Abstract
Objectives
The aim of this study was to analyze the risk factors for acute events after systemic-to-pulmonary shunt (SPS) and to investigate the effectiveness of pulmonary blood flow regulation with a metal clip.
Methods
The case histories of 116 patients (78 biventricular [BV] and 38 single ventricle [SV] physiology) who underwent SPS between 2010 and 2021 were retrospectively reviewed. Our strategy was to delay SPS until 1 month of age; pulmonary blood flow (PBF) regulation by partial clipping of the graft, if needed. Cases of aortic cross-clamping were excluded from this study.
Results
CPB was used in 49 (42%) patients: the median age at SPS was 1 month (2 days to 16 years), and the sternotomy approach in 65. Discharge survival was 98.3% (114/116); hospital death occurred in 1.7% due to coronary ischemia. Inter-stage mortality occurred in 1.7% (shunt thrombosis, 1; pneumonia, 1). Pre-discharge acute events occurred in 7 patients (6.0%): thrombosis 3, pulmonary over-circulation 2, and coronary ischemia 2. Multiple logistic regression analysis revealed that pulmonary atresia with intact ventricular septum (PA/IVS) (p = 0.0253) was an independent risk factor for acute events. Partial clipping of the graft was performed in 24 patients (pulmonary atresia 15) and clip removal was performed by catheter intervention in 9 patients; no coronary ischemic events and graft injury occurred in these patients.
Conclusion
Surgical outcomes after SPS were acceptable and metal clip regulation of pulmonary blood flow appears to be safe and effective. PA/IVS was still a significant risk factor for acute events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The systemic-to-pulmonary artery shunt (SPS) was introduced as a palliative procedure for patients requiring a reliable pulmonary blood source, and is still widely used in both biventricular (BV) and single ventricular (SV) physiology [1, 2]. The main goal of this palliative procedure is to balance the systemic and pulmonary circulation.
Despite improvements in the perioperative management of patients with congenital heart disease, significant mortality and morbidity has been reported in relation to the SPS, with in-hospital mortality rates of 7.2–19% [3,4,5,6,7,8]. According to the previous reports, the main causes of in-hospital mortality were temporary pulmonary over-circulation, shunt thrombosis, and coronary ischemia. Several previous studies reported risk factor for mortality of the SPS procedure: neonatal period, 3.0 mm graft, median sternotomy approach, PA/IVS, SV physiology, low BW; therefore, it is desirable to establish a safer surgical strategy for these high-risk patients [5,6,7,8].
To improve the surgical outcomes of SPS, the following surgical strategy was adopted for SPS in 2010: (1) set the timing of SPS after the neonatal period by maintaining the patent ductus arteriosus (PDA) under continuous prostaglandin E1 (PGE1) infusion, (2) use the median sternotomy approach for patients with SV patients and BV patients requiring CPB, (3) avoiding the use of 3.0 mm grafts, and (4) controlling PBF by graft size and partial clipping of the SPS graft (the shunt clip can be removed by catheter intervention if needed).
The purpose of this preliminary study was to investigate the recent surgical outcomes of the SPS procedure, risk factors of acute events, and evaluate whether PBF control with the metal clip is safe and effective.
Materials and methods
Study group
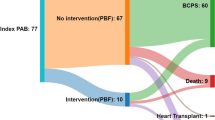
This study was approved by the Institutional Review Board of Niigata University Hospital. Due to the retrospective nature of this study, the requirement for individual patient consent was waived. The case histories of 116 pediatric patients who underwent SPS procedures (Blalock-Taussig shunt [BTS] in 115 patients; central shunt [CS] in 1 patient) in our institution between 2010 and 2021 were retrospectively reviewed. To eliminate the effect of another major procedure, cases of concomitant major procedures with aortic cross-clamping (Norwood operation, unifocalization of collaterals, Starnes operation, and atrioventricular valve repair) were excluded from this study. Patients who underwent SPS immediately after pulmonary artery banding were also excluded from this study. Figure 1 shows the CONSORT flow diagram of this study with inclusion information.
