Abstract
Phytases are the special class of enzymes which have excellent application potential for enhancing the quality of food by decreasing its inherent anti-nutrient components. In current study, a protease-resistant, acidic phytase from Aspergillus aculeatus APF1 was partially purified by ammonium sulfate fractionation followed by chromatography techniques. The molecular weight of partially purified phytase was in range of 25–35 kDa. The purified APF1 phytase was biochemically characterized and found catalytically active at pH 3.0 and 50 °C. The Km and Vmax values of APF1 phytase for calcium phytate were 3.21 mM and 3.78 U/mg protein, respectively. Variable activity was observed with metal ions and among inhibitors, chaotropic agents and organic solvents; phenyl glyoxal, potassium iodide, and butanol inhibited enzyme activity, respectively, while the enzyme activity was not majorly influenced by EDTA, urea, ethanol, and hexane. APF1 phytase treatment was found effective in dephytinization of flour biofortified wheat genotypes. Maximum decrease in phytic acid content was noticed in genotype MB-16-1-4 (89.98%) followed by PRH3–30-3 (82.32%) and PRH3–43-1 (81.47%). Overall, the study revealed that phytase from Aspergillus aculeatus APF1 could be effectively used in food and feed processing industry for enhancing nutritional value of food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytic acid (PA) is one of the vital storage forms of phosphorous (P) comprising 1–5% by weight in cereal grains, oil seeds, legumes, wheat bran, rice bran, and nuts [1,2,3,4]. It represents 60–90% of total plant phosphorous and during the maturation period it accumulates in seeds, where it is stored in the form of globoid crystal within the protein bodies [5]. The unique structure of phytic acid provides it strong chelating potential to bind with positively charged divalent cations such as zinc, iron, copper, calcium, magnesium etc. to form insoluble complexes which negatively modulate the absorption and digestion of these cations in humans as well as in animals; moreover, in people with poor nutritional status, it results in deficiencies of minerals and trace elements [6]. Though inositol and P are stored in plant seeds in the form of phytate, but due to chelation of P, it remains unavailable for the monogastric animals and human beings as their gut is devoid of digestive enzymes [7]. A major cause of “Hidden hunger” in population of developing countries, dependent on cereals as major food, is attributed to this anti-nutritional property of PA. Various interventions to address the problem of micronutrient deficiency provide emphasis on Biofortification of cereals and increasing bioavailability of micronutrients through reducing PA content therein. Phytases (myo-inositol 1,2,3,4,5,6-hexakisphosphate phosphohydrolase) are most crucial class of phosphatases which catalyze step by step elimination of phosphates from phytate [8, 9], thus releasing divalent cations and myo-inositol free for absorption and utilization. It is present in number of organisms including plants, animals, bacteria, yeasts, and fungi, but fungal sources are most widely reported for industrial phytases with Aspergillus sp. as one of the best producers of extracellular phytase [5, 10, 11]. In this study, a fungal isolate Aspergillus aculeatus APF1 was utilized for purification and characterization of an acidic phytase followed by its application towards reducing the content of anti-nutritional factor in biofortified cereals for its potential application in alleviating micronutrient deficiency in developing countries.
Materials and Methods
Fungal Culture and Fermentation Conditions
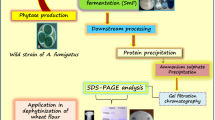
The fungus, Aspergillus aculeatus APF1 (GenBank accession No. KX865278.1), was isolated from decaying fruit in Baru Sahib, Sirmaur H.P. (Latitude 30.8244 and longitude 77.26855). The fungal culture was grown on Czapek Dox Agar (CDA) media (pH 6.0) at 30 °C for 72–96 h.
Phytase Production and Extraction
For inoculum preparation, 50 ml of Czapek Dox Agar (CDA) slants (pH 6.0) was prepared in 250 ml Erlenmeyer flask, point inoculation was done from pure fungal culture of Aspergillus aculeatus APF1, and flasks were kept at 30 °C for 96 h. Spores from 4-day-old CDA slants were harvested using normal saline with 0.1% Tween-80, and 14.00 × 108 spores/ml obtained was used as inoculum for solid-state fermentation (SSF). For phytase production, thoroughly washed and dried wheat bran (5 g) as a sole media source was used as solid substrate and moistened with 10 ml distilled water for SSF. The flasks were autoclaved at 121 °C, 15 psi for 20 min, cooled and inoculated with 2 ml fresh inoculum and incubated at 30 °C for 72 h. The fermented substrate was suspended in 50 ml normal saline containing 0.1% Tween-80 and kept on shaking at 200 rpm for 1 h. The mixture was filtered through double-layered muslin cloth, the extract thus obtained was centrifuged at 10,000 rpm for 10 min at 4 °C to remove debris, and the supernatant was further used in phytase assays.
Determination of Phytase Activity
Phytase activity was assayed by determining the amount of phosphate liberated [12] using calcium phytate as the substrate [13, 14]. One unit of phytase is defined as the amount of enzyme required to liberate 1 nmol of inorganic phosphate ml−1 s−1 under the assay conditions using KH2PO4 as the standard. Quantitative estimation of soluble protein at various stages of enzyme production and purification was done by Bradford’s method using bovine serum albumin (BSA) as standard [15]. Each experiment was carried out in triplicates, and the values reported are the average of three such experiments.
