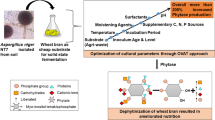
Abstract
The need for exogenous phytase in animal feed processing is increasing due to its ability to breakdown anti-nutrients in grains which constitute a large proportion of animal feeds. This study investigated the production of phytase using indigenously isolated Aspergillus sp. and the characterization of the purified phytase. The Aspergillus sp. was isolated from a poultry site using phytate screening medium (PSM). Quantitative estimation of phytase production was carried out using 2 × 108 spores/100 mL basal medium containing 0.5% sodium phytate as substrate at 30 °C and 150 rpm for 5 days under submerged fermentation condition. The extracted crude enzyme was purified using ammonium sulphate and gel filtration chromatography using Sephadex G-75 gel. The enzyme was characterized by investigating the effect of temperature, pH, and nutrient sources on the purified enzyme. Molecular identification confirmed the isolate as Aspergillus flavus (accession number of MT899184). The total activity observed in crude faction (609 U/mL) reduced to 187.5 U/mL in the ammonium sulphate fraction and then to 77.6 U/mL in the Sephadex G-75 fraction. Optimum temperature and pH were 40 °C and 6, respectively. The enzyme remained active for 5 min at both 70 °C and 80 °C. However, at 100 °C, all activity was lost. Glucose was the preferred carbon source and had higher activity (0.185 U/mL) but was unable to utilize sucrose. This study concludes that this isolate may be exploited for industrial production of phytase which has great application in animal feed industries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phytates and its isomers (myo-inositol phosphate esters (IP5 to IP2), even at low levels, constitute anti-nutritional factors that can have adverse effects on protein and mineral digestibility in animals particularly monogastric animals (Lee et al., 2020). These monogastric animals are unable to utilize all the nutrients in their feed because of the deficiency of their digestive system, particularly as they lack the enzyme phytase (Asmare, 2014). Being the main form by which phosphorus in cereals, leguminous crops, oil seeds, and nuts is stored (Gupta et al., 2015), commercial production of monogastric animal feeds requires the addition of exogenous phytase. Phytase is the most valuable commercial enzyme in poultry feed production (Lamp et al., 2020) and constitutes the major cost in the commercial production of poultry feed (Kim et al., 2019). The enzyme is able to hydrolyze the phytic acid present in the feed, thus improving the nutritional value of feed and also decreasing the amount of phosphorus excreted by animals (Muslim et al., 2018). Also phytase can be derived from other sources; however, plants are unable to produce extracellular phytase (Richardson et al., 2004). Microorganisms remain the most significant sources for production of enzymes (Atolagbe et al., 2016). They are generally regarded as cheaper means of producing industrial enzymes, since they can utilize readily available waste materials. In fungi, phytase production has been reported in species like Aspergillus niger (Afinah et al., 2010), Aspergillus flavus (Gaind & Singh, 2015) and Aspergillus fumigatus (Mittal et al., 2013). Although in most cases solid-state fermentation technique is regarded most suitable for commercial phytase production using Aspergillus species (Saithi and Tongta et al., 2016). In this study, submerged fermentation is employed due to the ease of product recovery which will minimize the cost of downstreaming processing and ultimately the overall cost of enzyme production. The aim of this study was therefore to characterize the phytase produced by a local fungal isolate to determine its suitability for inclusion as an exogenous supplement in animal feeds.
2 Materials and Methods
2.1 Sample Collection
A total of 100 g of soil samples were randomly collected from ten different spots (at 10 g per spot) of a poultry site in Lagos state (6°27′14.65“ N 3°23’40.81” E), Nigeria. Samples were collected from the areas on which the poultry droppings fell. They were collected in black polythene bags and transported same day to the Covenant University, Microbiology Laboratory for analyses.
