Abstract
Systemic-to-pulmonary artery shunt placement is an established palliative procedure for congenital heart disease, but it is associated with high morbidity and mortality. Data of all patients with biventricular circulation who underwent systemic-to-pulmonary artery shunt implantation between 2000 and 2016 were reviewed. Endpoints of the study were shunt failure and shunt-related mortality. Shunt failure was defined as any shunt dysfunction requiring intervention or reoperation. Shunt-related mortality was defined as death due to shunt dysfunction. A total of 217 shunts (central shunt, n = 131, Blalock-Taussig shunt, n = 86) were implanted in 178 patients. The median age of the patients was 98 days [1 day to 1.2 years]. Corrective surgery was performed at a median time of 0.6 years [3 months to 7 years] after shunt placement. Shunt failure was diagnosed in 21 patients (9.6%) at a median time of 14.6 days [0 days to 2 years]. Causes of shunt failure were stenosis (n = 11; 5%) and thrombosis (n = 10; 4.6%). The rate of freedom from shunt failure was 89.9 ± 2.6% at 1 year, the rate of shunt-related mortality was 3% (n = 5), and the rate of freedom from shunt-related mortality at 1 year was 97.5 ± 1%. Platelet transfusion was required in 43 patients (20%), all for postoperative thrombocytopenia. Perioperative platelet transfusion (p = 0.03) and shunt size of 3 mm (p = 0.03) were identified as risk factors for shunt failure. Shunt size of 3 mm was also identified as a risk factor for shunt-related mortality. The ideal shunt size in patients with biventricular circulation requiring a systemic-to-pulmonary artery shunt is 3.5 mm or larger. Platelet transfusion increases the risk of shunt failure and should be avoided. Type of shunt and diagnosis have no influence on morbidity or mortality after shunt placement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Systemic-to-pulmonary artery shunt placement is a well-established palliation in the surgical management of selected neonates and infants with complex congenital heart disease. Systemic-to-pulmonary artery shunts were first described by Blalock and Taussig in 1945 (subclavian to pulmonary artery shunt) [1], Potts in 1946 (descending aorta to left pulmonary artery shunt) [2], and Waterston and Cooley in 1962 and 1966 (ascending aorta to right pulmonary artery shunt) [3, 4]. In 1975, de Leval modified the technique using a polytetrafluoroethylene interposition graft known as the modified Blalock-Taussig shunt (mBTS) [5].
Although considered a “simple” surgical procedure, shunt placement is nonetheless associated with reported mortality rates of 10% or more [6,7,8]. This limitation has advanced the strategy of early primary correction of heart defects that are amenable to a biventricular repair [9]. Even in tetralogy of Fallot (TOF) and pulmonary atresia with ventricular septal defect (PA/VSD), there are some centers that advocate the avoidance of first-step palliation [10,11,12,13] because of the expected high mortality after shunt placement. According to the literature, patients with univentricular hearts exhibit higher mortality after shunt placement than patients with biventricular physiology [9, 14]. Shunt-dependent survival is strongly influenced by the development of shunt-related circulatory complications, which, particularly in functionally single-ventricle hearts, may result in a dismal outcome [9, 14].
The clinical outcome of corrective surgery is determined by the morbidity and mortality induced by the surgical procedure on one hand, and by the complexity of the underlying heart disease on the other [9]. Shunt placement is indicated in patients with hypoplastic pulmonary arteries to promote growth [15] and might be a better option than primary repair in these patients.
We sought to review our outcomes in patients with biventricular circulation who underwent systemic-to-pulmonary artery shunt placement in order to identify risk factors for postoperative complications.
Materials and Methods
All patients with biventricular circulation who underwent shunt placement and subsequent corrective surgery between 2000 and 2016 at the German Heart Centre in Munich were included in the study. Patients were identified from our clinical database and all available medical records and operative notes were reviewed. Indications for shunt placement were duct-dependent pulmonary circulation without antegrade blood flow, hypoplastic pulmonary arteries, hypoxic spells, and prematurity.
All patients were examined regularly after surgery at our outpatient clinic or by the referring pediatric cardiologist.
We defined two endpoints of the study:
-
Shunt failure, defined as shunt thrombosis or stenosis
-
Shunt-related mortality, defined as death as a consequence of shunt failure.
The shunt size was determined according to the patient’s weight. The goal was a shunt/size index (mm/kg) of < 1.5. If the shunt/size index was ≥ 1.5, the shunt was considered oversized.