SPS procedures and post-operative anticoagulation management
According to our institutional strategy, SPS was delayed until 1 month of age via PGE1 infusion as often as possible. We selected the thoracotomy approach on the opposite side of the aortic arch when the patient had a BV physiology and did not require CPB; we used the sternotomy approach in patients with an SV physiology or who required CPB support. The innominate or subclavian artery was used for inflow of the BTS, and the ascending aorta was used for CS inflow. The Gore-Tex expanded polytetrafluoroethylene graft (W.L Gore & Associates. Inc. Newark, Delaware, United States) was used in all patients who underwent the BTS and CS procedures, and the size of the shunt graft was selected according to the patient’s body weight (BW), cardiac anatomy, and ventricular function. We routinely ligated the PDA immediately after construction of the SPS in patients who underwent the median sternotomy approach, and PGE1 infusion was discontinued in all cases. After achieving hemostasis, continuous intravenous heparin infusion was initiated at 10 units/kg/hour to maintain the activating coagulation time between 170 and 200 s, and enteral aspirin of 5 mg/kg/day was replaced. Details of the study patients are shown in Table 1.
Partial clipping of the SPS graft
We performed partial clipping of the SPS graft as previously described by Kuduvalli et al. [9] and Napoleone et al. [10]. When pulmonary over-circulation was suspected, partial clipping of the shunt graft was applied either during the operation or post-operatively. The hemostatic clip (Ligaclip, small; Ethicon. Inc., Raritan, New Jersey, United States) was placed with a graft size reduction of less than one-third of the original diameter (Fig. 2). The degree of clipping was determined according to the blood pressure and oxygen saturation level of the arterial blood (SaO2) which ranged from 70 to 80% in room air or a diastolic/systolic blood pressure ratio of the systemic arterial pressure > 0.4, ensuring care that the clips do not interfere with surrounding organs (such as the ascending aorta). To avoid a steep caliber change of the shunt graft, we controlled PBF via the number of clips. If cyanosis deteriorated after the SPS operation, the clip was removed by ballooning inside the shunt graft via catheter intervention (Fig. 2C).
A Intra-operative finding of partial clipping of the SPS shunt. Arrow showing the partial clipping. B Post-operative computed tomography of the patient with heterotaxy who underwent SPS, PA angioplasty, and partial clipping of the graft. Arrow shows graft clipping. C Clip removal by catheter intervention. Arrow shows graft clipping. D SaO2 change before and after clip removal by catheter intervention. SPS systemic-to-pulmonary shunt, PA pulmonary artery, SaO2 oxygen saturation level of arterial blood
Statistical analysis
Data were expressed as mean ± standard deviation for normally distributed continuous variables, or as median for skewed continuous variables, according to the Shapiro–Wilk test. Testing for differences between variables was performed using the unpaired Student’s t-test to compare between cases with and without acute events, and the paired t-test for changes in SaO2 before and after clip removal and the diastolic/systolic blood pressure ratio of the systemic arterial pressure before and after partial clipping. Stepwise logistic regression was used to determine the risk factors of acute events after SPS procedure All the statistical tests were two sided, and p-values < 0.05 were considered as statistically significant. Statistical analysis was performed with SPSS statistical software (Version 16.0; SPSS Inc., Chicago, Illinois, United States).
Results
Patients’ characteristics and operative details
In this cohort, 116 patients underwent SPS as the main procedure, including BTS and CS in 115 patients and 1 patient, respectively. The median age at SPS operation was 1 month (2 days–16 years), and the median BW at SPS was 4.0 (2.6–16.1) kg, with seven patients (6%) weighing < 3.0 kg. Despite the institutional strategy described above, 15 patients required the SPS procedure during the neonatal period due to severe cyanosis; there were no preoperative deaths while waiting for SPS. There were 43 (37%) patients with pulmonary atresia, and 13 patients (11%) had a genetic disorder. Based on the echocardiography results, 78 and 38 patients had a BV and SV physiology, respectively. There were 9 patients with pulmonary atresia with intact ventricular septum (PA/IVS). Details of patient’s characteristics are shown in Table 1.
