Abstract
Vitamin D has a crucial role in preserving musculoskeletal health and modulating the immune system; the latter has been a subject of great interest in recent years. Vitamin D deficiency is common in the general population and even more so among patients with various autoimmune diseases. Vitamin D deficiency has been previously linked to various autoimmune diseases, including multiple sclerosis, type 1 diabetes, inflammatory bowel diseases, and rheumatoid arthritis. In this chapter, the association between vitamin D deficiency and various autoimmune diseases will be discussed, as well as the possible therapeutic implications derived from this relationship.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Autoimmune diseases are characterized by an abnormal response of the immune system toward antigens of the body’s own cells due to lack of self-tolerance. The various diseases differ from one another in the specific immune cells involved in the autoimmune process, the self-antigen targeted by the affected immune system, and, consequently, their signs and symptoms.
There are more than 80 known autoimmune diseases; their prevalence in the general population is estimated to be 5–20%. Genetic, immunological, hormonal, and environmental factors which may contribute to or increase the probability of autoimmune disease have been researched in recent years. One of the environmental factors studied has been vitamin D [1, 2].
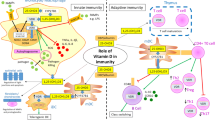
In recent years, evidence has accumulated regarding vitamin D’s immunomodulatory features and its significant role in various processes of the immune system [1, 3]. Vitamin D receptors (VDR) were found in immune cells, including macrophages, dendritic cells, B cells, and T cells [1, 4, 5]; in vitro studies demonstrated the vitamin’s direct effect on their activity [6].
The vitamin D receptor is a nuclear hormone receptor. The gene for this receptor incorporates many polymorphisms, four of which have been extensively studied: TaqI, BsmI, ApaI, and FokI. These polymorphisms may affect the receptor’s function and thereby affect the serum levels of active vitamin D [7]. Several of these polymorphisms were previously linked to autoimmune diseases [8, 9].
Modern living has shifted human activity away from the sunlight, and consequently exposed us to a variety of autoimmune diseases whose prevalence is constantly on the rise. Among the diseases found to be related to vitamin D deficiency are multiple sclerosis, type I diabetes, inflammatory bowel diseases, and rheumatoid arthritis [1, 10, 11].
Vitamin D Deficiency
The definition of vitamin D deficiency is controversial in the medical literature; an accepted definition is a serum level of 25-hydroxy-vitamin D (25(OH)D—the measured form of vitamin D in the blood) under 30–40 ng/mL, as lower levels lead to activation of the parathyroid glands, which results in bone absorption and damage [12] as well as increased risk for malignancies, cardiovascular diseases, and autoimmune diseases [13]. Some researchers further divide deficiencies into insufficiency (21–29 ng/mL) and deficiency (under 20 ng/mL) [7, 14,15,16]; in some publications, the definition of vitamin D deficiency is even lower [12, 17]. An optimal level for the functioning of the immune system, within the normal range of serum vitamin D concentration, is yet to be determined [7].
Vitamin D deficiency is more common in patients with autoimmune diseases than in healthy subjects [18,19,20,21]. One risk factor for vitamin D deficiency is certain medications used for treatment of autoimmune diseases, such as glucocorticoids; kidney damage is another risk factor, one that can also be the result of autoimmune diseases [12, 22].
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE ) is a chronic systemic autoimmune disease. Its manifestations involve almost all systems of the body, including the musculoskeletal, cardiovascular, and central nervous systems, as well as the kidneys and the skin. Signs and symptoms of the disease vary between patients, as does the severity. SLE is more common in women than in men, in a ratio of about 7:1 [23].
For SLE patients, sunlight is a double-edged sword, while sunlight produces vitamin D, which has a beneficial immunomodulatory effect, ultraviolet (UV) exposure in lupus patients worsens the disease’s manifestations, primarily the cutaneous symptoms; therefore sun avoidance is advised [24].
Vitamin D deficiency is more common in lupus patients than in healthy subjects [20, 21]. Similar findings were demonstrated in teenagers and young adults diagnosed with juvenile-onset systemic lupus erythematosus (JoSLE) [25, 26]. There are several possible explanations for the difference between healthy subjects and patients with lupus. As mentioned previously, SLE patients are instructed to avoid exposure to sunlight due to photosensitivity, contributing to their vitamin D deficiency. In addition, the disease causes renal dysfunction, which interferes with the activation of the vitamin. Lastly, medical treatments for lupus, such as steroids, can harm the metabolism of the vitamin [4, 16, 27, 28].