After surgery, the patients were examined regularly by echocardiography and all patients were seen every 4–6 weeks in our outpatient clinic after discharge and were examined by echocardiography. Angiography was routinely planned and performed in all patients at 3–6 months after shunt implantation prior to further surgery. If there were signs of reduced shunt flow on echocardiography, angiography was performed sooner.
Surgical Technique
Shunt placement was performed through a median sternotomy with cardiopulmonary bypass in the majority of the cases. The brachiocephalic trunk was exposed and the right pulmonary artery (PA) dissected up to the hilum. A bolus of 100 IU/kg heparin was administered if surgery was not performed on bypass. The brachiocephalic trunk was clamped with a side-biting clamp and a longitudinal arteriotomy was performed. An obliquely fashioned thin-wall Gore-Tex stretch vascular graft (W. L. Gore & Associates, Inc., AZ, USA) was sutured end-to-side to the brachiocephalic trunk. The clamp was released and good shunt flow ascertained. After trimming the shunt to the right length, it was anastomosed to the right PA. In case of a right descending aortic arch, the shunt was anastomosed to the left PA. A central shunt was placed for anatomical reasons or at the discretion of the attending surgeon. The anatomy was considered unsuitable for a mBTS if the PAs were hypoplastic. Central shunt placement was performed interposing a thin-wall Gore-Tex stretch vascular graft between the ascending aorta and the PA in a similar fashion, using the pulmonary trunk or bifurcation in cases of hypoplastic PA. We have routinely used Propaten brand heparin-coated shunts since 2011.
Postoperative anticoagulation was performed according to a standard protocol as follows:
-
For shunt size < 4 mm, intravenous heparin 5000 IU/m2/day beginning 6 h after surgery until removal of the central venous catheter; thereafter oral aspirin 3–5 mg/kg.
-
For shunt size > 4 mm, intravenous heparin 5000 IU/m2/day beginning 6 h after surgery until removal of the central venous catheter and no postoperative aspirin.
Statistical Analysis
Statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). Frequencies are given as absolute numbers and percentages. Continuous data are given as medians with ranges or as means with standard deviation. Comparison of categorical variables was carried out by using the two-tailed χ2-test or the Fisher exact test, as appropriate. Comparison of continuous variables was carried out by using the t-test for independent samples. The Kaplan–Meier method was used to estimate the probability of freedom from shunt failure and shunt-related mortality. The Cox-regression analysis was used to identify risk factors for events. For all tests, a p value ≤ 0.05 was considered statistically significant.
Results
Patients
A total of 217 shunts were implanted in 178 patients. The median patient age at time of surgery was 98 days [1 day to 1.2 years]. Only one patient received a shunt beyond the infant age. This patient, who had PA/VSD and multiple aortopulmonary collateral arteries (MAPCA), underwent successful corrective surgery 5 years after shunt implantation. Initial patient characteristics are summarized in Table 1.
mBTS was performed in 131 patients (60%; 116 [88.5%] right PA and 15 [11.5%] left PA) and 86 patients (40%) had central shunt placement. Shunt size ranged from 3 to 6 mm (Fig. 1). One in three patients (33%, n = 72) received heparin-coated shunts. The mean shunt/size index (mm/kg) was 1.0 ± 0.3; 146 patients had shunt/size index > 1 and 12 had shunt/size index ≥ 1.5. All shunt characteristics are summarized in Table 2.
Four patients (1.8%; three with acute cardiac failure and one with acute hypoxemia) required postoperative extracorporeal membrane oxygenation (ECMO), which was implanted postoperatively in the ICU during cardiopulmonary resuscitation (CPR) in all cases. The shunt was partially clipped in three patients at the time of ECMO implantation in order to secure adequate blood flow for ECMO.
Forty-three patients (20%) with thrombocytopenia required platelet transfusion for postoperative bleeding. The indication was a thrombocytopenia. Other coagulopathy or polycythemia was not associated. All 43 patients received also fresh frozen plasma (FFP) and packed red blood cells (PRBC). Further 40 patients received FFP and PRBC, but no platelets.
All of the patients on ECMO required platelet transfusion. Five patients (two on ECMO) needed reoperation for postoperative bleeding.
A total of 199 angiographies were performed after surgery; 178 were scheduled prior to further surgery and 21 were performed emergently after finding severely reduced shunt flow on echocardiography.
There were 147 (83%) patients who underwent corrective surgery within a median time of 0.6 years [3 months to 7 years] after shunt placement. The patient who had corrective surgery at 5 years after shunt placement was initially considered not to be suitable for biventricular repair.