2.2 Screening and Selection of Phytase-Producing Fungi from Poultry Soil
The selected fungi were quantitatively screened for the production of phytase following Lee et al. (2005). Soil suspension (1 mL) was plated out onto phytase-screening medium (D-glucose – 15.0 g; sodium phytate – 3.0 g; NH4NO3–5.0 g; MgSO4.7H2O – 0.5 g; KCl – 0.5 g; FeSO4.7H2O – 0.01 g; MnSO4.4H2O – 0.01 g; and Agar – 15.0 g in 1 L) (Qasim et al., 2016). Soil suspension was prepared by mixing 0.5 g of soil sample in 10 mL of 9% saline solution (Gontia-Mishra et al., 2013). The plates were left to incubate at 30 °C for 5 days. Fungal isolate was selected based on zone of hydrolysis.
2.3 Morphological Identification
Selected fungal isolate was purified by subculturing into potato dextrose agar (PDA) plates and subjected to lactophenol cotton blue test and identified based on morphological and microscopic characteristics referenced to fungi compendium (Domsch et al., 1980).
2.4 Molecular Identification
The DNA isolation was carried out following the CTAB method described by Dellaporta et al. (1983). PCR amplification of fungal internal transcribed spacer region was performed using the primer set ITS 1 and ITS 4 with the following sequences: ITS 1 (5’-TCCGTAGGTGAACCTGCG G-3′ and ITS 4 (5’-TCCTCCGCTTATTGATATGC-3′) (Alves et al., 2018). A 50 μL reaction master mix containing 0.5 μL Taq DNA polymerase (Transtat China), 5 μL 10X dilution of the manufacturer’s buffer (Transtat China), 4 μL deoxynucleoside triphosphates (dNTPs), 1 μL each of primers ITS 1 and ITS 4, and 36.5 μL sterile DNAse free water was employed for the PCR amplification. The reaction conditions were as follows: initial denaturation at 95 °C for 10 min, 30 amplification cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min (determined after gradient PCR), and primer extension at 72 °C for 90 min 1:30 min, followed by a final extension at 72 °C for 10 min. Aliquots of amplicons (7 μL) were analysed in 1% (w/v) agarose gel (Sigma, USA) by horizontal gel electrophoresis. DNA bands were visualized by UV excitation after staining with ethidium bromide (0.5 μL). Amplicon was purified according to the protocol described on EasyPure® PCR purification kit and quantified using Nanodrop 2000 Spectrophotometer. Then, appropriate concentration was packaged and sent for Sanger sequencing at Eurofins Genomics, Ebersberg, Germany. Forward and reverse sequencing reads were quality checked and assembled using reference sequence NR_121481.1 obtained from the NCBI Genbank database. Assembled reads were then compared against the NCBI Fungal ITS database, using the NCBI BLAST software, to check similar isolates. Multiple alignments were performed using the Geneious aligner (Kearse et al., 2012) and phylogenetic tree computed following Tamura-Nei distance model (Tamura and Nei, 1993) and Neighbor-Joining method with no outgroup.
2.5 Phytase Production
The selected fungus was quantitatively screened for the production of phytase by submerged fermentation (SmF). One mL of spore suspension containing 2 x 108 spores was inoculated into 100 mL phytase screening broth (PSB) (PSM without agar) and incubated in a shaking water bath at 30 °C for 5 days at 150 rpm. The fungal biomass was separated from the medium using Whatman No. 4 filter paper, and cell-free filtrate was employed for phytase assay (Qasim et al., 2016). The phytase activity was investigated by incubating 1 mL of crude phytase at 37 °C for 30 min in 1 mL 0.2 M sodium acetate buffer (pH 5.5) with 0.5% sodium phytate. The reaction was stopped by adding 1 mL of trichloroacetic acid (15% [w/v]). After the addition of 2 mL of freshly prepared colouring reagent (3.66 g of FeSO4·7H2O, 0.5 g of (NH4)6Mo7O24·4H2O, and 1.6 mL of concentrated H2SO4 in 50 mL of distilled water), the chilled sample on ice was incubated for 10 min at 30 °C. The released phosphate was determined at 750 nm with a UV-VIS spectrophotometer (Lee et al., 2005). Protein quantification was carried following Lowry et al. (1951).