At the time of SPS, the median sternotomy approach was used in 65 patients (56%) and thoracotomy approach was 51 patients (44%), respectively. CPB was used in 49 patients (42%); concomitant procedures were pulmonary artery plasty in 22 patients, and intra-pulmonary artery septation in 1 patient. Details of SPS procedure are shown in Table 2. The single patient with a 3.0 mm graft was an exceptional case; this patient had PA/IVS with right ventricle (RV)-dependent coronary circulation, and had undergone primary bidirectional Glenn (BDG) anastomosis at 2 months of age to avoid SPS-induced coronary ischemia. She underwent additional BTS for progressive cyanosis 2 weeks after BDG anastomosis.
Partial clipping of the SPS
In this study cohort, 24 patients underwent partial clipping of the SPS graft (intra-operative, 19; post-operative, 5; pulmonary atresia in 15) (Fig. 2A, B). The number of clips was 1 in 15 patients, 2 in 8 patients, and 3 in 1 patient. The diastolic/systolic blood pressure ratio of the systemic arterial pressure significantly increased after clipping from 0.48 ± 0.07 to 0.53 ± 0.07 (p < 0.001). Ten patients had a BV physiology, and 14 patients had SV physiology, of whom 9 patients underwent clip removal via catheter intervention (ballooning within the SPS graft) (Fig. 2C). Catheter clip removal significantly increased SaO2 from 75.0% ± 4.6% to 83.3% ± 3.1% (p = 0.0018) (Fig. 2D). Shunt thrombosis after partial clipping occurred in two patients; one patient underwent emergent clip removal by catheter intervention immediately after thrombosis without any complication, while another patient suddenly died after discharge (inter-stage mortality). No graft injury related to clipping or the catheter removal procedure occurred.
In-hospital mortality and acute events
The overall discharge survival was 114/116 (98.3%). Prior to discharge, two (1.7%) patients died prior to 2013 due to coronary ischemia occurring 6 days and 30 days after operation, respectively. Both patients had PA/IVS with sinusoidal communication, and did not undergo clip regulation; therefore, we introduced clip regulation of the SPS flow as of 2013.
Pre-discharge acute events defined as shunt thrombosis, pulmonary over-circulation, or sudden circulatory collapse, occurred in seven (6.0%) patients. Shunt thrombosis occurred in three patients before discharge: two required revision of the SPS, and one underwent clip removal by catheter intervention.
Two patients required cardiac resuscitation for sudden pulmonary over-circulation on post-operative day 1; they underwent emergent exploration in the intensive care unit, and partial clipping of the SPS graft was performed. They recovered from cardiac collapse and were discharged without any neurological complications. Two cases of coronary ischemia were the aforementioned in-hospital deaths. Risk factors for pre-discharge acute events were the median sternotomy approach (7/7 vs. 0/109; p = 0.016), SV physiology (6/7 vs. 32/109; p = 0.002), and PA/IVS (4/7 vs. 5/109; p < 0.001). Shunt size, shunt size/BW ratio, pulmonary atresia, genetic disorders, and CPB use were not significant risk factor for pre-discharge acute events. Multivariate analysis revealed that PA/IVS (p = 0.0253) was an independent risk factor for in-hospital acute events after the SPS procedure (Table 3).
Inter-stage mortality
Two patients died before the next stage of the operation (1.7%). One patient with tetralogy of Fallot after BTS suffered from pneumonia and suddenly died 6 months after surgery. Another patient with PA/IVS after BTS and partial clipping was discharged without complication, but died of shunt thrombosis 1 month after BTS. Subsequently, 103 patients (89%) patients underwent the planned next-stage operation (BV repair, 72 patients; BDG, 31 patients).