In various molecular studies, vitamin D was found to have an attenuating effect on various immune cells that participate in the pathogenesis of the disease, including neutrophils [29], dendritic cells [30], and T regulatory cells (Treg) [31,32,33].
A study by Schoindre et al. examined the association between 25(OH)D levels and disease activity and flares. The investigators found an association between low levels of the vitamin and a higher SLE activity; however, they did not find a similar association with risk of flares during the 6 months following the measurement [17]. A study by Lertratanakul et al. presented the complementing finding that higher vitamin levels are associated with a lower disease activity [34]. Similar findings were also reported in studies performed on adults in Australia [35], India [36], Egypt [37], and Jamaica [15], as well in a study on adolescents [25]. These findings were also supported in recent literature reviews [4, 16, 28, 38]. Vitamin D levels were also associated with other manifestations of the disease, such as cognitive impairment [39].
However, in other studies of more limited scale, the investigators were unable to show a correlation between vitamin D deficiency and disease activity measured in different disease activity scales [14, 26, 27, 40, 41]. No link was found between vitamin D levels and SLE flares, even when flares were self-reported [20].
In a literature review published in 2014, intervening variables for the association between vitamin D levels and SLE manifestations were analyzed. The most significant variables that were found include medications (hydroxychloroquine, steroids, and vitamin D supplements), body mass index (BMI), renal function, and proteinuria [40].
The assumed link between vitamin D deficiency and lupus, which was supported by some studies, has led to attempts to better understand its nature and direction in interventional studies using vitamin D supplements. The conventional doses in the literature are 800, 2000, and 4000 IU per day, with some studies suggesting adjustment of the dose to patient baseline vitamin levels and individual risk factors (BMI, use of steroids, etc.) [4, 7].
In a large-scale cohort study, vitamin D supplementation was given to lupus patients with 25(OH)D levels lower than 40 ng/mL (50,000 IU of D2 per week and 200 calcium/D3 units each, two times a day). Improvement in proteinuria was shown in patients with higher 25(OH)D levels, and a correlation was observed between disease activity and the change in 25(OH)D values. Notably, this correlation was only seen in patients who were vitamin D deficient at the beginning of the study. No effect on disease activity was found after increasing the vitamin D levels above 40 ng/mL [42]. Similar findings were shown in adolescents and young adults; vitamin D supplements (50,000 IU per week, for 24 weeks) decreased disease activity and improved fatigue [43].
In contrast to the previously mentioned study, a recent review of the literature did not reach definite conclusions regarding the effectiveness of vitamin D supplements on patients [7]. A more recent interventional study, conducted in 2015, also failed to demonstrate vitamin D supplements effectiveness: although a high-dose regimen elevated serum levels of vitamin D, no significant difference was found between the treatment groups in disease activity and serological markers [44].
Currently, there is no across-the-board recommendation to give vitamin D supplements to lupus patients, apart from those patients that are treated with steroids, for whom a dose of 800–1000 units per day is recommended [45].
Type 1 Diabetes Mellitus
Type 1 diabetes mellitus , previously known as juvenile diabetes or insulin-dependent diabetes, is an autoimmune disease that is usually diagnosed during childhood or adolescence, typically before the age of 30. It is caused by an autoimmune response against beta cells in the pancreas, leading to their gradual destruction and to insulin deficiency [46].
The association between diabetes type 1 and vitamin D deficiency was investigated in an epidemiological study. In this study, researchers investigated the relationship between ultraviolet B (UVB) irradiance in different locations worldwide and the incidence of type 1 diabetes in children. Researchers assumed that UVB irradiation, as the primary source of vitamin D in the human body, could be used as a rough estimation of the vitamin’s circulating levels. They demonstrated a correlation between the disease’s rates and the patients’ distance from the equator: higher rates of the disease were found at higher geographic latitudes, in which UVB is scarce (R 2 = 0.25, p < 0.001) [47].
In a cohort study from Finland, 10,366 children were followed for 31 years, from 1966 to 1997. The researchers wished to investigate whether vitamin D supplementation in early life influenced the risk for development of type 1 diabetes later in life. They found that regular administration of vitamin D supplementation (2000 IU per day) in the first year of life was associated with reduced risk for the disease )0.12, 95% CI 0.03–0.51 relative risk (RR((. They also found that children suspected of suffering from rickets, which is caused by vitamin D deficiency, during their first year of life were more susceptible to develop type 1 diabetes later on) RR 3.0, CI 1.0–9.0 ([48].
