polymorphism relation to celiac disease.
Type I Diabetes Mellitus
Type I diabetes mellitus (TID) is a metabolic disorder presented with insulin deficiency due to destruction of insulin-producing islet cells in the pancreas. This is in contrast to type II diabetes mellitus (TIID), formerly termed non-insulin-dependent diabetes mellitus (NIDDM) or adult-onset diabetes, which is a metabolic disorder characterized by high blood glucose in the context of insulin resistance and relative insulin deficiency. Experimental and epidemiological evidence established a link between vitamin D deficiency and an increased incidence of both TID and TIID. A higher incidence of hypovitaminosis D was observed in patients with both types of diabetes, and lower levels of vitamin D were related to poor control of type I disease [88]. Pancreatic tissues, particularly the insulin-producing beta-cells, similarly to cells of the immune system, express the vitamin-D receptor (VDR) and vitamin D-binding protein. In addition, vitamin D promotes calcium absorption and utilization, which is necessary for beta cells function and insulin secretion [88, 89]. Last but not least, allelic variations in genes involved in vitamin D metabolism and VDR are associated with glucose intolerance, insulin secretion, insulin sensitivity, and inflammation [90]. Taking it all together, it has been accepted that vitamin D plays a role in the pathogenesis of diabetes mellitus. However, whether increasing vitamin D status would reduce the risk of diabetes is yet to be answered. In non-obese diabetic mice and other models of T1D, pharmacologic doses of 1,25-dihydroxyvitamin-D, the active form of vitamin D, prevented insulitis and the appearance of overt T1D [90]. Recently, Sorensen and colleagues [91] evaluated the odds of type 1 diabetes among children born to mother with vitamin D deficiency. More than twofold increase in the risk of TID was observed in offspring of women with the lowest levels of 25-OH D compared with the offspring of those with levels above the upper quartile. However, studies on vitamin D supplementation for patients with established TID have yielded conflicting results, and the required doses, length of treatments, and other therapeutic factors are yet unknown [92, 93].
Crohn's Disease
Crohn's disease is one of two main inflammatory bowel diseases. The pathogenesis of inflammatory bowel disease is that abnormal intestinal inflammations occur in genetically susceptible individuals following exposure to various environmental factors. Although vitamin D deficiency occurs in inflammatory bowel disease, it is currently unclear to what extent it is associated with the pathogenesis of diseases as well as with seasonality and genetic factors. Lately, serum 25-hydroxy vitamin D concentrations were found to be significantly lower in Crohn's disease patients than in control subjects during different seasons (i.e., winter and summer) [94]. Other studies have shown that up to 65 % of patients with Crohn's disease have low serum 25-hydroxy vitamin D concentrations, and 45 % of these patients have metabolic bone disease [95]. Joseph et al. [95] have evaluated 34 patients with Crohn's disease and 34 matched controls. 25 (OH) vitamin D levels were significantly lower in patients with Crohn's disease as compared to controls (16.3 ± 10.8 vs 22.8 ± 11.9 ng/ml, respectively; P < 0.05). Moreover, the severity of disease activity as assessed by the Harvey Bradshaw score negatively correlated with vitamin D levels (correlation coefficient −0.484, significance P < 0.004) [95]. In a second study [18], administration of the active form of vitamin D 1, 25(OH) D2 was compared to plain vitamin D in managing osteoporosis in patients with Crohn's disease. The activity of Crohn's disease was also measured clinically and by laboratory parameters. At week 6, the Crohn's Disease Activity Index scores and concentration of C-reactive protein significantly decreased (P < 0.05), while a prominent short-term beneficial effect on bone metabolism was observed [18].
The VDR gene represents a strong candidate susceptibility gene for inflammatory bowel disease as the VDR gene maps to a region on chromosome 12 that has been strongly linked with these autoimmune diseases. In a European study of 158 patients with ulcerative colitis, 245 with Crohn's disease, and 164 cadaveric renal allograft donor controls, significantly more homozygotes for the TaqI polymorphism at codon 352 of exon 8 (genotype "tt") were found among patients with Crohn's disease compared to those with ulcerative colitis or controls (odds ratio 1.99; 95 % confidence interval, 1.14–3.47; p = 0.017) [96].