2.6 Phytase Purification
2.6.1 Ammonium Sulphate Precipitation
Ammonium sulphate precipitation was employed to purify the crude phytase using a modification of the methods of Sanni et al. (2019) and Kalsi et al. (2016). Crude phytase was purified by precipitation using 70% ammonium sulphate. The ammonium sulphate-crude phytase mixture was incubated overnight at 4 °C with gentle stirring. The resulting solution was centrifuged at 21,000 g for 20 min to obtain precipitated proteins in pellet form. The pellet was then dissolved in 10 mL of 0.1 M acetate buffer pH 6.0 and dialyzed using a pre-acetylated cellophane tubing against three changes of the same buffer solution for 24 h at 4 °C, to desalt the precipitated enzyme. The precipitated phytase sample was assayed for phytase activity as previously described by Lee et al. (2005) and protein content determined following Lowry et al. (1951).
2.6.2 Gel Filtration Chromatography
The dialyzed crude phytase from the ammonium sulphate precipitation was further purified using the modifications methods of Thyagarajan et al. (2014) and Ajith et al. (2019). A chromatographic column of (50 × 1.5 cm) was packed with Sephadex G-75 gel and glass wool placed in the lower part of the column. The Sephadex G-75 (16.7 g) was dissolved in 250 mL of the phosphate buffer pH 7.0 (following manufacturer’s instruction of 1 g in 15 mL) and then boiled in a water bath for 5 min for swelling and degassing. The gel was allowed to settle for 18 h. The column was washed with phosphate buffer pH 7. After column equilibrium, 5 mL of ammonium sulphate phytase fraction was loaded gently on the surface of the gel and eluted with Tris-HCL buffer pH 7.8 at a 20 mL/h flow rate. Aliquots of 2 mL fractions of the eluent were collected; then the optical density at 280 nm and phytase activity (U/mL) was determined for each fraction. The phytase activity was determined following Lee et al. (2005), and protein content was determined following Lowry et al. (1951).
2.6.3 Effect of Carbon and Nitrogen Sources on Phytase Production
The effect of nutrient sources on phytase production was assessed by addition of 1% sucrose, in place of glucose and 0.1% of a different nitrogenous salt, (NH4)2SO4 instead of NH4NO3. Phytase activity was determined following Lee et al. (2005).
2.6.4 Effect of Temperature on Phytase Activity
Optimum temperature for phytase activity was determined by subjecting the enzyme to different temperatures (30–70 °C). This was carried out by varying the incubation temperature at a 10 °C interval during the activity determination. Phytase activity was determined following the descriptions (Lee et al., 2005).
2.6.5 Effect of pH on Phytase Activity
Optimum pH was established by subjecting the phytase to varying pH across a pH range of 3 to 8. Sodium acetate buffer was prepared at different pH during the activity determination, while incubation temperature was held at the optimum temperature determined earlier for the phytase. Phytase activity was determined following the descriptions by Lee et al. (2005).
2.6.6 Thermal Stability of Phytase
Thermal stability of the phytase was determined by subjecting the phytase to temperatures of 70 °C, 80 °C, and 100 °C for 5, 10, 20, and 30 min. The phytase activity was determined following the descriptions by Lee et al. (2005).