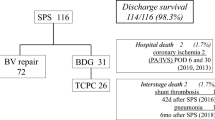
There were 39 patients who required a second shunt implantation before corrective surgery. These patients were doubly included in the study: eight with shunt failure and 31 who outgrew the initial shunt but still matched the inclusion criteria. A total of 31 patients died; 21 remained in shunt failure, and 147 achieved the corrective surgery. The course of all patients with implanted shunts is depicted in Fig. 2.
Mortality
The 30-day mortality rate was 6.7% (n = 12). Shunt-related mortality was confirmed in five patients (2.8%), all after shunt thrombosis. Four of these patients (4/5) died after CPR and one (1/5) had the shunt clipped on ECMO. The other 7 (7/12) patients died of myocardial failure (n = 5) or septicemia (n = 2). In all seven of these, autopsy confirmed a patent shunt. There were 19 patients who died after hospital discharge, all of acute cardiac failure.
The rate of freedom from shunt-related mortality at 1 year was 97.5 ± 1% (Fig. 3).
Shunt Failure and Treatment
A total of 21 shunts (9.6%) remained in a failure at a median of 14.6 days [0 days to 2 years]. Angiography showed shunt stenosis in 11 patients, which was treated with balloon angioplasty (n = 4), stent implantation (n = 4), or surgical shunt replacement (n = 3).
Shunt thrombosis was diagnosed by angiography in five patients. The treatment in all of these was reoperation for shunt exchange. Additionally, shunt thrombosis was diagnosed at autopsy in another five patients.
The course of the patients with shunt failure is depicted in Fig. 4. The rate of freedom from shunt failure at 1 year was 89.9 ± 2.6% (Fig. 5).
Risk Factors for Shunt Failure and Shunt-Related Mortality
Perioperative platelet transfusion (p = 0.03) and shunt size of 3 mm (p = 0.03) were identified as risk factors for shunt failure. Shunt size of 3 mm was also a risk factor for shunt-related mortality (p = 0.01).
Underlying diagnosis, shunt type, patient weight and sex, and transfusion of other blood products did not significantly influence the risk for shunt failure or shunt-related mortality (Table 3).
Discussion
Placement of a systemic-to-pulmonary artery shunt can be a life-saving procedure for patients with congenital heart defects associated with diminished or absent pulmonary blood flow. However, despite numerous improvements in diagnosis, intensive care medicine, and intraoperative management in recent decades, the overall outcome of the mBTS remains unsatisfactory. Our retrospective analysis of shunt placements in patients with biventricular circulation during 15 years showed a 30-day mortality rate of 6.7% after initial shunt placement. This analysis featured a large single-center cohort of 217 shunts implanted in 178 patients with biventricular circulation. The shunt-related mortality was only 3% (n = 5), but a total of 21 patients (9.6%) developed shunt failure. We found that the size of shunt influenced the probability of a stenosis or thrombosis and increased the risk of shunt-related mortality. Transfusion of platelets also worsened the postoperative prognosis in terms of greater risk of shunt failure.
A number of single-center reports from an earlier era evaluated the outcomes of shunt placement. These studies reported mortality rates ranging from 3.7 to 14% [16,17,18,19,20], and more recent studies have confirmed that mBTS placement remains a high-risk procedure. Petrucci and colleagues examined outcomes in 1273 patients (62% with biventricular heart defects) from the multi-institutional Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database and identified an overall mortality rate of 7.2% [14], and in 2014, Bove et al. reported an 8.7% overall hospital mortality rate in 150 neonates after shunt surgery [9]. In our study, the overall 30-day mortality rate after shunt surgery was 6.7% (n = 12), but the shunt-related mortality rate was only 3%. Our report included only patients with biventricular circulation. We found a lack of publications analyzing the reasons for shunt-related mortality. In our analysis, autopsy results showed that 7 of 12 patients who died with a shunt in situ died of reasons unrelated to the shunt itself.
Regarding our finding that shunt size of 3 mm is a risk factor for shunt-related mortality, it is well known that both a patient’s weight and the use of smaller shunts are significantly related to poor outcomes after shunt placement [21, 22]. However, weight alone had no influence on shunt-related mortality in our cohort, whereas the 3-mm shunt size increased the risk of shunt-related mortality. This finding suggests a benefit for shunt oversizing, although other authors have reported that excessive pulmonary blood flow can also contribute to mortality [6, 9]. We could not identify an increased risk of mortality related to shunt oversizing in our cohort. Furthermore, patients with additional blood flow, such as MAPCA, who should have a higher risk of pulmonary flooding, did not have a higher risk of mortality.