Partial Purification of Phytase from Aspergillus aculeatus APF1
Crude phytase was concentrated by ammonium sulfate fractionation (20–80%). The concentrated sample was dialyzed against 0.1 M sodium acetate buffer (pH 5.0) and loaded on SP-Sepharose ion exchange matrix (10 ml 20 × 1.5 cm, GE Healthcare, USA). Bound phytase was eluted with discontinuous gradient of 0.1–1 M NaCl in the same buffer. The fractions showing phytase activity were collected and concentrated by lyophilization for gel filtration chromatography. Concentrated sample was loaded on gel filtration chromatography column packed with P-60 matrix (BioRad, USA; 45 ml, 300 × 15 mm). The protein was eluted with sodium acetate buffer (0.1 M, pH 5.0). Fractions of 2 ml were collected with the flow rate of 20 ml/h and their absorbance was recorded at 280 nm. All fractions were checked for phytase activity as per given procedure. Active fractions were loaded on SDS-PAGE and checked for their homogeneity. Fractions showing high phytase activity were pooled, lyophilized, and used for analysis for biochemical properties.
Biochemical Characterization of Partially Purified APF1 Phytase
The partially purified enzyme was characterized for its biochemical properties including optimum pH, optimum temperature, pH and temperature stability, Km, Vmax and its activity in presence of other effector molecules such as metal ions and organic solvents.
Effect of pH on Phytase Activity and Stability
The effect of pH on enzyme activity was studied by using three buffer systems (0.1 M) of different pH, i.e., glycine-HCl buffer (pH 2.0, 2.5, 3.0), sodium acetate buffer (pH 4.0, 5.0, 6.0), and Tris–HCl buffer (pH 7.0–8.0). The reaction mixture was incubated at 50 °C for 10 min and enzyme activity was determined. The phytase activity at pH 5.0 was taken as control, and maximum phytase activity was considered as 100%. It was expressed as % relative activity. The pH stability of phytase at 50 °C was determined at pH ranging from 2.5 to 8.0 after per-incubating the enzyme at these buffers for 2 h at 4 °C and the residual activity was determined as compared to enzyme sample without this treatment.
Effect of Temperature on Phytase Activity and Stability
The effect of temperature on phytase activity was studied by incubating the reaction mixture at various temperatures (30, 40, 50, 60, 70, and 80 °C). The maximum phytase activity was taken as 100% and activity was expressed as % relative activity.
Thermostability of phytase was determined by incubating enzyme at 50 °C and 80 °C over a period of 180 min followed by subsequent phytase assay. The residual activity was determined as compared to enzyme sample without this treatment.
Determination of Michaelis-Menton Constant Km and Vmax Values
To determine the Km and Vmax constants, different concentration (0.2 mM to 5.0 mM) of calcium phytate as substrate was taken in separate reaction mixture tubes and it was incubated with phytase enzyme at 50 °C for 10 min. Phytase activity was determined as per given procedure and Lineweaver Burk plot was drawn between 1/v vs 1/[S] to determine the values of kinetic constants Km and Vmax.
Effect of Modulators on APF Phytase Activity
The effect of several modulators on phytase activity was studied, and the resulting phytase activity was expressed as relative activity (%) as compared to control enzyme sample in which no modulator was added, taking as 100%.
The effect of number of metal ions was analyzed; the enzyme sample was incubated at 50 °C in the presence of different metal ions like Na+, Ca2+, Fe2+, Mg2+, Al3+ at concentration of 1 mM and 5 mM; and phytase activity was determined as per standard procedure. In a separate treatment, various organic solvents (acetone, butanol, ethanol, isopropanol, hexane) were added in the separate reaction mixture tubes at two different concentration, i.e., 5% and 10%, to determine the relative phytase activity (%). Effect of EDTA and phenyl glyoxol was also analyzed by adding 0.01 and 0.05 mM concentration of these inhibitors in the reaction mixture, and phytase activity was estimated. In another treatment, the enzyme was incubated with 1 and 2 mM of chaotropic agents (Urea and potassium iodide) at 50 °C and residual activity was determined as compared to control without these agents. Considering the importance of resistance to proteolytic digestion during food applications, APF1 phytase was incubated with two proteases, i.e., trypsin and pepsin (0.5%), for varying time (30–120 min) at 37 °C, and residual activity (%) was determined as compared to enzyme without proteases treatments.