Primary Biliary Cirrhosis
Primary biliary cirrhosis (PBC) is an autoimmune disease of the liver, with female to male ratio of at least 9:1. This liver disease is marked by slow progressive destruction of the small bile ducts (bile canaliculi) within the liver, and eventually, liver scarring, fibrosis, and cirrhosis. PBC is a model autoimmune disease characterized by a highly directed immune response to pyruvate dehydrogenase (PDC-E2) [97, 98]. Similarly to other autoimmune diseases, a mosaic of genetic and environmental factors was suggested to play a role in its pathogenesis. VDRs’ polymorphisms were documented to be associated with increased susceptibility to PBC in Japanese, Italian, and Polish populations [98, 99]. Additionally, we have recently analyzed a cohort of 78 patients with PBC, which demonstrated significantly lower levels of vitamin D compared to healthy matched controls. Moreover, vitamin D levels correlated with markers of PBC disease activity (unpublished data).
Autoimmune Skin Diseases
Hypovitaminosis D was reported in patients with autoimmune bullous skin diseases [100], as well as in patients with alopecia areata. In contrast to former studies, VDR polymorphisms were not associated with the presence of alopecia [101]. The significance of these observations requires further studies.
Conclusions
Epidemiological, genetic, and basic studies indicate a potential role of vitamin D in the pathogenesis of certain autoimmune diseases, most of which demonstrate a correlation between low levels of vitamin D and disease appearance or manifestations. The presence of VDR polymorphisms may further support such plausible pathogenic link. Nevertheless, the current available data hold many inconsistencies, and a major limitation of present cross-sectional studies is that reverse causation is also likely as low vitamin D level may be the consequence, not the cause. Therefore, further studies addressing the mechanisms by which vitamin D status affect autoimmunity as well as large epidemiological studies are in need.
Vitamin D supplementation is crucial for the maintenance of bone density. Such supplementation was suggested to be also beneficial for the primary prevention of autoimmune diseases as diabetes mellitus and multiple sclerosis [11].The effects of treatment with vitamin D, or its analogues on established autoimmune diseases, have been addressed only in several small open-label studies and interventional trials. In an open-label observational study of 60 SLE patients in Spain who took vitamin D3 supplementation for 2 years, significant improvement was seen in subject fatigue as measured by a visual analog scale, but not with SLE disease activity measures [39]. Studies assessing vitamin D treatment for patients suffering from multiple sclerosis, rheumatoid arthritis, and Crohn's disease were detailed above.
Presently, there is a consensus regarding the target range for serum 25-OH-levels of vitamin D (i.e., at least 30 to 40 ng/mL), and the safety of supplementation doses of 2,000-4,000 IU/day [12]. However, the appropriate regimen for vitamin D therapy that will be sufficient to modulate immunological homeostasis (e.g., 10,000 IU/day or more) is yet to be determined. Also, novel vitamin D analogues with more pronounced immune modulation effects and lower activity on calcium metabolism are in the pipeline, and might represent a great innovative opportunity for the treatment of vitamin D deficiency in autoimmune disorders. Randomized controlled trials are necessary to establish the clinical efficacy of different vitamin D supplementation protocols as well as the required dose or increment in vitamin D serum levels. Nonetheless, evidence regarding the purported benefit of vitamin D supplementation on a multitude of health outcomes has been reported worldwide. Furthermore, given the fact that supplementation of vitamin D in its native form is harmless and inexpensive, we believe that time has come to advocate for a wider use of vitamin D supplementation.