3 Results and Discussions
3.1 Identification of Phytase-Producing Isolate
Based on zone of hydrolysis, isolate code H was selected as phytase-producing and identified morphologically as Aspergillus sp. (Table 1). Further identification by molecular techniques recognized the isolate as Aspergillus flavus (Fig.1). The amplified internal transcribed spacer region sequence of the fungi submitted to NCBI was allocated accession number MT899184 (Fig. 2). The isolate showed genetic relatedness to other Aspergillus species isolated from agricultural samples. Previous studies reporting phytase production by fungi exist which is consistent with this study (Ajith et al., 2019; Sanni et al., 2019; Ribeiro Corrêa et al., 2015; Gaind and Singh, 2015; Mittal et al., 2013).. Handa et al. (2020) noted that the genus Aspergillus is the best producers of phytase. The choice of members of the genus Aspergillus to produce phytase and indeed other industrially relevant enzyme may not be unconnected to their generally regarded as safe (GRAS) status, their ubiquitous nature, and being able to utilize wide variety of substrates and their minimal nutritional requirement (Obafemi et al., 2018; Saith et al., 2016). Phytase is inducible and produced in the presence of an anti-nutrient (Vasudevan et al., 2017). The high amount of anti-nutrient (phytate), in poultry environment owing to the inability of the birds to digest the phytate present in their feed grains, may have encouraged the growth of the phytate degrading fungi.
3.2 Phytase Production and Purification
Quantitative analysis of crude phytase showed a crude phytase with total activity of 609 U/mL and protein content of 314.6 mg/mL. Purification resulted in a consistent reduction in total activity but with a corresponding increase on phytase-specific activity from 1.9 U/mL to 18.4 U/mL in the crude enzyme fraction and the gel chromatography fraction, respectively (Table 2). The Sephadex G-75 chromatogram showed one very distinct elution peaks (Fig. 3).
Optimum phytase production was observed on day 5 as similarly reported by Neira-Vielma et al. (2018) and Qasim et al. (2016). Although differing report was presented by Sanni et al. (2019) where optimum phytase production was observed at 216 h (9 days) for A. niger and 144 h (6 days) for A. flavus. Shivanna and Govindarajulu (2014) also reported optimal activity on the sixth day. This prolonged fermentation time may be as a result of the inducible nature of the enzyme; hence a prolonged lag phase might be required by the fungi to produce the enzyme. Generally, fungi species have been reported to be slow growers requiring about 2–5 days for optimal growth depending on the nutrient medium in which they are grown (Meletiadis et al., 2001). The use of SmF in this study is corroborated by the report of Jain et al. (2016). SmF is associated with ease in the recovery of product (Sethi et al., 2016). As the aim of this study to maximize profit and reduce production cost to the barest minimum, a fermentation technique which will reduce the cost is highly encouraged. Additionally, SmF has extra advantage of easy control of fermentation conditions such as aeration, pH, and temperature (Ornela and Guimaraes, 2019). The method is also the most commonly employed method for phytase production (Jain et al., 2016).
The quantitative assay of the cell-free supernatant showed an activity within the range of 609 U/mL. This result was higher than the report of Shivanna and Govindarajulu (2014) where they reported activity of 9.2 U/mL and 8.8 U/mL using a 6-day-old culture of A. niger and A. ficuum, respectively, under SmF. For total phytase activity, there was a continuous reduction from 609 U/mL in the crude phytase step to 77.6 U/mL in the Sephadex column fraction with each purification step. For specific phytase activity, there was a continuous increase from 1.9 U/mL in the crude phytase to 4.5 U/mL in the ammonium sulphate fraction and 18.4 U/mL in the Sephadex G-75 fraction. These values were higher when compared with the specific phytase activity of crude extract (1.13 U/mL), microfiltration fraction (1.15 U/mL), ultrafiltration fraction (1.93 U/mL), and DEAE Sepharose fraction (8.38 U/mL) reported by Neira-Vielma et al. (2018) when solid-state fermentation (SSF) was employed. Saithi and Tongta (2016) had earlier reported the limitation of SSF over SmF.