Application of APF1 Phytase Using Biofortified Wheat Derivatives
In this study, biochemically characterized phytase from A. aculeatus APF1 was applied on flour of various wheat derivatives for the hydrolysis of insoluble phytates and PA content was analyzed after enzyme treatment. Among different wheat samples, PBW343LrP, PRH3-30-3, PRH3-43-1, PRH3-15-12, 46-1-15-15-3-1, MB-16-1-4, 17-1-2-5-4-9-3 were selected to study the effect of dephytinization. Concentrated phytase from Aspergillus aculeatus strain APF1 (2 units, used in glycine-HCl buffer, 0.1 M, pH 2.5) was separately added to defined amount of flour (0.25 g) of experimental samples and incubated at 37 °C for 2 h. Control for all samples was also taken where no such treatment was given. Thereafter, phytic acid content in each sample was evaluated using the following procedure. After incubation, the finely ground wheat flour sample (0.25 g) under treatment was added with 10 ml of 2.4% HCl, vortexed, and kept for overnight at room temperature. The samples were mixed thoroughly at 150 rpm for 1 h at 30 °C and centrifuged at 8000 rpm for 15 min. Supernatant was collected from this extract, 1800 μl was taken into a test tube, and 600 μl Wade reagent (0.09 g sulphosalicylic acid + 0.009 g ferric chloride in 30 ml distilled water) was added. The reaction mixture was kept at room temperature for 20 min and OD was taken at 500 nm. The samples untreated with phytase were considered as control, and comparison was made on the basis of decrease in PA content with the enzyme-treated samples [16]. The standard curve of phytic acid (Sigma Aldrich) was used for analysis.
Results and Discussion
Purification of APF1 Phytase
Purification is sequential separation of unwanted impurities from the crude extract and isolation of desired macromolecule such as protein or enzyme. It is a multi-step process, which includes concentration of crude extract by salt precipitation, followed by lyophilization and separation through ion exchange, gel-filtration chromatography [2, 14, 17]. Protein solubility is influenced by ionic salts; at low ionic concentrations, the solubility of protein tends to increase, known as “salting-in” while at very high ionic strength protein, solubility decreases, a phenomenon termed as “salting-out”. Thus, positively charged ammonium ions interact with negative charge of phytase and aggregate the enzyme, so, salting out has been routinely used for separation and purification of proteins [18].
A purification fold of 1.42, 32.67, and 44.59 was obtained after dialysis, ion exchange chromatography, and gel-filtration chromatography, respectively. The recovery of enzyme was 25.54%, 14.90%, and 5.15% after dialysis, SP-Sepharose anion exchange chromatography and BioGel P-60 gel-filtration chromatography, respectively (Table 1). The partially purified phytase thus obtained was visualized by two bands on SDS-PAGE, and the molecular weight was in the range of 25–35 kDa (Fig. 1).
The increasing specific activity at each purification step indicated purification of protein and the same was revealed by SDS-PAGE. The partially purified phytase thus obtained was visualized as two bands on SDS-PAGE and their molecular weights were in between 25 and 35 kDa. Similar kind of purification strategy i.e., ammonium sulfate precipitation, followed by ion exchange chromatography and gel filtration chromatography was used by Sapna and Singh [14], for purification of phytase from Aspergillus Oryzae SBS50. Dan et al. [19] used ammonium sulfate precipitation, DEAE Sepharose, and Sephadex G-100 size-exclusion column chromatography for phytase purification from B. licheniformis. Ammonium sulfate precipitation, anion exchange chromatography, and gel filtration chromatography were used for phytase purification from Weissela halotolerans [18].
The two bands in gel filtration fraction were considered as positive, and the SDS-PAGE excised bands were analyzed using commercial MALDI TOF sequencing services. The analysis of MALDI TOF resulted into sequences of protein fragments, but there was no or very less similarity with the already existing phytase sequences. Due to lack of sequence information of phytase from Aspergillus aculeatus in the protein data bank, BLASTp analysis showed no homology with available protein sequences, though one fragment showed similarity with hypothetical Aspergillus aculeatus protein.
The results of SDS PAGE shows the molecular weight of APF1 phytase is in the range of 25–35 kDa. The molecular mass of microbial phytases ranges from 14 to 500 kDa [13, 14, 17, 20, 21]. The molecular weight of purified phytases from Pleurotuseryngii was 14 kDa [20]. The phytase purified from Aspergillus niger7A-1 had molecular weight of 89 kDa [17] while that from Aspergillus oryzae SBS50 was about 80 kDa [14]. The molecular weight of phytases isolated from A. niger has wide range, i.e., 39 kDa [22], 66 kDa [23, 24], and 85 kDa [25].
Characterization of APF1 Phytase
The Km and Vmax values of partially purified Aspergillus aculeatus APF1 phytase for calcium phytate were 3.21 mM and 3.78 U/mg protein, respectively. Variations in Km and Vmax of phytase were observed as both the Km and Vmax values for phytase from A. niger 7A-1 were 220 μM and 25 μmol/min [17], A. oryzae were 1.14 mM and 58.82 μmol/ml/min [14], A. niger were 0.929 μM and 52.36 nkat/cm3 [22], while of A. niger ATCC 9142 were 100 μM and 7 nmols/s [26], respectively.