References
Arnson Y, Amital H, Shoenfeld Y (2007) Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis 66(9):1137–1142
Holick MF (2007) Vitamin D, deficiency. N Engl J Med 357(3):266–281
Peelen E, Knippenberg S, Muris AH, Thewissen M, Smolders J, Tervaert JW et al (2011) Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev 10(12):733–743
Toubi E, Shoenfeld Y (2010) The role of vitamin D in regulating immune responses. Isr Med Assoc J 12(3):174–175
Pelajo CF, Lopez-Benitez JM, Miller LC (2010) Vitamin D and autoimmune rheumatologic disorders. Autoimmun Rev 9(7):507–510
Shapira Y, Agmon-Levin N, Shoenfeld Y (2010) Mycobacterium tuberculosis, autoimmunity, and vitamin D. Clin Rev Allergy Immunol 38(2–3):169–177
Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE (2007) Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179(3):1634–1647
Cutolo M, Plebani M, Shoenfeld Y, Adorini L, Tincani A (2011) Vitamin D endocrine system and the immune response in rheumatic diseases. Vitam Horm 86:327–351
Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C et al (2011) 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol 31(17):3653–3669
Khoo AL, Koenen HJ, Chai LY, Sweep FC, Netea MG, van der Ven AJ et al (2012) Seasonal variation in vitamin D(3) levels is paralleled by changes in the peripheral blood human T cell compartment. PLoS One 7(1):e29250
Antico A, Tampoia M, Tozzoli R, Bizzaro N (2012) Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev 12(2):127–136
Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ et al (2010) Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 9(11):709–715
Broder AR, Tobin JN, Putterman C (2010) Disease-specific definitions of vitamin D deficiency need to be established in autoimmune and non-autoimmune chronic diseases: a retrospective comparison of three chronic diseases. Arthritis Res Ther 12(5):R191
Shoenfeld N, Amital H, Shoenfeld Y (2009) The effect of melanism and vitamin D synthesis on the incidence of autoimmune disease. Nat Clin Pract Rheumatol 5(2):99–105
Oren Y, Shapira Y, Agmon-Levin N, Kivity S, Zafrir Y, Altman A et al (2010) Vitamin D insufficiency in a sunny environment: a demographic and seasonal analysis. Isr Med Assoc J 12(12):751–756
Shapira Y, Agmon-Levin N, Shoenfeld Y (2010) Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun 34(3):J168–J177
Carmi G, Amital H (2011) The geoepidemiology of autoimmunity: capsules from the 7th International Congress on Autoimmunity, Ljubljana, Slovenia, May 2010. Isr Med Assoc J 13(2):121–127
Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL et al (2009) Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis 15(11):1656–1662
Cutolo M, Pizzorni C, Sulli A (2011) Vitamin D endocrine system involvement in autoimmune rheumatic diseases. Autoimmun Rev 11(2):84–87
Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y (2009) Infections and autoimmunity—friends or foes? Trends Immunol 30(8):409–414
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR et al (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311(5768):1770–1773
Barzilai O, Sherer Y, Ram M, Izhaky D, Anaya JM, Shoenfeld Y (2007) Epstein–Barr virus and cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann N Y Acad Sci 1108:567–577
Yenamandra SP, Lundin A, Arulampalam V, Yurchenko M, Pettersson S, Klein G et al (2009) Expression profile of nuclear receptors upon Epstein–Barr virus induced B cell transformation. Exp Oncol 31(2):92–96
Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS (2006) Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev 5(2):114–117
Cutolo M, Otsa K (2008) Review: vitamin D, immunity and lupus. Lupus 17(1):6–10
Borba VZ, Vieira JG, Kasamatsu T, Radominski SC, Sato EI, Lazaretti-Castro M (2009) Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int 20(3):427–433
Kim HA, Sung JM, Jeon JY, Yoon JM, Suh CH (2011) Vitamin D may not be a good marker of disease activity in Korean patients with systemic lupus erythematosus. Rheumatol Int 31(9):1189–1194
Ruiz-Irastorza G, Egurbide MV, Olivares N, Martinez-Berriotxoa A, Aguirre C (2008) Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatol (Oxford) 47(6):920–923
Amital H, Szekanecz Z, Szucs G, Danko K, Nagy E, Csepany T et al (2010) Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis 69(6):1155–1157