Discussion
While SPS has become an established palliative procedure which provides adequate PBF for patients with congenital heart disease, significant operative mortality rate is still reported [3,4,5,6,7,8]. Previous studies have reported several risk factors for operative and inter-stage mortality after the SPS procedure, including neonatal period, 3.0 mm graft, median sternotomy approach, PA/IVS, SV physiology, low BW, genetic disorders, pulmonary atresia, CPB use, heterotaxy syndrome, and delayed heparin initiation [3,4,5,6,7,8, 11,12,13]. Further improvements in surgical techniques and safe strategies for SPS are therefore warranted. With the aim of minimizing the risks of the SPS procedure, we have adopted the following surgical strategies: (1) delaying the timing of SPS until 1 month of age as often as possible, (2) avoiding the use of SPS grafts 3.0 mm in size, (3) median sternotomy approach for SV patients or BV patients who required CPB, and (4) regulating PBF by partial clipping of the graft and remove the clips by catheter intervention, if necessary. Since the discharge survival rate of the present study was 98.3%, comparing favorably with previously reported rates of 85–94%, we believe that our institutional surgical strategy for SPS was safe and acceptable.
Timing of the SPS procedure
As pulmonary vascular resistance is highly variable in the neonatal period, especially in patients who undergo CPB [14, 15], we set the timing of SPS to after the first month of life as often as possible. It has been reported that open heart surgery in neonates can cause circulatory collapse due to a sudden increase or decrease in pulmonary vascular resistance immediately after operation [4, 8]. Since stenting of the arterial duct is still an uncommon treatment in Japan, we maintained patency of the PDA under PGE1 infusion for 1 month. We have therefore become accustomed to the long-term administration of PGE1 in Japan, and death while awaiting SPS and the significant adverse effects of PGE1 were not observed in the present study. Additionally, we aggressively performed simultaneous pulmonary artery angioplasty at the time of the SPS procedure under CPB to promote symmetric growth of the pulmonary artery tree if needed; thus, we set the timing of the SPS procedure to 1 month after birth, when the pulmonary resistance stabilizes.
Shunt graft size
As the use of a 3.0 mm shunt graft has been reported to be a risk factor for operative mortality due to shunt thrombosis and reintervention [4,5,6, 12], this was avoided. The PBF is defined by peripheral vascular resistance, the pressure gradient between proximal and distal pressure, and the diameter of the shunt graft, inevitably forcing the use of small-sized conduits in infants with low BW. To address this issue, we used a relatively larger graft size (3.5 mm), and restricted the PBF by adding partial shunt clipping if necessary. We believe that the use of larger sized grafts reduces the risk of shunt thrombosis and allows for fine regulation of PBF with metal clips. In addition, as we delayed the operation until after 1 month of age according to the institutional strategy, weight gain was achieved during the waiting period, and small-caliber grafts were less likely to be needed during the study period.
Partial clipping of the shunt graft
As previously described by Kuduvalli et al. [9], we applied partial clipping of the SPS shunt either during the operation or post-operatively. The degree of partial clipping was determined by hemodynamics and the oxygen saturation level of the arterial blood, similar to that of pulmonary artery banding.
A major advantage of this technique is that PBF can be easily adjusted intra-operatively and can be changed as many times as needed. Pulmonary vascular resistance varies rapidly, especially during the neonatal period and immediately after CPB, making it difficult to accurately determine and regulate PBF intra-operatively only by the size and length of the graft. Several techniques have been reported for regulation of SPS flow [16,17,18], but no technique has been yet widely used. We believe that graft partial clipping technique allows us to regulate PBF according to the changes of pulmonary vascular resistance as appropriate, not only intra-operatively, but also after operation.