The optimum pH at which phytase is active is one of the most important characteristics of the enzyme as it determines the catalytic efficiency as well as the future applications of enzyme. The search for an ideal phytase includes its effectiveness in the stomach of humans and animals during digestion process, where the pH ranges from 1.5 to 3.5; the phytase which is active at acidic pH will enhance its benefits in the food industry. Partially purified phytase from Aspergillus aculeatus APF1 was most active at acidic pH 3.0 (Fig. 2). Mullaney and Ullah [27] reported that Histidine acid phosphatases (phyB), isolated from A. niger NRRL 3135, showed high phytase activity at 2.5 pH, and the studies revealed that substrate specificity site (SSS) of phyB is composed of negatively charged glutamate and aspartate. At pH 2.5, SSS remains uncharged and easily accommodates negatively charged phytate as substrate [28]. This could be the reason for A. aculeatus phytase to be active at 3.0 pH. Phytase purified from Aspergillus nigervan Teighem [29] and A. niger [24] also exhibited maximum enzyme activity at 2.5 pH. Similarly, phytases from A. niger [22] and Rhizopus oligosporus [30] were active in the range of 2.6–3.5 pH.
The ability of enzyme to remain functional at high temperature is the significant characteristic required in food and feed industry. Thermostability of phytase may improve its supplementation benefits in the food processing industry, as the enzymes during food and feed processing is exposed to high temperatures to prevent contamination. The phytase purified from Aspergillus aculeatus was most active at 50 °C (Fig. 3), which is in accordance with previously reported phytase from Aspergillus oryzae SBS50 [14] and Weissela halotolerans [18]. Other reports suggested that optimum temperature at which phytase exhibited maximum activity was in the range of 55–60 °C is in Aspergillus niger 7A-1 [17] and P. oxalicum PJ3 [2], A. niger NCIM 563 [31], Sporotrichum thermophile [13], A. niger [25]. Purified phytase was more stable at 50 °C as compared to 80 °C and retained its 20% activity after 5 min, 10% activity after 10 min, and gradually lost its activity after 60 min at 50 °C (Fig. 4). Similar results were reported by Wyss et al. [28] in the phytase purified from A. niger T213. Variation in thermostability is mainly attributed to involvement of multiple factors such as amino acid composition, aromatic amino acid interactions, disulphide bridges, hydrogen bonds, hydrophobic interactions, presence and orientation of secondary structures (α and β motifs and hairpins), interactions with metal ions, and the extent of glycosylation to regulate the thermostable nature of phytases [28, 32].
Metal ions are known to play pivotal role in regulation of enzyme catalysis, and microbial phytases are not the exception. Though the activity of fungal phytases is not strongly dependent on metal ions as it is observed in bacterial phytases, the addition of few divalent cations affects the phytase activity. It is a well known fact that association of Ca2+ ions with β-propeller phytases positively regulates their activity in Bacillus sp.; such dependency is rarely noticed in fungal phytases [33, 34]. In our study, Aspergillus aculeatus phytase was treated with 1 mM and 5 mM concentration of metal salts and it was concluded that Na+ ions had no effect on phytase activity, Ca2+, and Fe2+ when used in low molar concentration, and their effect was almost negligible but when used at molar concentration of 5 mM, they slightly inhibited the phytase activity. Mg2+ inhibited the enzyme activity at both concentrations and Al3+ strongly inhibited the phytase activity. When aluminum salt was added, no phytase activity was recorded. The following pattern of inhibition of enzymatic activity was observed when phytase was supplied with metal ions, Al3+ > Mg2+ > Fe2+ > Ca2+ > Na+. Similar results were reported by Vats and Banerjee [29]; the phytase activity of Aspergillus niger van Teighem was severely inhibited by Al3+ ions at 5 mM and except Ca2+ ions, the Cu2+, Mg2+, Mn2+, Ni2+, and Zn2+ ions have shown inhibitory effects. Contrary to this finding, a recent report on A. niger 7A 1 showed that Ca2+ ions exerted negative effect on phytase activity while other metal ions (Cu2+, Mg2+, Mn2+, Hg2+, Cd2+, Ba2+, Zn2+) displayed a significant positive stimulatory effect [17]. The phytase activity of A. oryzae was found stimulated in the presence of Ca2+, Mg2+, Co2+ while negatively affected by Cu2+, Fe2+, and Fe3+ [14]. The interaction of metal ions with phytase purified from A. niger CFR 335 showed that the enzyme was activated by Cu2+ > Ca2+ > Mg2+ > Zn2+ while inhibited by Mn2+ > Fe3+ > Al3+ [23]. The ions of Zn and Al strongly inhibited the phytase activity of A. niger 307 [22]. All these reports supported our findings that Al3+ and Mg2+ have inhibitory effect on phytase activity. Most likely, the binding of these ions at the active site of enzyme modifies the conformational orientation of phytase, hence, resulting in inhibition of enzymatic activity. Another plausible explanation for negative modulation could be that metal ions directly interact with the substrate and phytic acid, and the insoluble metal-phytate complexes thus formed interfere with the action of enzyme and liberation of phosphorus which is the measure of enzymatic activity in the assay [13, 14, 22].