Similarly, a large-scale population-based study from multiple centers in Europe demonstrated a reduction in risk for type 1 diabetes in countries where vitamin D supplementation during infancy is prevalent [49]; a meta-analysis done on this subject reached similar conclusions [50]. A different study from Norway concluded that children whose mothers who suffered from vitamin D deficiency during their pregnancy were more than twice as likely to develop type 1 diabetes [51]. These findings suggest the great importance of sufficient levels of vitamin D during infancy, neonatal, and even prenatal periods.
Vitamin D supplementation improved glycemic control of type 1 diabetes, as measured by Hemoglobin A1C levels, in pediatric patients who suffered from vitamin D deficiency [52]. Normal vitamin D levels in diabetes patients were correlated with lower prevalence of macroalbuminuria, a marker for diabetic nephropathy, but not with other complications of the disease, such as retinopathy and cardiovascular diseases [53].
Animal models studies also support this association, and further demonstrate a direct effect of vitamin D on the pancreas and its function. Zeitz generated mice with inactive mutant VDR. Compared to wild type mice, the mutant mice had elevated glucose levels and reduced insulin secretion in response to glucose administration [54].
Multiple Sclerosis
Multiple sclerosis (MS) is an inflammatory disease that causes demyelination of the central nervous system. The disease usually has a relapsing and remitting course; its clinical manifestations include sensory disturbances, motor weakness, diplopia, gait and balance disturbance, vertigo, and bladder dysfunction [55]. These symptoms are believed to be caused by inflammation, demyelination, and axonal damage [56].
An extensive review, done in 2008, suggests that distance from the equator is the strongest risk factor for MS, seemingly due to UV radiation exposure. Also, emigration from cold countries, with relatively low UV irradiation (e.g., United Kingdom), to sunny ones (e.g., South Africa) decreased the risk for MS [57]. A seasonal pattern was described for the clinical course of MS, which, at least in part, can be explained by vitamin D levels [58].
Munger et al. analyzed data from two very large cohort studies of women: “Nurses’ Health Study” (92,253 participants) and “Nurses’ Health Study II” (95,310 participants). Dietary and supplementary vitamin D intake was assessed at baseline and every 4 years. The study found that the risk for developing MS was lower in women with high vitamin D intake, compared to women with low intake (RR 0.67, 95% CI 0.40–1.12; p = 0.03), and vitamin D supplementation was inversely associated with MS risk. Notably, high dietary intake of vitamin D alone did not generate similar association [59].
MHC (major histocompatibility complex) class II are a family of molecules that is found in specific cells of the immune system known as antigen-presenting cells. Genetic variations in these molecules greatly influence the function of the immune system and were previously associated with autoimmunity [60]. MHC class II allele HLA-DRB1*15, which was previously linked to increased risk for MS, is relatively common among individuals of Northern European descent [61]. Ramagopalan et al. wished to demonstrate a mechanism by which genetic and environmental factors of MS interact. In their molecular study, they showed a direct impact of vitamin D on HLA-DRB1*1501, as the vitamin influences its expression in lymphocytes [62].
Vitamin D was reported to be an early predictor of MS activity and progression: slower progression and low disease activity were seen in patients with high serum vitamin D levels at the time of MS diagnosis. An increase of 50 nmol/L in average serum vitamin D levels in the first year from diagnosis was associated with less active lesions in magnetic resonance imaging (MRI) scans and lower relapse rate [63,64,65].
In an interventional study, increased levels of transforming growth factor-β1(TGF-β1) were measured in response to vitamin D supplementation in MS patients after 6 months’ treatment [66].
Inflammatory Bowel Diseases
Inflammatory bowel diseases (IBD ) are characterized by chronic inflammation of the gastrointestinal tract. The underlying mechanism for these diseases remains unknown, although the mechanism seems to involve overactivation of the immune system in response to certain antigens in individuals with genetic predisposition. Clinical manifestations of IBD vary and can include abdominal pain, diarrhea, weight loss, and anemia. There are two main diseases in this category: Crohn’s disease (CD) and ulcerative colitis (UC); the two differ in their clinical, histological, and epidemiological characters [67, 68].
Vitamin D deficiency was found in 49.8% of IBD patients in a retrospective cohort study and severe deficiency in 10.9% of them. Moreover, low levels of vitamin D were associated with higher disease activity and lower quality of life in patients with CD (though not patients with UC) [69]. One factor that may mediate this association is malabsorption, a common condition in IBD patients, which can influence the absorption of vitamin D from food. However, the main source for vitamin D in the human body is its synthesis in the skin; therefore the influence of malabsorption as a mediator is limited. Another possible mediator for this association is the regulatory effect of vitamin D on the innate immune system’s response to intestinal microbiota; the intestinal microbiota is one of the environmental factors that has been associated with IBD in recent year [70].