Lopez-Robles C, Rios-Fernandez R, Callejas-Rubio JL, Ortego-Centeno N (2011) Vitamin D deficiency in a cohort of patients with systemic lupus erythematous from the South of Spain. Lupus 20(3):330–331
Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R et al (2001) Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 44(10):2331–2337
Wu PW, Rhew EY, Dyer AR, Dunlop DD, Langman CB, Price H et al (2009) 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Rheum 61(10):1387–1395
Luo XY, Yang MH, Wu FX, Wu LJ, Chen L, Tang Z et al (2012) Vitamin D receptor gene BsmI polymorphism B allele, but not BB genotype, is associated with systemic lupus erythematosus in a Han Chinese population. Lupus 21(1):53–59
Ozaki Y, Nomura S, Nagahama M, Yoshimura C, Kagawa H, Fukuhara S (2000) Vitamin-D receptor genotype and renal disorder in Japanese patients with systemic lupus erythematosus. Nephron 85(1):86–91
Sakulpipatsin W, Verasertniyom O, Nantiruj K, Totemchokchyakarn K, Lertsrisatit P, Janwityanujit S (2006) Vitamin D receptor gene BsmI polymorphisms in Thai patients with systemic lupus erythematosus. Arthritis Res Ther 8(2):R48
Kornberg A, Blank M, Kaufman S, Shoenfeld Y (1994) Induction of tissue factor-like activity in monocytes by anti-cardiolipin antibodies. J Immunol 153(3):1328–1332
Orbach H, Zandman-Goddard G, Amital H, Barak V, Szekanecz Z, Szucs G et al (2007) Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci 1109:385–400
Agmon-Levin N, Blank M, Zandman-Goddard G, Orbach H, Meroni PL, Tincani A et al (2011) Vitamin D: an instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann Rheum Dis 70(1):145–150
Lindqvist PG, Epstein E, Olsson H (2009) Does an active sun exposure habit lower the risk of venous thrombotic events? A D-lightful hypothesis. J Thromb Haemost 7(4):605–610
Boissier MC, Semerano L, Challal S, Saidenberg-Kermanac'h N, Falgarone G (2012) Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun 39(3):222–8
Welsh P, Peters MJ, Sattar N (2011) Is vitamin D in rheumatoid arthritis a magic bullet or a mirage? The need to improve the evidence base prior to calls for supplementation. Arthritis Rheum 63(7):1763–1769
Zold E, Szodoray P, Nakken B, Barath S, Kappelmayer J, Csathy L et al (2011) Alfacalcidol treatment restores derailed immune-regulation in patients with undifferentiated connective tissue disease. Autoimmun Rev 10(3):155–162
Kroger H, Penttila IM, Alhava EM (1993) Low serum vitamin D metabolites in women with rheumatoid arthritis. Scand J Rheumatol 22(4):172–177
Cutolo M, Otsa K, Laas K, Yprus M, Lehtme R, Secchi ME et al (2006) Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol 24(6):702–704
Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D (2007) Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum 56(7):2143–2149
Straube S, Derry S, Moore RA, McQuay HJ (2010) Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev 2010(1):CD007771
Laragione T, Shah A, Gulko PS (2012) The vitamin D receptor regulates rheumatoid arthritis synovial fibroblast invasion and morphology. Mol Med 18(1):194–200
Baker JF, Baker DG, Toedter G, Shults J, Von Feldt JM, Leonard MB (2012) Associations between vitamin D, disease activity, and clinical response to therapy in rheumatoid arthritis. Clin Exp Rheumatol 30(5):658–64
Hiraki LT, Munger KL, Costenbader KH, Karlson EW (2012) Dietary intake of vitamin d during adolescence and risk of adult onset systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res (Hoboken) 64(12):1829–36
Harari M, Dramsdahl E, Shany S, Baumfeld Y, Ingber A, Novack V et al (2011) Increased vitamin D serum levels correlate with clinical improvement of rheumatic diseases after Dead Sea climatotherapy. Isr Med Assoc J 13(4):212–215
Wen H, Baker JF (2011) Vitamin D, immunoregulation, and rheumatoid arthritis. J Clin Rheumatol 17(2):102–107
Leventis P, Patel S (2008) Clinical aspects of vitamin D in the management of rheumatoid arthritis. Rheumatol (Oxford) 47(11):1617–1621
Ibn Yacoub Y, Amine B, Laatiris A, Wafki F, Znat F, Hajjaj-Hassouni N (2012) Bone density in Moroccan women with systemic scleroderma and its relationships with disease-related parameters and vitamin D status. Rheumatol Int 32(10):3143–8
Gambichler T, Chrobok I, Hoxtermann S, Kreuter A (2010) Significantly decreased serum 25-hydroxyvitamin d levels in a large German systemic sclerosis cohort. J Rheumatol 38(11):2492–2493, author reply 4