The effect of inhibitors, chaotropic agents, and organic solvents was also studied. The treatment of phytase with inhibitors displayed that EDTA did not affect the enzyme activity contrary to inhibition by phenyl glyoxal. The results were in accordance with reports on phytase from A. oryzae [14] and Sporotrichum thermophile [35], where no effect of EDTA was observed on phytase activity. In support of the inhibitory effect of phenyl glyoxal on APF1 phytase, Aspergillus ficuum phytase [36] and phytases from rhizobacterial isolates Klebsiella and Enterobacter were also inhibited by phenylglyoxal [37]. These results indicate that phytase purified from Aspergillus aculeatus might belong to Histidine acid phosphatases (HAPs) class. The active site of HAPs is occupied with arginine residues which plays pivotal role in interaction of substrate with enzyme. Phenylglyoxal is an arginine modifier which reacts with sensitive arginine residues at even very low concentrations, resulting in reduced enzyme-substrate affinity and low product formation. Decrease in phytase activity with increasing concentration of phenylglyoxal again implicated the involvement of Arg residues in phosphohydrolytic cleavage of phytate [38]. Addition of urea and potassium iodide (KI) has apparently moderate effect on phytase activity. KI inhibited the enzyme activity up to a certain extent. All the enzymes restore their active conformation with the help of several non-covalent interactions such as van der Walls forces and H-bonding. The modulation of enzymatic activity, after exposure of chaotropic agents, suggests the influence of structural conformations of phytase by non-covalent forces [39]. In concise with the present study, the chaotropic agents such as urea and KI also inhibited the enzymatic activity of phytase from A. oryzae [14] and S. thermophile [13]. When organic solvents were added in the reaction mixture in two concentrations (5% and 10%), it was observed that butanol strongly inhibited the enzyme activity, acetone and isopropanol slightly affected the activity, while ethanol and hexane have no effect on phytase activity. These results might indicate that during the enzymatic catalysis, hydrophobic amino acid residues do not play significant role at the active site of A. aculeatus phytase [14]; hence, these organic solvents did not affect the enzyme activity except butanol (Table 2).
For successful commercial application, phytase should resist the action of proteases in the digestive tract of human and other monogastric animals. Purified APF1 phytase is resistant against pepsin and trypsin treatment (Table 3). Similarly, phytase from A. oryzae [14] and A. ficuum NTG-23 [40] was also resistant to digestion by pepsin and trypsin. The phytase from A. niger UVF-1 retained more than 90% residual activity and displayed strong resistance against both pepsin and trypsin treatment [41]. Phytase from Citrobacter koseri PM-7 showed good tolerance, up to 98.5% towards proteolytic enzymes pepsin, trypsin, and chymotrypsin [42].
Application of APF1 Phytase Treatment on Dephytinization in Selected Wheat Genotypes
The effect of phytase on dephytinization of the biofortified derivatives of wheat was studied. After phytase treatment, the phytic acid content in all the experimental samples was decreased. The observations showed efficacy of phytase action and a decrease in phytic acid in the range of 70.66–89.98% with APF1 phytase (Fig. 5) APF1 phytase was found effective and after phytase treatment, maximum decrease in PA content was noticed in MB-16-1-4 (89.98%) followed by PRH3–30-3 (82.32%) and PRH3–43-1 (81.47%).
In the last few decades, extensive research has been done on the development of high iron and zinc biofortified crops but the results are not as fruitful as expected due to the reduced mineral bioavailability [43, 44]. Therefore, the use of enzymatic method to hydrolyze phytates from the biofortified crop grains is prerequisite to enhance the bioavailability of micronutrients. Moreover, to overcome the negative interactions of PA, application of phytase for degradation of phytate in the cereal crops has been recommended [45, 46]. The use of microbial phytases as well as cereal phytases played important role in enhancing mineral and phosphorous content in food and feed items [47, 48]. Considering demerits of low micronutrient content in cereal crops, biofortified wheat derivatives developed at the Eternal University, Baru Sahib, were used for evaluation of PA content in these biofortified wheat grains after treatment with fungal phytase. Efficacy of APF1 phytase in reducing phytate content in the treated samples revealed that it could be used under optimal conditions during food processing for reducing phytate content. Promising results were obtained and up to 89% phytate reduction by APF1 phytase was obtained. According to the results, the reducing phytate concentration over initial is a key indicator of success of experiment and further experiments can be carried out at different enzyme concentrations and pH as it is also known to be a key player in micronutrient dialyzability/bioavailability [49, 50]. Genotype MB-16-1-4 was found to be most affected by APF1 phytase treatment for PA degradation. In most of the treatments for the outcome of phytase on phytic acid, the quantitative comparisons and complete understanding of the results obtained in different studies (regarding phytate degradation) is not always deducible because different outcome is influenced by different methodologies [51]. When mixture of A. niger phytase and E. coli phytase was used for degradation of PA in bran, 91–97% decrease in PA content was observed [47]. In another study, exogenous wheat phytase addition to wheat flour followed by analysis of phytate degradation reveals decrease in phytate content from 35 to 69% [52]. According to Hellström et al. [53], addition of A. ficuum phytase, prior to ingestion, leads to 89% phytate degradation after 48 h fermentation while in the control, it was degraded up to 51% after fermentation. Addition of fungal phytase during whole wheat bread making degraded ~ 97% of phytate and increased dialyzable Fe up to 17% [54]. The variation of response of phytase with different biofortified genotypes might be attributed to inherent properties of material used. Current issues of concern for all exogenous enzymes include variability in response, where these are significantly influenced by variability of substrate and interactive factors [55]. The phytase-mediated beneficial effect might depend upon inherent content of calcium, phosphorus, vitamin D, the ratio of calcium to phosphorus, and the level of endogenous phytase activity present in the ingredients used [55,56,57].