To examine the relationship between vitamin D and IBD, a team created an experimental animal model of IBD by creating interleukin 10 (IL-10) knockout (KO) mice, who developed the inflammatory bowel disease spontaneously. They created three groups: a control group, an experimental group that was vitamin D deficient, and an experimental group that was deficient early in life, but later supplemented with the vitamin. Both experimental groups showed severe clinical manifestations of the disease, however, vitamin D supplementation ameliorated symptoms of the disease. Mice in the control group did not develop the disease [71]. Other supporting evidence for this association can be found in another animal model experiment, in which IL-10 KO mice, as well as VDR KO mice, developed severe IBD with high mortality rates [72].
Inflammatory bowel diseases have extra intestinal manifestations and a deteriorating effect on bone health; IBD patients are more prone to suffer from osteoporosis and fractures. One suggested explanations for these issues is malabsorption of calcium from digested food. It has also been suggested that inflammatory mediators impact the activity of osteoblasts or bone-forming cells [73]. Hence, in IBD patients, vitamin D supplementation importance is doubly important, both for modulation of the immune system and for preserving bone health .
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic and systemic autoimmune disease that is usually characterized by symmetric polyarticular inflammation. Non-articular involvement of the disease can be seen in almost all systems of the body, including the skin, eye, lung, heart, kidney, and blood vessels.
Vitamin D deficiency is common among male RA patients, and even more so in patients who developed anti-cyclic citrullinated peptide antibodies—a specific, yet nonsensitive, marker of the disease [74].
When vitamin D levels and RA symptoms were compared between populations of Northern and Southern Europe, an inverse association was found between vitamin D levels and RA disease activity. Notably, this association was found only during the summer months [75].
A cohort study, comprised of 29,368 women over the course of 11 years, showed that intake of vitamin D supplementations was inversely associated with the risk of RA (p = 0.03), while dietary intake of vitamin D showed a similar trend but did not reach significance (p = 0.16) [76]. In a different study that analyzed data from the “Nurses’ Health Study,” no association was found between dietary intake of vitamin D and RA risk [77].
All three studies imply a fundamental difference between dietary and supplementary intake of vitamin D. This difference could be explained by the retrospective design of the studies and the methodological difficulty in assessing dietary intake of vitamin D, in contrast to intake of supplements.
Psoriasis
Psoriasis is a chronic inflammatory skin disorder. One of the disease’s manifestations is hyperproliferation of the skin, which results in erythematous plaques with or without silver scale over them.
Lower levels of vitamin D deficiency were observed in psoriasis patients compared to healthy control patients in a case-control study by Orgaz-Molina et al. [78]. Also, VDR polymorphism in ApaI was associated with psoriasis, as well as with early onset of the disease [79].
In addition to the immunomodulatory effect of vitamin D, another important feature of the vitamin was discovered—as an antiproliferative agent, specifically in keratinocytes. Previous studies demonstrated that keratinocytes have VDR and that activated vitamin D inhibited their proliferation and stimulated their maturation in vitro [80], making activated vitamin D and its analogues good therapeutic agents for psoriasis [81]. Therefore, topical use of vitamin D analogs (calcipotriol, calcitriol, and tacalcitol) is an essential part of psoriasis treatment [82].
Other Autoimmune Diseases
Vitamin D deficiency was previously associated with several other autoimmune diseases.
Primary biliary cholangitis (primary biliary cirrhosis—PBC) and autoimmune hepatitis (AIH) are chronic autoimmune inflammatory diseases of the liver. Different genetic polymorphisms of VDR were associated with both diseases, BsmI polymorphisms in PBC patients (χ 2 = 9.49, p = .009) and FokI in AIH patients (χ 2 = 9.71, p = .008) [8].
VDR polymorphism (BsmI and TaqI) was also associated with autoimmune thyroid diseases (AITD ) [83]. Moreover, vitamin D deficiency was found to be more common among patients with autoimmune thyroid diseases compared to patients with non-autoimmune thyroid diseases and to healthy controls; vitamin D deficiency also correlated with presence of antithyroid antibodies [84].
Low levels of vitamin D were inversely associated with disease’s severity, both in Behçet’s disease, a type of immune-mediated small-vessel systemic vasculitis [85], and in systemic sclerosis (SS), an autoimmune connective tissue disease [86]. Vitamin D levels were found to be lower in diffuse cutaneous type of SS (dcSSc) compared to limited cutaneous type (lcSSc) and negatively associated with patients’ skin thickness [87].