3.3 Phytase Characterization
Upon characterization, the phytase showed an optimum temperature and pH of 40 °C (Fig. 4) and 6 (Fig. 5), respectively. The thermal stability of the phytase over temperatures 70–80 °C showed that the phytase could withstand both temperatures for 5 minutes (Figs. 6 and 7), but had no activity at 100 °C (Fig. 8). For effect of carbon and nitrogen sources, results obtained showed that phytase production when glucose was employed as carbon source was 0.185 U/mL but no activity was observed using sucrose as carbon source (Fig. 9). However, the isolate could not utilize ammonium sulphate as an alternative nitrogen source to ammonium nitrate for phytase production (Fig. 9). Fungal phytases have a broad spectrum of optimal temperature ranging between 37 °C and 67 °C (Jatuwong et al., 2020). The optimal pH recorded in this study was at pH 6.0. Most fungal phytases are reported to be active at optimal pH within the acidic range between 2.0–6.0 (Pedri et al., 2015). However, there are studies reporting optimal activity around the neutral pH (Gaind and Singh, 2015), pH 6.5 (Thayagarajan et al., 2014), and pH 8–9 (Sanni et al., 2019). Different microorganisms exhibit varying optimum pH as pH greatly affects the transport of nutrients across the cell membrane of the organisms, thus contributing to cell growth and production of desired products (Gaind and Singh, 2015).
Effect of carbon and nitrogen sources on the production of phytase produced by A. flavus (isolate H). (GLUC Medium containing glucose as carbon source and ammonium nitrate as nitrogen source, SUC Medium containing sucrose as carbon source and ammonium nitrate as nitrogen source, AMM Medium containing glucose as carbon source and ammonium sulphate as nitrogen source)
The isolate in this study showed optimal activity when glucose was employed as carbon source compared to sucrose, while ammonium sulphate showed better activity as nitrogen source as supported by the results of Pedri et al. (2015). Glucose is a simple sugar, hence may require less energy to metabolize. When exposed to temperature of 70 °C, the phytase in this study retained its activity after 5 min, similarly at 80 °C. This result is of utmost importance since inclusion of this enzyme in animal feed processing means that it can withstand a temperature of 70 °C and above and still remain active. Although pelleting commonly occurs at temperatures of 80 °C, reducing the temperature to about 65 °C does not in any alter the overall effect of the process (Boroojeni et al., 2016); therefore phytase produced in this current study can find application as feed supplement if the feed is pelleted at temperatures 80 °C and below. Phytase is regarded as a thermo-tolerant enzyme; the results from the enzyme produced in this study are similar to the reports of Neira-vielma et al. (2018), where the enzyme retained more than 70% activity at 80 °C for 1 min, 40% activity for 2 min, and 9% after 5 min.
4 Conclusion
This present study has demonstrated the ability of Aspergillus flavus MT899184, local poultry isolate to degrade sodium phytate under submerged fermentation technique by producing phytase enzyme. There was continuous increase in specific activity of the phytase produced with each purification step. The phytase has optimal conditions of 40 °C and 6.0 of temperature and pH, respectively. The enzyme also demonstrated enzymatic activity after 5 minutes of heating at both 70 °C and 80 °C. These properties make it suitable as an exogenous additive in poultry feed. The properties of this enzyme also suggest that the feed formulation processes specifically the pelleting will not have adverse effects on the feed characteristics. The exogenous addition of this enzyme also makes its application easy without technical hitches, thus user friendly for easy utilization by the local farmers.