In conclusion, a promising phytase from Aspergillus aculeatus APF1 for application potential in treatment of cereals-based food was partially purified and characterized for its biochemical properties. The properties of APF1 phytase such as its acidophilic nature, resistance to protease digestion, and potential to reduce phytate content revealed that it could be used in human food applications based on outcome of cell line and animal model studies. Further research on application of APF1 phytase on varying foods along with analysis of micronutrient dualizability and bioavailability will be of great importance for efficient utilization of this enzyme.
References
Farouk, A., Banaja, A., Ahamed, N. T., AlZahrani, O., & Bazaid, S. (2015). Inducible secretion of phytate-degrading enzymes from bacteria associated with the medical plant Rosa damascena cv. Taifi using rice bran. African Journal of Biotechnology, 14, 425–433.
Lee, S. H., Cho, J., Bok, J., Kang, S., Choi, Y., & Lee, P. C. (2015). Characterization, gene cloning, and sequencing of a fungal phytase, PhyA, from Penicillium oxalicum PJ3. Preparative Biochemistry and Biotechnology, 45(4), 336–347.
Moreira, K. A., Herculano, P. N., Maciel, M., & Porto, T. S. (2014). Optimization of phytase production by Aspergillus japonicus Saito URM 5633 using cassava bast as substrate in solid state fermentation. African Journal of Microbiology Research, 8, 929–938.
Singh, B., & Satyanarayana, T. (2015). Fungal phytases: characteristics and amelioration of nutritional quality and growth of non-ruminants. Journal of Animal Physiology and Animal Nutrition, 99(4), 646–660.
Singh, B., Kunze, G., & Satyanarayana, T. (2011). Developments in biochemical aspects and biotechnological applications of microbial phytases. Biotechnology and Molecular Biology Reviews, 6, 69–87.
Silva, E. O., & Bracarense, A. P. F. (2016). Phytic acid: from antinutritional to multiple protection factor of organic systems. Journal of Food Science, 81, 1357–1362.
Holm, P. B., Kristiansen, K. N., & Pedersen, H. B. (2002). Transgenic approaches in commonly consumed cereals to improve iron and zinc content and bioavailability. The Journal of Nutrition, 132, 514–516.
Kumar, V., Sangwan, P., Verma, A., & Agrawal, S. (2014). Molecular and biochemical characteristics of recombinant β-propeller Phytase from Bacillus licheniformis strain PB-13 with potential application in aquafeed. Applied Biochemistry and Biotechnology, 173(2), 646–659.
Lei, X. G., Weaver, J. D., Mullaney, E., Ullah, A. H., & Azain, M. J. (2013). Phytase, a new life for an “old” enzyme. Annual Review on Animal Biosciences, 1, 283–309.
Gaind, S., & Singh, S. (2015). Production, purification and characterization of neutral phytase from thermotolerant Aspergillus flavus ITCC 6720. International Biodeterioration & Biodegradation, 99, 15–22.
Vohra, A., & Satyanarayana, T. (2003). Phytases: microbial sources, production, purification, and potential biotechnological applications. Critical Reviews in Biotechnology, 23(1), 29–60.
Fiske, C. H., & Subbarow, Y. (1925). The colorimetric determination of phosphorus. Journal of Biological Chemistry, 66, 375–400.
Singh, B., & Satyanarayana, T. (2009). Characterization of a HAP–phytase from a thermophilic mould Sporotrichum thermophile. Bioresource Technology, 100, 2046–2051.
Sapna, & Singh, B. (2017). Purification and characterization of a protease-resistant phytase of Aspergillus oryzae SBS50 whose properties make it exceptionally useful as a feed supplement. International Journal of Biological Macromolecules, 103, 458–466.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Gao, Y., Shang, C., Maroof, M., Biyashev, R., Grabau, E., Kwanyuen, P., Burton, J., & Buss, G. (2007). A modified colorimetric method for phytic acid analysis in soybean. Crop Science, 47, 1797–1803.
Neira-Vielma, A. A., Aguilar, C. N., Ilyina, A., Contreras-Esquivel, J. C., Carneiro-da-Cunha, M. D. G., Michelena-Álvarez, G., & Martínez-Hernández, J. L. (2018). Purification and biochemical characterization of an Aspergillus niger phytase produced by solid-state fermentation using triticale residues as substrate. Biotechnology Reports, 17, 49–54.
Demir, Y., Şenol Kotan, M., Dikbaş, N., & Beydemir, Ş. (2017). Phytase from Weissella halotolerans: purification, partial characterisation and the effect of some metals. International Journal of Food Properties, 20, 2127–2137.
Dan, S. K., Nandi, A., Banerjee, G., Ghosh, P., & Ray, A. K. (2015). Purification and characterization of extracellular phytase from Bacillus licheniformis isolated from fish gut. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 85, 751–758.