Another advantage of clip-based PBF adjustment is that the clips can be post-operatively removed via catheter intervention. Napoleone et al. [10]. reported a technique for post-operative clip removal using 5–0 polypropylene sutures, describing its effectiveness and safety. In addition, as the relative shunt flow decreases with weight gain, we performed post-operative clip removal by catheter intervention to increase the PBF in a stepwise fashion. Notably, we have not experienced any graft injury resulting from clip removal by catheter intervention.
We adopted the following criteria to determine the degree of clipping: in patients with SV physiology, the SaO2 was around 80% (pulmonary-to-systemic blood flow ratio almost equal to 1.0); in patients with both SV and BV physiology, the systemic diastolic pressure was > 40% of the systolic pressure. We used not only SaO2 and but also the diastolic/systolic blood pressure ratio as indicators of PBF. It is sought that SaO2 does not necessarily reflect PBF, since some cases of CPB have temporarily impaired oxygenation. As the excessive reduction of diastolic pressure leads to myocardial ischemia, we restricted of blood flow with clips with reference to the diastolic/systolic pressure ratio. In the present study, the diastolic/systolic pressure ratio of patients who underwent partial clipping of the graft increased significantly to more than 0.53 immediately after clipping, and no coronary ischemic events were observed in these patients. Therefore, a set target of the diastolic/systolic pressure ratio even higher than 0.5 may be appropriate especially in patients with PA/IVS.
Although the use of 3.0 mm-sized grafts is avoided by adding PBF regulation with metal clips, the risk of shunt thrombosis due to clips cannot be ruled out. In fact, graft thrombosis occurred in two cases in the present study in which PBF was restricted by partial clipping; one patient was successfully treated by emergent clip removal and ballooning by catheter intervention, while the other patient died suddenly after discharge. We have since placed multiple (1–3) clips within one-third of the diameter to prevent thrombus formation due to the steep caliber change caused by a single clip, and have not experienced similar shunt occlusion.
Another important point is the graft size for partial clipping. Partial clipping should be avoided whenever possible because of the risk of thrombus occlusion. In this study, partial clipping was performed in 15 patients with 3.5 mm grafts, which may have avoided the use of 3.0 mm grafts. On the other hand, partial clipping was also performed in nine cases with 4.0 mm grafts, and these cases could have been addressed by downsizing to 3.5 mm grafts. In the present study, graft thrombosis occurred only in the partial clipping cases with 3.5 mm grafts, and the risk of thrombosis may be low even with partial clipping of 4.0 mm grafts, but the number of cases in this study was not large enough to investigate this issue.
Median sternotomy approach or thoracotomy approach
Although the median sternotomy approach for SPS is now widely used in many centers, it has been identified as a risk factor for acute events immediately after SPS in the previously reported studies [19]. We selected the thoracotomy approach for patients with BV physiology who did not require CPB, and the median sternotomy approach for patients with SV or BV physiology requiring CPB. We aggressively performed pulmonary artery (PA) angioplasty and CPB simultaneously in patients with PA coarctation via the median sternotomy approach, as symmetric PA growth is important, especially in patients with SV physiology [20]. Approaches through median incisions tend to cause excessive shunt flow, as the anastomosis of the graft inflow is in the proximal brachiocephalic artery; we thus applied partial clipping to the median sternotomy approach patients if needed. Conversely, we prefer the thoracotomy approach in patients who do not require PA plasty. As pulmonary over-circulation is rare in patients using the thoracotomy approach, the regulation of PBF with partial clipping was not required in the present study. Our institutional surgical strategy for tetralogy of Fallot is that the timing of the definitive repair should be around 6 months or more of age to preserve the autologous pulmonary valve as far as possible. We, therefore, performed the SPS procedure through lateral thoracotomy in patients with BV physiology and severe cyanosis within the first 6 months of life to minimize the risk of acute events after the procedure.