Possible Underlying Mechanism
In the past decades, since the discovery of VDR on activated T cells [5], there has been growing interest in the various effects of vitamin D on T cells.
Type 1 T helper cells (Th1) and their subset of cytokine, including IL-2, IL-12, interferon gamma (IFN-γ), and tumor necrosis factor (TNF), have been previously associated with autoimmune processes. Specifically, Th1 are involved in organ-specific autoimmune diseases [88, 89] like many of the diseases mentioned above. Another subset of T helper cells, Th17, which was discovered more recently, also has a crucial role in various autoimmune conditions [90].
Vitamin D was shown to have an inhibitory effect on Th1 cells [91], leading to decrease in Th1 cytokine production [18, 91, 92]. Some studies also suggest vitamin D promotes an immunologic shift toward Th2, by increasing Th2 cytokines [91, 93]; however, there are conflicting reports on this matter, as some studies suggest vitamin D has an inhibiting effect on Th2 cytokines as well [94]. Th17 cells are also influenced by vitamin D; the activated form of vitamin D was shown to modulate Th17 activity and ameliorate symptoms of related autoimmunity [95].
T regulatory cells (Treg) are a subset of CD4+ T cells that also express CD25. They have an important role in modulation of the immune system, specifically in autoimmune diseases that are mediated by autoreactive T cells [96]. Previous studies have found that vitamin D increased the population of Treg cells, both in animal models [97] and in humans [92, 98].
Evidence suggests B cells are also affected by vitamin D. In an in vitro study, vitamin D inhibited autoantibody production and secretion [99].
Dendritic cells are antigen-presenting cells that activate naïve T cells and stimulate B cells for growth and differentiation [100, 101]. Differentiation and maturation of dendritic cells is modulated and inhibited by vitamin D, as well as their activity, which results in increased tolerance in autoimmune conditions [102,103,104].
Conclusion
Maintaining normal levels of vitamin D is important as vitamin D has a crucial role in regulation of the immune system.
Vitamin D deficiency is common in patients with autoimmune diseases. Epidemiologically, this deficiency was found to be a risk factor for various diseases, including MS, type 1 diabetes, IBD, and RA.
Many molecular studies concluded that through different mechanisms, vitamin D has an immunomodulatory effect on various cells of the immune system; however, the interaction between those mechanisms is yet to be determined. Although great progress has been made on this subject in recent years, there are still some missing parts of the puzzle.
Clinically, vitamin D deficiency affects the activity of numerous autoimmune diseases outcomes: end organ damage in SLE patients, glycemic control in type 1 diabetes patients, MRI-detected brain lesions and relapse frequency in MS patients, disease activity and quality of life in IBD patients, disease activity and serologic markers in RA patients, and others.
However, interventional studies done mostly on SLE patients failed to reach definite conclusions regarding treatment with vitamin D supplements for the disease, except for those performed on patients treated with steroids. Determining the safety, efficacy, and establishment of the exact doses for vitamin D treatment in patients with autoimmune diseases requires additional extensive interventional studies. Future studies should address possible intervening variables such as initial and final levels of the vitamin, VDR genetics, and sun exposure. However, since vitamin D has little, if any, known side effects in autoimmune patients, and its cost is rather low, vitamin D treatment should be considered based on existing evidence.
Abbreviations
- AIH:
-
Autoimmune hepatitis
- AITD:
-
Autoimmune thyroid diseases
- BMI:
-
Body mass index
- CD:
-
Crohn’s disease
- dcSSc:
-
Diffuse cutaneous systemic sclerosis
- IBD:
-
Inflammatory bowel diseases
- JoSLE:
-
Juvenile-onset systemic lupus erythematosus
- KO:
-
Knockout
- lcSSc:
-
Limited cutaneous systemic sclerosis
- MRI:
-
Magnetic resonance imaging
- MS:
-
Multiple sclerosis
- PBC:
-
Primary biliary cholangitis
- RA:
-
Rheumatoid arthritis
- RR:
-
Relative risk
- SLE:
-
Systemic lupus erythematosus
- SS:
-
Systemic sclerosis
- TGF:
-
Transforming growth factor
- Th:
-
T helper cells
- Treg:
-
T regulatory cells
- UC:
-
Ulcerative colitis
- UV:
-
Ultraviolet
- UVB:
-
Ultraviolet B
- VDR:
-
Vitamin D receptor
References
Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66(9):1137–42.
Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmun Rev. 2007;7(1):59–64.
Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678–88.
Mok CC. Vitamin D and systemic lupus erythematosus: an update. Expert Rev Clin Immunol. 2013;9(5):453–63.
Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308–10. The Endocrine Society.