Li, M., Wang, H., & Bun Ng, T. (2013). Isolation of a phytase with distinctive characteristics from an edible mushroom. Pleurotus eryngii, Protein and Peptide Letters, 20(4), 459–466.
Segueilha, L., Lambrechts, C., Boze, H., Moulin, G., & Galzy, P. (1992). Purification and properties of the phytase from Schwanniomyces castellii. Journal of Fermentation and Bioengineering, 74, 7–11.
Sariyska, M., Gargova, S., Koleva, L., & Angelov, A. (2005). Aspergillus niger phytase: purification and characterization. Biotechnology & Biotechnological Equipment, 19, 98–105.
Gunashree, B., & Venkateswaran, G. (2015). Extracellular phytase from Aspergillus niger CFR 335: purification and characterization. Journal of Food Science and Technology, 52(7), 4558–4564.
Soni, S., Magdum, A., & Khire, J. (2010). Purification and characterization of two distinct acidic phytases with broad pH stability from Aspergillus niger NCIM 563. World Journal of Microbiology and Biotechnology, 26(11), 2009–2018.
Greiner, R., Silva, L. G. D., & Couri, S. (2009). Purification and characterization of an extracellular phytase from Aspergillus niger 11T53A9. Brazilian Journal of Microbiology, 40(4), 795–807.
Casey, A., & Walsh, G. (2003). Purification and characterization of extracellular phytase from Aspergillus niger ATCC 9142. Bioresource Technology, 86(2), 183–188.
Mullaney, E. J., & Ullah, A. H. (2003). The term phytase comprises several different classes of enzymes. Biochemical and Biophysical Research Communications, 312, 179–184.
Wyss, M., Pasamontes, L., Rémy, R., Kohler, J., Kusznir, E., Gadient, M., Müller, F., & van Loon, A. P. (1998). Comparison of the thermostability properties of three acid phosphatases from molds: Aspergillus fumigatus Phytase, A. niger Phytase, and A. niger pH 2.5 acid phosphatase. Applied and Environmental Microbiology, 64(11), 4446–4451.
Vats, P., & Banerjee, U. (2005). Biochemical characterisation of extracellular phytase (myo-inositol hexakisphosphate phosphohydrolase) from a hyper-producing strain of Aspergillus niger van Teighem. Journal of Industrial Microbiology and Biotechnology, 32(4), 141–147.
Azeke, M. A., Greiner, R., & Jany, K. D. (2011). Purification and characterization of two intracellular phytases from the tempeh fungus Rhizopus oligosporus. Journal of Food Biochemistry, 35, 213–227.
Bhavsar, K., Kumar, V. R., & Khire, J. (2011). High level phytase production by Aspergillus niger NCIM 563 in solid state culture: response surface optimization, up-scaling, and its partial characterization. Journal of Industrial Microbiology & Biotechnology, 38(9), 1407–1417.
Hesampour, A., Siadat, S. E. R., Malboobi, M. A., Mohandesi, N., Arab, S. S., & Ghahremanpour, M. M. (2015). Enhancement of thermostability and kinetic efficiency of Aspergillus niger PhyA phytase by site-directed mutagenesis. Applied Biochemistry and Biotechnology, 175(5), 2528–2541.
Jain, J., & Singh, B. (2016). Characteristics and biotechnological applications of bacterial phytases. Process Biochemistry, 51, 159–169.
Kumar, V., Yadav, A. N., Verma, P., Sangwan, P., Saxena, A., Kumar, K., & Singh, B. (2017). β-Propeller phytases: diversity, catalytic attributes, current developments and potential biotechnological applications. International Journal of Biological Macromolecules, 98, 595–609.
Ranjan, B., Singh, B., & Satyanarayana, T. (2015). Characteristics of recombinant phytase (rSt-Phy) of the thermophilic mold Sporotrichum thermophile and its applicability in dephytinizing foods. Applied Biochemistry and Biotechnology, 177(8), 1753–1766.
Tang, J., Leung, A., Leung, C., & Lim, B. L. (2006). Hydrolysis of precipitated phytate by three distinct families of phytases. Soil Biology and Biochemistry, 38, 1316–1324.
Patel, K. J., Singh, A. K., Nareshkumar, G., & Archana, G. (2010). Organic-acid-producing, phytate-mineralizing rhizobacteria and their effect on growth of pigeon pea (Cajanus cajan). Applied Soil Ecology, 44, 252–261.
Singh, B., Sharma, K., Kumari, A., Kumar, A., & Gakhar, S. (2018). Molecular modeling and docking of recombinant HAP-phytase of a thermophilic mould Sporotrichum thermophile reveals insights into molecular catalysis and biochemical properties. International Journal of Biological Macromolecules, 115, 501–508.
Gulati, H., Chadha, B., & Saini, H. (2007). Production, purification and characterization of thermostable phytase from thermophilic fungus Thermomyces lanuginosus TL-7. Acta Microbiologica et Immunologica Hungarica, 54(2), 121–138.