Rios Fernandez R, Fernandez Roldan C, Callejas Rubio JL, Ortego Centeno N (2010) Vitamin D deficiency in a cohort of patients with systemic scleroderma from the south of Spain. J Rheumatol 37(6):1355, author reply 6
Vacca A, Cormier C, Piras M, Mathieu A, Kahan A, Allanore Y (2009) Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. J Rheumatol 36(9):1924–1929
Seriolo B, Molfetta L, Cutolo M (2011) Seasonal variations in serum levels of 25-hydroxyvitamin D in patients with systemic sclerosis. Clin Rheumatol 30(3):445–446
Arnson Y, Amital H, Agmon-Levin N, Alon D, Sanchez-Castanon M, Lopez-Hoyos M et al (2011) Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature. Autoimmun Rev 10(8):490–494
Caramaschi P, Dalla Gassa A, Ruzzenente O, Volpe A, Ravagnani V, Tinazzi I et al (2010) Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol 29(12):1419–1425
Muller K, Oxholm P, Sorensen OH, Thymann M, Hoier-Madsen M, Bendtzen K (1990) Abnormal vitamin D3 metabolism in patients with primary Sjogren's syndrome. Ann Rheum Dis 49(9):682–684
Szodoray P, Horvath IF, Papp G, Barath S, Gyimesi E, Csathy L et al (2010) The immunoregulatory role of vitamins A, D and E in patients with primary Sjogren's syndrome. Rheumatol (Oxford) 49(2):211–217
Agmon-Levin N, Kivity S, Tzioufas AG, Lopez Hoyos M, Rozman B, Efes I et al (2012) Low levels of vitamin-D are associated with neuropathy and lymphoma among patients with Sjogren's syndrome. J Autoimmun 39(3):234–239
Cutolo M (2008) Vitamin D, or hormone D deficiency in autoimmune rheumatic diseases, including undifferentiated connective tissue disease. Arthritis Res Ther 10(6):123
Zold E, Szodoray P, Gaal J, Kappelmayer J, Csathy L, Gyimesi E et al (2008) Vitamin D deficiency in undifferentiated connective tissue disease. Arthritis Res Ther 10(5):R123
Hajas A, Sandor J, Csathy L, Csipo I, Barath S, Paragh G et al (2011) Vitamin D insufficiency in a large MCTD population. Autoimmun Rev 10(6):317–324
Robinson AB, Thierry-Palmer M, Gibson KL, Rabinovich CE (2012) Disease activity, proteinuria, and vitamin D status in children with systemic lupus erythematosus and juvenile dermatomyositis. J Pediatr 160(2):297–302
Sadovnick AD (2012) Genetic background of multiple sclerosis. Autoimmun Rev 11(3):163–166
Sellner J, Kraus J, Awad A, Milo R, Hemmer B, Stuve O (2011) The increasing incidence and prevalence of female multiple sclerosis—a critical analysis of potential environmental factors. Autoimmun Rev 10(8):495–502
Hanwell HE, Banwell B (2011) Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim Biophys Acta 1812(2):202–212
Ascherio A, Munger KL, Simon KC (2010) Vitamin D and multiple sclerosis. Lancet Neurol 9(6):599–612
Simpson S Jr, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H et al (2010) Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 68(2):193–203
Smolders J, Thewissen M, Theunissen R, Peelen E, Knippenberg S, Menheere P et al (2011) Vitamin D-related gene expression profiles in immune cells of patients with relapsing remitting multiple sclerosis. J Neuroimmunol 235(1–2):91–97
Sternberg Z (2012) Autonomic dysfunction: a unifying multiple sclerosis theory, linking chronic cerebrospinal venous insufficiency, vitamin D(3), and Epstein–Barr virus. Autoimmun Rev 12(2):250–9
Stein MS, Liu Y, Gray OM, Baker JE, Kolbe SC, Ditchfield MR et al (2011) A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 77(17):1611–1618
Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R et al (2010) A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 74(23):1852–1859
Smolders J, Peelen E, Thewissen M, Cohen Tervaert JW, Menheere P, Hupperts R et al (2010) Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One 5(12):e15235
Simon KC, Munger KL, Xing Y, Ascherio A (2010) Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler 16(2):133–138
Agliardi C, Guerini FR, Saresella M, Caputo D, Leone MA, Zanzottera M et al (2011) Vitamin D receptor (VDR) gene SNPs influence VDR expression and modulate protection from multiple sclerosis in HLA-DRB1*15-positive individuals. Brain Behav Immun 25(7):1460–1467
Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Danko K et al (2011) Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol 8(3):243–247