Peelen E, Knippenberg S, Muris A-H, Thewissen M, Smolders J, Tervaert JWC, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev. 2011;10(12):733–43. Elsevier.
Schneider L, Dos Santos ASP, Santos M, da Silva Chakr RM, Monticielo OA. Vitamin D and systemic lupus erythematosus: state of the art. Clin Rheumatol. 2014;33(8):1033–8.
Vogel A, Strassburg CP, Manns MP. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology. 2002;35(1):126–31. Wiley Online Library.
Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med. 2004;229(11):1136–42. SAGE Publications.
Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol. 2013;45(2):256–66.
Rosen Y, Daich J, Soliman I, Brathwaite E, Shoenfeld Y. Vitamin D and autoimmunity. Scand J Rheumatol. 2016;18:1–9. Taylor & Francis.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev. 2010;9:709–15.
de Souza VA, Bastos MG, Fernandes NMDS, Mansur HN, Raposo NRB, de Souza DMK, et al. Association of hypovitaminosis D with systemic lupus erythematosus and inflammation. J Bras Nefrol. 2014;36(4):430–6.
McGhie T, Deceulaer K, Walters C, Soyibo A, Lee M. Vitamin D levels in Jamaican patients with systemic lupus erythematosus. Lupus. 2014;23:1–5.
Yap KS, Morand EF. Vitamin D and systemic lupus erythematosus: continued evolution. Int J Rheum Dis. 2015;18(2):242–9.
Schoindre Y, Jallouli M, Tanguy M-L, Ghillani P, Galicier L, Aumaitre O, et al. Lower vitamin D levels are associated with higher systemic lupus erythematosus activity, but not predictive of disease flare-up. Lupus Sci Med. 2014;1(1):e000027.
Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223(3):230–3. Wiley Online Library.
Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6):1678S–88S. Am Soc Nutrition.
Squance ML, Reeves GEM, Tran HA. Vitamin D levels are associated with expression of SLE, but not flare frequency. Int J Rheumatol. 2014;2014:1–10.
Sabio JM, Vargas-Hitos JA, Martinez-Bordonado J, Navarrete-Navarrete N, Diaz-Chamorro A, Olvera-Porcel C, et al. Association between low 25-hydroxyvitamin D, insulin resistance and arterial stiffness in nondiabetic women with systemic lupus erythematosus. Lupus. 2014;24:155–63.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56(6):2092–4. Wiley Online Library.
Winkelmann RR, Kim GK, Del Rosso JQ. Treatment of cutaneous lupus erythematosus: review and assessment of treatment benefits based on Oxford Centre for Evidence-based Medicine Criteria. J Clin Aesthet Dermatol. 2013;6(1):27–38. Matrix Medical Communications.
Stagi S, Cavalli L, Bertini F, de Martino M, Cerinic MM, Brandi M, et al. Vitamin D levels in children, adolescents, and young adults with juvenile-onset systemic lupus erythematosus: a cross-sectional study. Lupus. 2014;23:1059–65.
Garf KE, Marzouk H, Farag Y, Rasheed L, Garf AE. Vitamin D status in Egyptian patients with juvenile-onset systemic lupus erythematosus. Rheumatol Int. 2015;35(9):1535–40.
Chaiamnuay S, Chailurkit L, Narongroeknawin P, Asavatanabodee P, Laohajaroensombat S, Chaiamnuay P. Current daily glucocorticoid use and serum creatinine levels are associated with lower 25(OH) vitamin D levels in Thai patients with systemic lupus erythematosus. J Clin Rheumatol. 2013;19(3):121–5.
Azrielant S, Shoenfeld Y. Eppur Si Muove: vitamin D is essential in preventing and modulating SLE. Lupus. 2016;25(6):563–72.
Handono K, Sidarta YO, Pradana BA, Nugroho RA, Hartono IA, Kalim H, et al. Vitamin D prevents endothelial damage induced by increased neutrophil extracellular traps formation in patients with systemic lupus erythematosus. Acta Med Indones - Indones J Intern Med. 2014;46(3):189–98.
Wahono CS, Rusmini H, Soelistyoningsih D, Hakim R, Handono K, Endharti AT, et al. Effects of 1,25(OH)2D3 in immune response regulation of systemic lupus erythematosus (SLE) patient with hypovitamin D. Int J Clin Exp Med. 2014;7(1):22–31.
Toubi E, Shoenfeld Y. The role of vitamin D in regulating immune responses. Isr Med Assoc J. 2010;12(3):174–5.