Zhang, G., Dong, X., Wang, Z., Zhang, Q., Wang, H., & Tong, J. (2010). Purification, characterization, and cloning of a novel phytase with low pH optimum and strong proteolysis resistance from Aspergillus ficuum NTG-23. Bioresource Technology, 101(11), 4125–4131.
Monteiro, P. S., Guimarães, V. M., Melo, R. R. D., & Rezende, S. T. D. (2015). Isolation of a thermostable acid phytase from Aspergillus niger UFV-1 with strong proteolysis resistance. Brazilian Journal of Microbiology, 46(1), 251–260.
Tripathi, P., Garg, S., Panwar, D., Kaira, G. S., Kumar, R., & Kapoor, M. (2017). Phytase from Citrobacter koseri PM-7: cost-effective production using agro-industrial residues. Biochemical Characterization and Application in de-Phytinization, Waste and biomass Valorization, 8, 1105–1118.
Díaz-Gómez, J., Twyman, R. M., Zhu, C., Farré, G., Serrano, J. C., Portero-Otin, M., Muñoz, P., Sandmann, G., Capell, T., & Christou, P. (2017). Biofortification of crops with nutrients: factors affecting utilization and storage. Current Opinion in Biotechnology, 44, 115–123.
Saltzman, A., Birol, E., Bouis, H. E., Boy, E., De Moura, F. F., Islam, Y., & Pfeiffer, W. H. (2013). Biofortification: progress toward a more nourishing future. Global Food Security, 2, 9–17.
Kalsi, H. K., Singh, R., Dhaliwal, H. S., & Kumar, V. (2016). Phytases from Enterobacter and Serratia species with desirable characteristics for food and feed applications, 3. Biotech, 6, 64.
Kaur, R., Saxena, A., Sangwan, P., Yadav, A. N., Kumar, V., & Dhaliwal, H. S. (2017). Production and characterization of a neutral phytase of Penicillium oxalicum EUFR-3 isolated from Himalayan region. Nusantara Bioscience, 9, 68–76.
Nielsen, A. V., & Meyer, A. S. (2016). Phytase mediated mineral solubilization from cereals under in vitro gastric conditions. Journal of the Science of Food and Agriculture, 96(11), 3755–3761.
Vashishth, A., Ram, S., & Beniwal, V. (2017). Cereal phytases and their importance in improvement of micronutrients bioavailability, 3. Biotech, 7, 42.
Gupta, R. K., Gangoliya, S. S., & Singh, N. K. (2015). Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. Journal of Food Science and Technology, 52(2), 676–684.
Weaver, C. M., & Kannan, S. (2002). Phytate and mineral bioavailability. Food Phytate, 211–223.
Nielsen, A., Tetens, I., & Meyer, A. (2013). Potential of phytase-mediated iron release from cereal-based foods: a quantitative view. Nutrients, 5(8), 3074–3098.
Akhter, S., Saeed, A., Irfan, M., & Malik, K. A. (2012). In vitro dephytinization and bioavailability of essential minerals in several wheat varieties. Journal of Cereal Science, 56, 741–746.
Hellström, A. M., Almgren, A., Carlsson, N. G., Svanberg, U., & Andlid, T. A. (2012). Degradation of phytate by Pichia kudriavzevii TY13 and Hanseniaspora guilliermondii TY14 in Tanzanian togwa. International Journal of Food Microbiology, 153(1-2), 73–77.
Sanz-Penella, J. M., Laparra, J. M. S., Sanz, Y., & Haros, M. (2012). Assessment of iron bioavailability in whole wheat bread by addition of phytase-producing bifidobacteria. Journal of Agricultural and Food Chemistry, 60(12), 3190–3195.
Bedford, M. R. (2000). Exogenous enzymes in monogastric nutrition—their current value and future benefits. Animal Feed Science and Technology, 86, 1–13.
Barrier-Guillot, B., Casado, P., Maupetit, P., Jondreville, C., Gatel, F., & Larbier, M. (1996). Wheat phosphorus availability: 2—in vivo study in broilers and pigs; relationship with endogenous phytasic activity and phytic phosphorus content in wheat. Journal of the Science of Food and Agriculture, 70, 69–74.
Qian, H., Kornegay, E., & Denbow, D. (1997). Utilization of phytate phosphorus and calcium as influenced by microbial phytase, cholecalciferol, and the calcium: total phosphorus ratio in broiler diets. Poultry Science, 76(1), 37–46.
Funding
Financial support was provided by the Department of Biotechnology (DBT), Govt. of India (Grant No. BT/AGR/BIOFORTI/PHII/NIN/2011) to carry out this work; Ministry of Food Processing Industries (MoFPI), Govt. of India, provided “infrastructural facility development” at Eternal University; and Head, Department of Microbiology, Maharshi Dayanand University, Rohtak permitted leading author to carry out part of this work at the Department.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saxena, A., Verma, M., Singh, B. et al. Characteristics of an Acidic Phytase from Aspergillus aculeatus APF1 for Dephytinization of Biofortified Wheat Genotypes. Appl Biochem Biotechnol 191, 679–694 (2020). https://doi.org/10.1007/s12010-019-03205-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03205-9