Twig G, Shina A, Amital H, Shoenfeld Y (2012) Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun 38(2–3):J275–J281
Goswami R, Marwaha RK, Gupta N, Tandon N, Sreenivas V, Tomar N et al (2009) Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: a community-based survey. Br J Nutr 102(3):382–386
Effraimidis G, Badenhoop K, Tijssen JG, Wiersinga WM (2012) Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur J Endocrinol 167(1):43–48
Lin WY, Wan L, Tsai CH, Chen RH, Lee CC, Tsai FJ (2006) Vitamin D receptor gene polymorphisms are associated with risk of Hashimoto's thyroiditis in Chinese patients in Taiwan. J Clin Lab Anal 20(3):109–112
Stefanic M, Papic S, Suver M, Glavas-Obrovac L, Karner I (2008) Association of vitamin D receptor gene 3'-variants with Hashimoto's thyroiditis in the Croatian population. Int J Immunogenet 35(2):125–131
Stefanic M, Karner I, Glavas-Obrovac L, Papic S, Vrdoljak D, Levak G et al (2005) Association of vitamin D receptor gene polymorphism with susceptibility to Graves' disease in Eastern Croatian population: case–control study. Croat Med J 46(4):639–646
Garcia-Manzanares A, Tenias JM, Lucendo AJ (2012) Bone mineral density directly correlates with duodenal Marsh stage in newly diagnosed adult celiac patients. Scand J Gastroenterol 8–9(47):927–36
Lerner A, Shapira Y, Agmon-Levin N, Pacht A, Ben-Ami Shor D, Lopez HM et al (2012) The clinical significance of 25OH-vitamin D status in celiac disease. Clin Rev Allergy Immunol 42(3):322–330
Boucher BJ (2011) Vitamin D, insufficiency and diabetes risks. Curr Drug Targets 12(1):61–87
Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C (2011) Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol 347(1–2):106–120
Takiishi T, Gysemans C, Bouillon R, Mathieu C (2010) Vitamin D and diabetes. Endocrinol Metab Clin North Am 39(2):419–446, table of contents
Sorensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC (2012) Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes 61(1):175–178
Bizzarri C, Pitocco D, Napoli N, Di Stasio E, Maggi D, Manfrini S et al (2010) No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care 33(9):1962–1963
Aljabri KS, Bokhari SA, Khan MJ (2010) Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Ann Saudi Med 30(6):454–458
McCarthy D, Duggan P, O'Brien M, Kiely M, McCarthy J, Shanahan F et al (2005) Seasonality of vitamin D status and bone turnover in patients with Crohn's disease. Aliment Pharmacol Ther 21(9):1073–1083
Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A (2009) 25 (OH) vitamin D level in Crohn's disease: association with sun exposure & disease activity. Indian J Med Res 130(2):133–137
Simmons JD, Mullighan C, Welsh KI, Jewell DP (2000) Vitamin D receptor gene polymorphism: association with Crohn's disease susceptibility. Gut 47(2):211–214
Selmi C, Meroni PL, Gershwin ME (2012) Primary biliary cirrhosis and Sjogren's syndrome: autoimmune epithelitis. J Autoimmun 39(1–2):34–42
Kempinska-Podhorecka A, Wunsch E, Jarowicz T, Raszeja-Wyszomirska J, Loniewska B, Kaczmarczyk M et al (2012) Vitamin d receptor polymorphisms predispose to primary biliary cirrhosis and severity of the disease in polish population. Gastroenterol Res Pract 2012:408723
Tanaka A, Nezu S, Uegaki S, Kikuchi K, Shibuya A, Miyakawa H et al (2009) Vitamin D receptor polymorphisms are associated with increased susceptibility to primary biliary cirrhosis in Japanese and Italian populations. J Hepatol 50(6):1202–1209
Marzano AV, Trevisan V, Eller-Vainicher C, Cairoli E, Marchese L, Morelli V et al (2012) Evidence for vitamin D deficiency and increased prevalence of fractures in autoimmune bullous skin diseases. Br J Dermatol 167(3):688–91
Akar A, Orkunoglu FE, Tunca M, Tastan HB, Kurumlu Z (2007) Vitamin D receptor gene polymorphisms are not associated with alopecia areata. Int J Dermatol 46(9):927–929
Author information
Authors and Affiliations
Corresponding author
Additional information
Nancy Agmon-Levin and Emanuel Theodor contributed equally to this article.
Rights and permissions
About this article
Cite this article
Agmon-Levin, N., Theodor, E., Segal, R.M. et al. Vitamin D in Systemic and Organ-Specific Autoimmune Diseases. Clinic Rev Allerg Immunol 45, 256–266 (2013). https://doi.org/10.1007/s12016-012-8342-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-012-8342-y