References
Afinah S, Yazid AM, Anis Shobirin MH, Shuhaimi M (2010) Phytase: application in food industry. International Food Research Journal 17(1): 13-21
Ajith S, Ghosh J, Shet D, ShreeVidhya S, Punith BD, Elangova AV (2019) Partial purification and characterization of phytase from Aspergillus foetidus MTCC 11682. AMB Express 9(1): 3
Alves RC, Borges IL, Dutra V, Garino Junior F, Miranda Neto EGD, Dantas AFM, Riet-Correa F, de-Galiza GJN (2018) Systemic infection by Aspergillus flavus in a mare. Acta Sci. Vet 46(supl): 345
Asmare B (2014) Effect of common feed enzymes on nutrient utilization of monogastric animals. International Journal Molecular Biology Research 5(4): 27–34
Atolagbe OM, Ajayi AA, Edegbo O (2016) Characterization of α-Amylase from Soursop (Annona muricata Linn.) Fruits Degraded by Rhizopus stolonifer. Pakistan Journal of Biological Sciences 9:77–81
Boroojeni FG, Svihus B, von Reichenbach HG, Zentek J (2016) The effects of hydrothermal processing on feed hygiene, nutrient availability, intestinal microbiota and morphology in poultry—A review. Animal Feed Science and Technology. 220: 187-215
Dellaporta SL, Wood J, Hicks JB (1983) DNA Extraction. Plant Molecular Biology Reporter 1(4): 19–21
Domsch K, Gams W, Anderson T (1980) Compendium of soil fungi (Vol. 1)
Gaind S and Singh S, 2015. Production, purification and characterization of neutral phytase from thermotolerant Aspergillus flavus ITCC 6720. International Journal of Biodeterioration and Biodegradation 99: 15–22
Gontia-Mishra I, Deshmukh D, Tripathi N, Bardiya-Bhurat K, Tantwai K, Tiwari S (2013). Isolation, morphological and molecular characterization of phytate-hydrolysing fungi by 18S rDNA sequence analysis. Brazilian Journal of Microbiology 44(1): 317
Gupta RK, Gangoliya SS, Singh NK (2015) Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. Journal of Food Science and Technology 52(2): 676
Handa V, Sharma D, Kaur A, Arya SK (2020) Biotechnological applications of microbial phytase and phytic acid in food and feed industries. Biocatalysis and Agricultural Biotechnology 25: 101600.
Jain J, Sapna, Bijender S (2016) Characteristics and biotechnological applications of bacterial phytases. Process Biochemistry 51: 159–169.
Jatuwong K, Suwannarach N, Kumla J, Penkhrue W, Kakumyan P, Lumyong S (2020) Bioprocess for Production, Characteristics, and Biotechnological Applications of Fungal Phytases. Frontiers in Microbiology 11: 188.
Kalsi HK, Singh R, Dhaliwal HS, Kumar V (2016) Phytases from Enterobacter and Serratia species with desirable characteristics for food and feed applications. 3 Biotech 6(1):64. doi: https://doi.org/10.1007/s13205-016-0378-x. Epub 2016 Feb 13
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C Drummond A (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12): 647–1649
Kim JW, Sanjayan N, Leterme P, Nyachoti CM (2019) Relative bioavailability of phosphorus in high-protein sunflower meal for broiler chickens and effects of dietary phytase supplementation on bone traits, growth performance, and apparent ileal digestibility of nutrients. Poultry Science 98(1): 298–305
Lamp AE, Ward NE, Wilson JW, Moritz JS (2020) In vitro phytase activity after steam conditioning and pelleting may not consistently correlate with in vivo measurements among commercial phytases. Journal of Applied Poultry Research 29(2): 420–434
Lee DH, Choi SU, Hwang YI (2005) Culture Conditions and Characterizations of a New Phytase-Producing Fungal Isolate, Aspergillus sp. L117. Mycobiology 33(4): 223–229
Lee SA, Lupatsch I, Gomes GA, Bedford MR (2020) An advanced Escherichia coli phytase improves performance and retention of phosphorus and nitrogen in rainbow trout (Oncorhynchus mykiss) fed low phosphorus plant-based diets, at 11 °C and 15 °C. Aquaculture 516: 734549
Lowry O, Rosebrough N, Farr A, Randal R (1951) Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193: 265-275
Meletiadis J, Mouton JW, Meis JF, Bouman BA, Donnelly JP, Verweij PE, Eurofung Network (2001) Colorimetric Assay for Antifungal Susceptibility Testing of Aspergillus Species. Journal of Clinical Microbiology 39(9): 3402–3408
Mittal A, Gupta V, Singh G, Yadav A, Aggarwal NK (2013) Phytase: A Boom in Food Industry. Acta Journal of Biosciences. 1(2): 158–169
Muslim SN, Ali AN, AL-Kadmy IM, Khazaal SS, Ibrahim SA, Al-Saryi NA, Al-Saadi LG, Muslim SN, Salman BK, Aziz SN (2018) Screening, nutritional optimization and purification for phytase produced by Enterobacter aerogenes and its role in enhancement of hydrocarbons degradation and biofilm inhibition. Microbial Pathogenesis 115: 159-67
Neira-Vielma AA, Aguilar CN, Ilyina A, Contreras-Esquivel JC, das Graça Carneiro-da-Cunha M, Michelena-Álvarez G, Martínez-Hernández JL (2018) Purification and biochemical characterization of an Aspergillus niger phytase produced by solid-state fermentation using triticale residues as substrate. Biotechnology Reports 17: 49–54.