One older (1995) multivariate risk analysis for mortality found that left mBTS and male sex were associated with increased mortality [20]. Pulmonary atresia with intact ventricular septum has also been identified as a significant risk factor for mortality after shunt surgery [14]. In our study, neither sex nor the nature of the underlying heart defect had any influence on postoperative mortality. On the other hand, other authors have, as we have, found that that shunt size of 3 mm is related to increased risk of shunt failure [21, 22]. It is assumed that a shunt size of at least 3.5 mm is required for sufficient flow and a respective flushing effect to prevent thrombus formation.
We found that perioperative transfusion of platelets increased the risk for shunt failure, which has not been previously reported. Forty-three patients (20%) required postoperative platelet transfusion to control bleeding, due to postoperative thrombocytopenia. Transfusion of other blood products such as fresh frozen plasma or packed red blood cells showed no influence on shunt failure and mortality. Heparin-coated polytetrafluoroethylene shunts have been introduced in an attempt to reduce acute shunt occlusion by inhibiting thrombus formation. This type of shunt has been available since 2011, and it comprised 33% of the shunts in our study. We did not detect an obvious advantage of heparin-coated shunts in our study, and a previous histopathological analysis of PTFE shunts showed that partial endothelialization and discrete pseudointima proliferation were equal in both heparin-coated and non-heparin-coated shunts [23].
We also could not find any association between anticoagulation regimens and shunt failure. All of our patients received an early postoperative intravenous heparin regimen after shunt surgery, and although the patients with shunt size < 4 mm received aspirin addition to heparin, the rate of shunt thrombosis was higher in these patients. Factors that may initiate thrombus formation during the early postoperative period with fresh surgical anastomoses include periods of low systemic blood pressure and pulmonary hypertension with consecutive stasis [6]. In contrast to our findings, Li et al. found a beneficial effect of aspirin after palliative shunt placement in infants. Patients who received aspirin had a lower risk of shunt thrombosis (p = 0.008) and death (p = 0.057) compared with those not receiving aspirin [24]. Another prospective study showed that hemodilution to a hematocrit value of 45% was also associated with a significantly higher shunt patency rate [25].
Conclusion
Type of shunt and preoperative diagnosis have no influence on morbidity or mortality after shunt placement in patients with biventricular circulation. The ideal shunt size in patients with biventricular circulation requiring a systemic-to-pulmonary artery shunt is 3.5 mm or larger. Platelet transfusion increases the risk of shunt failure and should be avoided.
References
Blalock A, Taussig HB (1984) Landmark article May 19, 1945: the surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia. By Alfred Blalock Helen B Taussig. JAMA 251:2123–2138
Potts WJ, Smith S, Gibson S (1946) Anastomosis of the aorta to a pulmonary artery in certain types of congenital heart disease. Case Rep Child Meml Hosp 5:704–718
Waterston DJ (1962) Treatment of Fallot’s tetralogy in children under 1 year of age. Rozhl Chir 41:181–183
Cooley DA, Hallman GL (1966) Intrapericardial aortic-right pulmonary arterial anastomosis. Surg Gynecol Obstet 122:1084–1086
de Leval MR, McKay R, Jones M, Stark J, Macartney FJ (1981) Modified Blalock-Taussig shunt. Use of subclavian artery orifice as flow regulator in prosthetic systemic-pulmonary artery shunts. J Thorac Cardiovasc Surg 81:112–119
Dirks V, Pretre R, Knirsch W, Valsangiacomo Buechel ER, Seifert B, Schweiger M et al (2013) Modified Blalock Taussig shunt: a not-so-simple palliative procedure. Eur J Cardio-thorac Surg 44:1096–1102
McKenzie ED, Khan MS, Samayoa AX, Vener DS, Ishak YM, Santos AB et al (2013) The Blalock-Taussig shunt revisited: a contemporary experience. J Am Coll Surg 216:699–704 discussion 04–46.