PA/IVS with sinusoidal communication
As in the previous reports, PA/IVS was an independent risk factor for acute events immediately after SPS in the present study [21, 22]. Since shunt circulation results in a decrease in arterial diastolic pressure, patients with severe sinusoidal communication or significant coronary obstruction are known to be at a high risk of circulatory collapse due to coronary ischemia. Therefore, we have restricted PBF with clips in cases of PA/IVS complicated with severe sinusoidal communication or significant coronary obstruction. More recently, we have also adopted a strategy of performing second stage palliation (BDG anastomosis) in early infancy for patients without a SPS. Within the present study period, one patient with RV-dependent coronary circulation underwent BDG anastomosis at 2-months of age without any coronary event.
Study limitation
This was a retrospective, single-center study with a small number of patient cohorts; thus, it may not be sufficient to identify risk factors of hospital or inter-stage mortality, and pre-discharge acute events. Additionally, excluding cases of aortic cross-clamping such as Norwood operation may account for the lower operative mortality rates compared with previous reports.; despite using logistic regression analysis for these acute events, confounding effects cannot be completely ruled out.
Conclusion
The outcomes of SPS according to our surgical strategy were acceptable compared to previous reports. The risk factor for acute event was still PA/IVS. PBF regulation with hemostatic clips was an easy and safe procedure, and post-operative catheter removal was safely performed.
Data availability
All patients were selected by the search term BTS or CS in the database of all patients with consultancy in Niigata University Hospital. Patient’s names were anonymized and each were given an unique code, which cannot be traced back. All data were collected in an encrypted excel file, only accessible for the authors. The data underlying this article cannot be shared publicly due to privacy of the patients and will only be shared on reasonable request to the corresponding author.
References
Blalock A, Taussig HB. Landmark article May 19, 1945: the surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia. JAMA. 1984;251:2123–38.
de Leval MR, McKay R, Jones M, Stark J, Macartney FJ. Modified Blalock-Taussig shunt. Use of subclavian artery orifice as flow regulator in prosthetic systemic-pulmonary artery shunts. J Thorac Cardiovasc Surg. 1981;81(1):112–9.
Dirks V, Prêtre R, Knirsch W, Valsangiacomo Buechel ER, Seifert B, Schweiger M, Hübler M, Dave H. Modified Blalock Taussig shunt: a not-so-simple palliative procedure. Eur J Cardiothorac Surg. 2013;44(6):1096–102.
Dorobantu DM, Pandey R, Sharabiani MT, Mahani AS, Angelini GD, Martin RP, Stoica SC. Indications and results of systemic to pulmonary shunts: results from a national database. Eur J Cardiothorac Surg. 2016;49(6):1553–63.
Alsoufi B, Gillespie S, Mori M, Clabby M, Kanter K, Kogon B. Factors affecting death and progression towards next stage following modified Blalock-Taussig shunt in neonates. Eur J Cardiothorac Surg. 2016;50(1):169–77.
Santro T, d’Udekem Y, Zannino D, Hobbes B, Konstantinov IE, Brizard C, Brink J. Determinants of acute events leading to mortality after shunt procedure in univentricular palliation. J Thorac Cardiovasc Surg. 2019;158(4):1144-1153.e6.
Alsoufi B, McCracken C, Schlosser B, Sachdeva R, Well A, Kogon B, Border W, Kanter K. Outcomes of multistage palliation of infants with functional single ventricle and heterotaxy syndrome. J Thorac Cardiovasc Surg. 2016;151(5):1369-77.e2.
Singh SP, Chauhan S, Choudhury M, Malik V, Talwar S, Hote MP, Devagourou V. Modified Blalock Taussig shunt: comparison between neonates, infants and older children. Ann Card Anaesth. 2014;17(3):191–7.
Kuduvalli M, McLaughlin KE, Trivedi DB, Pozzi M. Norwood-type operation with adjustable systemic—pulmonary shunt using hemostatic clip. Ann Thorac Surg. 2001;72:634–5.