Obafemi YD, Ajayi AA, Olasehinde GI, Atolagbe OM, Onibokun EA (2018) Screening and partial purification of amylase from Aspergillus niger isolated from deteriorated tomato (Lycopersicon esculentum mill.) Fruits. African Journal of Clinical and Experimental Microbiology 19: 47–57.
Ornelaa PH, de O, Guimarães LHS (2019) Purification and characterization of an alkalistable phytase produced by Rhizopus microsporus var. microsporus in submerged fermentation. Process Biochemistry 81: 70–76.
Pedri ZC, Lozano LMS, Hermann KL, Helm CV, Peralta RM, Tavares LBB (2015) Influence of nitrogen sources on the enzymatic activity and grown by Lentinula edodes in biomass Eucalyptus benthamii. Brazilian Journal of Biology 75(4): 0-0
Qasim SS, Shakir KA and Al-Shaibani AB (2016) Purification of Phytase produced from a Local fungal isolate and its Application in Food Systems. Iraqi Journal of Agricultural Sciences 47: 112–120
Ribeiro Corrêa T, de Queiroz MV, de Araújo EF (2015) Cloning, recombinant expression and characterization of a new phytase from Penicillium chrysogenum. Microbiology Research 170: 205–212.
Saithi S, Tongta A (2016) Phytase Production of Aspergillus niger on Soybean Meal by Solid-State Fermentation Using a Rotating Drum Bioreactor. Agricultural Science 11: 25–30.
Sanni DM, Lawal OT, Enujiugha VN (2019) Purification and characterization of phytase from Aspergillus fumigatus Isolated from African Giant Snail (Achatina fulica). Biocatalysis and Agricultural Biotechnology 17: 225–232
Sethi BK, Jana A, Nanda PK, Dasmohapatra PK, Sahoo SL, Kumar J (2016) Production of α-Amylase by Aspergillus terreus NCFT 4269.10 Using Pearl Millet and Its Structural Characterization. Frontiers in Plant Science 1: 639
Shivanna GB, Govindarajulu V (2014) Phytase Production by Aspergillus niger CFR 335 and Aspergillus ficuum SGA 01 through Submerged and Solid-State Fermentation. Science World Journal 2014: 1–6
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10(3): 512–526
Thyagarajan R, Namasivayamm KS, Narendrakumar G (2014) Partial purification of phytase from Hypocrea lixii SURT01, a poultry isolate. International Journal of Pharmacy and Biological Sciences 5(4): 680–687
Vasudevan UM, Krishna S, Jalaja V, Pandey A (2017) Microbial phytase: Impact of advances in genetic engineering in revolutionizing its properties and applications. Bioresource Technology 245: 1790–1799.
Acknowledgements
We also acknowledge Covenant University for support in payment of publication costs.
Disclaimer
None.
Conflict of Interest
None.
Funding
This study was funded by the International Foundation for Science (IFS) for its support through a grant awarded to Onibokun Adeola Elizabeth (Research grant agreement NO. I-3-C-6179-1).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Onibokun, E.A., Eni, A.O., Oranusi, S.U. (2022). Purification and Characterization of Phytase from a Local Poultry Isolate of Aspergillus flavus MT899184. In: Ayeni, A.O., Sanni, S.E., Oranusi, S.U. (eds) Bioenergy and Biochemical Processing Technologies. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-96721-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-96721-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96720-8
Online ISBN: 978-3-030-96721-5
eBook Packages: EnergyEnergy (R0)