Williams JA, Bansal AK, Kim BJ, Nwakanma LU, Patel ND, Seth AK et al (2007) Two thousand Blalock-Taussig shunts: a six-decade experience. Ann Thorac Surg 84:2070–2075; discussion 70–75
Bove T, Vandekerckhove K, Panzer J, De Groote K, De Wolf D, Francois K (2015) Disease-specific outcome analysis of palliation with the modified Blalock-Taussig shunt. World J Pediatr Congenit Heart Surg 6:67–74
Reddy VM, Liddicoat JR, McElhinney DB, Brook MM, Stanger P, Hanley FL (1995) Routine primary repair of tetralogy of Fallot in neonates and infants less than three months of age. Ann Thorac Surg 60:S592–S596
Arenz C, Laumeier A, Lutter S, Blaschczok HC, Sinzobahamvya N, Haun C et al (2013) Is there any need for a shunt in the treatment of tetralogy of Fallot with one source of pulmonary blood flow? Eur J Cardiothorac Surg 44:648–654
Hanley FL, Sade RM, Blackstone EH, Kirklin JW, Freedom RM, Nanda NC (1993) Outcomes in neonatal pulmonary atresia with intact ventricular septum. A multiinstitutional study. J Thorac Cardiovasc Surg 105:406–427; discussion 23–24
Watanabe N, Mainwaring RD, Reddy VM, Palmon M, Hanley FL (2014) Early complete repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. Ann Thorac Surg 97:909–915; discussion 14–15
Petrucci O, O’Brien SM, Jacobs ML, Jacobs JP, Manning PB, Eghtesady P (2011) Risk factors for mortality and morbidity after the neonatal Blalock-Taussig shunt procedure. Ann Thorac Surg 92:642–651; discussion 51–52
Ishikawa S, Takahashi T, Sato Y, Suzuki M, Murakami J, Hasegewa Y et al (2001) Growth of the pulmonary arteries after systemic-pulmonary shunt. Ann Thorac Cardiovasc Surg 7:337–340
Gold JP, Violaris K, Engle MA, Klein AA, Ehlers KH, Lang SJ et al (1993) A five-year clinical experience with 112 Blalock-Taussig shunts. J Card Surg 8:9–17
Gladman G, McCrindle BW, Williams WG, Freedom RM, Benson LN (1997) The modified Blalock-Taussig shunt: clinical impact and morbidity in Fallot’s tetralogy in the current era. J Thorac Cardiovasc Surg 114:25–30
Lamberti JJ, Carlisle J, Waldman JD, Lodge FA, Kirkpatrick SE, George L et al (1984) Systemic-pulmonary shunts in infants and children. Early and late results. J Thorac Cardiovasc Surg 88:76–81
Alkhulaifi AM, Lacour-Gayet F, Serraf A, Belli E, Planche C (2000) Systemic pulmonary shunts in neonates: early clinical outcome and choice of surgical approach. Ann Thorac Surg 69:1499–1504
Odim J, Portzky M, Zurakowski D, Wernovsky G, Burke RP, Mayer JE Jr et al (1995) Sternotomy approach for the modified Blalock-Taussig shunt. Circulation 92:II256–II261
Tsai KT, Chang CH, Lin PJ (1996) Modified Blalock-Taussig shunt: statistical analysis of potential factors influencing shunt outcome. J Cardiovasc Surg 37:149–152
Tamisier D, Vouhe PR, Vernant F, Leca F, Massot C, Neveux JY (1990) Modified Blalock-Taussig shunts: results in infants less than 3 months of age. Ann Thorac Surg 49:797–801
Horer J, Cleuziou J, Kasnar-Samprec J, Schreiber C, Balling G, Foth R et al (2014) A comparative histopathological study of heparin coated and uncoated polytetrafluoroethylene shunts in children with congenital heart defect. World J Pediatr Congenit Heart Surg 5:385–390
Li JS, Yow E, Berezny KY, Rhodes JF, Bokesch PM, Charpie JR et al (2007) Clinical outcomes of palliative surgery including a systemic-to-pulmonary artery shunt in infants with cyanotic congenital heart disease: does aspirin make a. difference? Circulation 116:293–297
Sahoo TK, Chauhan S, Sahu M, Bisoi A, Kiran U (2007) Effects of hemodilution on outcome after modified Blalock-Taussig shunt operation in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth 21:179–183
Funding
This study was funded by the Werner Reichenberger Foundation for Child Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or nation research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Vitanova, K., Leopold, C., Pabst von Ohain, J. et al. Risk Factors for Failure of Systemic-to-Pulmonary Artery Shunts in Biventricular Circulation. Pediatr Cardiol 39, 1323–1329 (2018). https://doi.org/10.1007/s00246-018-1898-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-1898-4