Napoleone C, Oppido G, Angeli E, Gargiulo G. Adjustable aorto-pulmonary shunt to prevent temporary pulmonary over-circulation. Pace Eur J Cardiothorac Surg. 2006;29(2):253–4.
Chittithavorn V, Duangpakdee P, Rergkliang C, Pruekprasert N. Risk factors for in-hospital shunt thrombosis and mortality in patients weighing less than 3 kg with functionally univentricular heart undergoing a modified Blalock-Taussig shunt. Interact Cardiovasc Thorac Surg. 2017;25(3):407–13.
Myers JW, Ghanayem NS, Cao Y, Simpson P, Trapp K, Mitchell ME, Tweddell JS, Woods RK. Outcomes of systemic to pulmonary artery shunts in patients weighing less than 3 kg: analysis of shunt type, size, and surgical approach. J Thorac Cardiovasc Surg. 2014;147(2):672–7.
Mohammadi S, Benhameid O, Campbell A, Potts J, Joza J, Al-Habib H, Sett S, Le Blanc J. Could we still improve early and interim outcome after prosthetic systemic-pulmonary shunt? A risk factors analysis. Eur J Cardiothorac Surg. 2008;34(3):545–9.
van Vonderen JJ, Roest AA, Siew ML, Walther FJ, Hooper SB, te Pas AB. Measuring physiological changes during the transition to life after birth. Neonatology. 2014;105(3):230–42.
Miller OI, Tang SF, Keech A, Pigott NB, Beller E, Celermajer DS. Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: a randomised double-blind study. Lancet. 2000;356(9240):1464–9.
Motohashi Y, Shimada R, Sasaki T, Katsumata T, Dan K, Tsutsui Y, Emot S. Development of a simple device enabling percutaneous flow regulation for a small vascular graft for a Blalock–Taussig shunt capable of flow regulation: complete translation of an original article originally published in Pediatric Cardiology and Cardiac Surgery (154–159, 2016: vol. 32). Gen Thorac Cardiovasc Surg. 2018;66(3):145–9.
Mohiuddin MW, Resig PP, Sexton KW, Douglas WI. Two-day control of pulmonary blood flow with an adjustable systemic-pulmonary artery shunt. ASAIO J. 2011;57(3):225–30.
Chikada M, Sekiguchi A, Oho S, Miyamoto T, Ishida R, Takayama H, Ishizawa A. Dilatable banding of a Blalock-Taussig shunt. Ann Thorac Surg. 2002;74:253–5.
Talwar S, Kumar MV, Muthukkumaran S, Airan B. Is sternotomy superior to thoracotomy for modified Blalock-Taussig shunt? Interact Cardiovasc Thorac Surg. 2014;18(3):371–5.
Brink J, MacIver R, Lee MG, Konstantinov IE, Cheung M, Brizard CP, d’Udekem Y. Neonatal pulmonary artery reconstruction during shunting to treat and prevent juxtaductal coarctation. Ann Thorac Surg. 2015;99(2):641–7.
Guleserian KJ, Armsby LB, Thiagarajan RR, del Nido PJ, Mayer JE. Natural history of pulmonary atresia with intact ventricular septum and right-ventricle-dependent coronary circulation managed by the single-ventricle approach. Ann Thorac Surg. 2006;81(6):2250–7.
Ashburn DA, Blackstone EH, Wells WJ et al. Congenital Heart Surgeons Study members. Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg. 2004;127(4): 1000–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shiraishi, S., Watanabe, M., Sugimoto, A. et al. Surgical outcomes of the systemic-to-pulmonary artery shunt: risk factors of post-operative acute events and effectiveness of regulation of pulmonary blood flow with metal clips. Gen Thorac Cardiovasc Surg (2024). https://doi.org/10.1007/s11748-024-02028-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11748-024-02028-8