Banica LM, Besliu AN, Pistol GC, Stavaru C, Vlad V, Predeteanu D, et al. Dysregulation of anergy-related factors involved in regulatory T cells defects in systemic lupus erythematosus patients: rapamycin and vitamin D efficacy in restoring regulatory T cells. Int J Rheum Dis. 2014;19:1–10.
Handono K, Marisa D, Kalim H. Association between the low levels of vitamin D and Treg function in systemic lupus erythematosus patients. Acta Med Indones. 2013;45(1):26–31.
Lertratanakul A, Wu P, Dyer A, Urowitz M, Gladman D, Fortin P, et al. 25-Hydroxyvitamin D and cardiovascular disease in patients with systemic lupus erythematosus: data from a large international inception cohort. Arthritis Care Res (Hoboken). 2014;66(8):1167–76.
Yap KS, Northcott M, Hoi AB-Y, Morand E, Nikpour M. Association of low vitamin D with high disease activity in an Australian systemic lupus erythematosus cohort. Lupus Sci Med. 2015;2(1):e000064.
Mandal M, Tripathy R, Panda AK, Pattanaik SS, Dakua S, Pradhan A, et al. Vitamin D levels in Indian systemic lupus erythematosus patients: association with disease activity index and interferon alpha. Arthritis Res Ther. 2014;16(1):R49.
Emerah AA, El-Shal AS. Role of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D level in Egyptian female patients with systemic lupus erythematosus. Mol Biol Rep. 2013;40(11):6151–62.
Sakthiswary R, Raymond AA. The clinical significance of vitamin D in systemic lupus erythematosus: a systematic review. PLoS One. 2013;8(1):1–6.
Tay SH, Ho CS, Ho RC-M, Mak A. 25-Hydroxyvitamin D 3 deficiency independently predicts cognitive impairment in patients with systemic lupus Erythematosus. PLoS One. 2015;10(12):e0144149. Public Library of Science.
Sahebari M, Nabavi N, Salehi M. Correlation between serum 25(OH)D values and lupus disease activity: an original article and a systematic review with meta-analysis focusing on serum VitD confounders. Lupus. 2014;23(11):1164–77.
Attar SM, Siddiqui AM. Vitamin D deficiency in patients with systemic lupus erythematosus. Oman Med J. 2013;28(1):42–7.
Petri M, Bello KJ, Fang H, Magder LS. Vitamin D in systemic lupus erythematosus: modest association with disease activity and the urine protein-to-creatinine ratio. Arthritis Rheum. 2013;65(7):1865–71.
Lima GL, Paupitz J, Aikawa NE, Takayama L, Bonfa E, Pereira RMR. A randomized double-blind placebo-controlled trial of vitamin D supplementation in adolescents and young adults with Juvenile-onset SLE: improvement in disease activity and fatigue scores. Arthritis Care Res (Hoboken). 2016;68(1):91–8.
Andreoli L, Dall’Ara F, Piantoni S, Zanola A, Piva N, Cutolo M, et al. A 24-month prospective study on the efficacy and safety of two different monthly regimens of vitamin D supplementation in pre-menopausal women with systemic lupus erythematosus. Lupus. 2015;24(4–5):499–506.
Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2010;62(11):1515–26.
Alberti KGMM, Zimmet PZFT. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. Wiley Online Library.
Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51(8):1391–8.
Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–3.
Dahlquist G. Vitamin D supplement in early childhood and risk for Type I (insulin- dependent) diabetes mellitus. Diabetologia. 1999;42(1):51–4.
Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512–7.
Sørensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61(1):175–8.
Mohammadian S, Fatahi N, Zaeri H, Vakili MA. Effect of vitamin d3 supplement in glycemic control of pediatrics with type 1 diabetes mellitus and vitamin d deficiency. J Clin Diagn Res. 2015;9(3):SC05–7.
Engelen L, Schalkwijk CG, Eussen SJPM, Scheijen JLJM, Soedamah-Muthu SS, Chaturvedi N, et al. Low 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 levels are independently associated with macroalbuminuria, but not with retinopathy and macrovascular disease in type 1 diabetes: the EURODIAB prospective complications study. Cardiovasc Diabetol. 2015;14(1):1–9.
Zeitz U. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17:509–11.
Rice CM, Cottrell D, Wilkins A, Scolding NJ. Primary progressive multiple sclerosis: progress and challenges. J Neurol Neurosurg Psychiatry. 2013;84(10):1100–6. BMJ Publishing Group Ltd.
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17.
Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008;7(3):268–77.
Watad A, Azrielant S, Soriano A, Bracco D, Abu Much A, Amital H. Association between seasonal factors and multiple sclerosis. Eur J Epidemiol. 2016;25:1–9. Springer.
Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–5.
Nepom GT, Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9(1):493–525. Annual Reviews 4139 El Camino Way, PO Box 10139, Palo Alto, CA 94303-0139, USA.
Silva AM, Pereira C, Bettencourt A, Carvalho C, Couto AR, Leite MI, et al. The role of HLA-DRB1 alleles on susceptibility and outcome of a Portuguese Multiple Sclerosis population. J Neurol Sci. 2007;258(1):69–74. Elsevier.
Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton SM, Dyment DA, et al. Expression of the multiple sclerosis-associated MHC class II allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009;5(2):1–6.
Auer DP, Schumann EM, Kümpfel T, Gössl C, Trenkwalder C. Seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;47(2):276–7.
Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014;71(3):306–14.
Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72(2):234–40.
Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134(0165–5728 (Print)):128–32.
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325(13):928–37. Mass Medical Soc.
Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(5):S3–9. Wiley Online Library.
Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35(3):308–16.
Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med. 2014;239(11):1524–30. SAGE Publications.
Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130(11):2648–52.
Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17(12):2386–92.
Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124(3):795–841.
Kerr GS, Sabahi I, Richards JS, Caplan L, Cannon GW, Reimold A, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38(1):53–9.
Cutolo M, Otsa K, Laas K, Yprus M, Lehtme R, Secchi ME, et al. Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol. 2006;24(6):702–4.
Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50(1):72–7.
Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2008;67(4):530–5.
Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, Ruiz JC, Arias-Santiago S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67(5):931–8. Elsevier.
Park B-S, Lee D-Y, Youn J-I, Park J-S, Kim I-G. Vitamin D receptor polymorphism is associated with psoriasis. J Invest Dermatol. 1999;112(1):113–6. Elsevier.
Smith EL, Walworth NC, Holick MF. Effect of 1α, 25-dihydroxyvitamin D 3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986;86(6):709–14. Elsevier.
Holick MF. Clinical efficacy of 1,25-dihydroxyvitamin D3 and its analogues in the treatment of psoriasis. Retinoids. 1998;14(1):12–7.
Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995;332(9):581–9. Mass Medical Soc.
Bizzaro G, Shoenfeld Y. Vitamin D and thyroid autoimmune diseases: the known and the obscure. Immunol Res. 2015;61(1–2):107–9. Springer Science & Business Media.
Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Dankó K, et al. Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol. 2011;8(3):243–7. Nature Publishing Group.
Do JE, Kwon SY, Park S, Lee E-S. Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet’s disease. Rheumatology. 2008;47(6):840–8. Br Soc Rheumatology.
Vacca A, Cormier C, Piras M, Mathieu A, Kahan A, Allanore Y. Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. J Rheumatol. 2009;36(9):1924–9.
Corrado A, Colia R, Mele A, Di Bello V, Trotta A, Neve A, et al. Relationship between body mass composition, bone mineral density, skin fibrosis and 25 (OH) vitamin D serum levels in systemic sclerosis. PLoS One. 2015;10(9):e0137912. Public Library of Science.
Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16(1):34–8. Elsevier.
Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol. 2000;85(1):9–21. Elsevier.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517.
Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HFJ, O’Garra A. 1α, 25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–80. Am Assoc Immnol.
Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183(9):5458–67. Am Assoc Immnol.
Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-β1 and IL-4. J Immunol. 1998;160(11):5314–9. Am Assoc Immnol.
Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168(3):1181–9. Am Assoc Immnol.
Joshi S, Pantalena L-C, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, et al. 1,25-Dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31(17):3653–69. Am Soc Microbiol.
Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160(3):1212–8. Am Assoc Immnol.
Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1α, 25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367–74. Am Diabetes Assoc.
Prietl B, Pilz S, Wolf M, Tomaschitz A, Obermayer-Pietsch B, Graninger W, et al. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? Isr Med Assoc J. 2010;12(3):136–9.
Linker-Israeli M, Elstner E, Klinenberg JR, Wallace DJ, Koeffler HP. Vitamin D 3 and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin Immunol. 2001;99(1):82–93. Elsevier.
Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3.
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18(Figure 1):767–811.
Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270(3):701–8. Elsevier.
Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88(2):227–33. Wiley Online Library.
Penna G, Adorini L. 1α, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–11. Am Assoc Immnol.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Azrielant, S., Shoenfeld, Y. (2018). Vitamin D and Autoimmune Diseases. In: Liao, E. (eds) Extraskeletal Effects of Vitamin D. Contemporary Endocrinology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-73742-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-73742-3_2
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-73741-6
Online ISBN: 978-3-319-73742-3
eBook Packages: MedicineMedicine (R0)