Abstract
Unplanned reinterventions following pulmonary artery banding (PAB) in single ventricle patients are common before stage 2 palliation (S2P) but associated risk factors are unknown. We hypothesized that reintervention is more common when PAB is placed at younger age and with a looser band, reflected by lower PAB pressure gradient. Retrospective single center study of single ventricle patients undergoing PAB between Jan 2000 and Dec 2020. The association with reintervention and successful S2P was modeled using exploratory cause-specific hazard regression. A multivariable model was developed adjusting for clinical and statistically relevant predictors. The cumulative proportion of patients undergoing reintervention were summarized using a competing risk model. 77 patients underwent PAB at median (IQR) 47 (24–66) days and 3.73 (3.2–4.5) kg. Within18 months of PAB, 60 (78%) reached S2P, 9 (12%) died, 1 (1%) transplanted and 7 (9%) were alive without S2P. Within 18 months of PAB 10 (13%) patients underwent reintervention related to pulmonary blood flow modification: PAB adjustment (n = 6) and conversion to Damus–Kaye–Stansel/Blalock–Taussig–Thomas shunt (n = 4). 6/10 (60%) reached S2P following reintervention. A trend toward higher intervention in patients with a genetic syndrome (p—0.06) and weight < 3 kg (p—0.057) at time of PAB was noted. Only genetic syndrome was a risk factor associated with poor outcome (p—0.025). PAB has a reasonable outcome in SV patients with unobstructed systemic and pulmonary blood flow, but with a high reintervention rate. Only a quarter of patients with genetic syndromes reach S2P and further study is required to explore the benefits from an alternative palliative strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Main pulmonary artery banding (PAB) is the preferred first stage palliation strategy for single ventricle heart lesions (SV) with unobstructed pulmonary blood flow [1,2,3]. PAB is usually required for some patients with double inlet left ventricle, tricuspid atresia, or unbalanced atrio-ventricular septal defects (AVSD) to restrict pulmonary blood flow and decrease pulmonary pressures to assure low pulmonary vascular resistance in preparation for the bidirectional cavopulmonary shunt (BCPS) and, ultimately, the Fontan operation. These goals are usually attained while avoiding exposure to cardiopulmonary bypass and interstage complications associated with conversion to a systemic-to-pulmonary artery shunt [4, 5].
Development of heart failure symptoms as pulmonary vascular resistance decreases is often used to time PAB placement with optimal tightness. Reintervention prior to BCPS is common and may stem from the inherent difficulties of intraoperative adjustment of PAB to achieve a balanced physiology [1]. The initial length of the PAB is usually calculated from the Trusler equation with further modifications guided by intraoperative physiology [3, 6, 7]. The tightness of the PAB is determined primarily by intraoperative systemic oxygen saturation, the pressure gradient across the PAB, distal pulmonary pressure, and lack of signs of increased ventricular afterload, including ventricular dysfunction or atrio-ventricular valve regurgitation. Suboptimal calibration may result in unanticipated reinterventions to further tighten the PAB because of excessive pulmonary blood flow or augment pulmonary blood flow in case of excessive cyanosis. Despite high reintervention rates and failure to reach BCPS [1], modifiable risk predictors of these complications have not been identified.
This study was undertaken to identify modifiable perioperative risk factors that predict reintervention or failure to reach BCPS. We hypothesized that younger age and a looser initial PAB, reflected by higher oxygen saturation and lower PAB gradient, was associated with poor clinical outcomes.
Materials and Methods
This single-center, retrospective study was approved by the institutional review board of the hospital (REB number 1000068839; Approved 2017-04-07), and informed patient consent for the publication of study data was waived due to it being a retrospective study. All patients with single ventricle physiology who underwent PAB as the first stage palliation between January 1, 2000, and December 31, 2020, were identified from cardiovascular surgery and SV databases. Patients were excluded only if they underwent banding of bilateral branch pulmonary arteries or ultimately underwent biventricular repair.
Surgical Procedure
Infants with SV physiology were presented at a multidisciplinary conference, and indication and timing of PAB were determined by consensus and institutional practices. Patients who required aortic arch reconstruction or coarctation repair typically underwent PAB and aortic arch procedure in the first week of life, whereas those with unobstructed biventricular outflow tracts the PAB was deferred until heart failure became clinically evident or concern for increasing PVR developed.
The PAB was originally constructed using a specially coated umbilical tape, which was replaced with a strip of a polytetrafluoroethylene graft trimmed to 2.5 to 3.5 mm width (GORE-TEX® Vascular Graft, W. L. Gore & Associates, Inc., Flagstaff, Arizona) in April 2019. The baseline PAB length was determined by the Trusler’s rule, 20 (biventricular) or 24 (SV) mm plus 1 mm for every kg of body weight, depending on patient’s anatomy and physiology and partly on surgeon’s discretion [8]. Further modifications were guided by intraoperative physiology, with the team targeting an echocardiographic peak pressure gradient of at least 25–35 mmHg or a systolic pulmonary artery pressure of less than 50% of systemic pressure by direct needle measurement, no increase in atrio-ventricular valve regurgitation, and preserved ventricular function, and systemic oxygen saturations of approximately 80%. The PAB position was confirmed by echocardiography to avoid distortion of branch pulmonary arteries or creation of new pulmonary valve insufficiency. The PAB was secured with 5–0 or 6–0 polypropylene sutures (PROLENE, Ethicone Inc. © Johnson & Johnson Medical N.V., Belgium) to the pulmonary artery adventitia by at least two sutures to prevent distal dislodgement. Different surgical approaches were taken depending on the concomitant procedures. Infants with unobstructed dual outflow tracts had PAB performed via a limited upper sternotomy, while a full median sternotomy under deep hypothermic circulatory arrest with selective cerebral perfusion was performed for infants who required a concomitant aortic arch reconstruction; PAB and atrial septectomy were performed during re-warming the patient. Patients who only required surgical atrial septectomy underwent standard normothermic cardiopulmonary bypass with bicaval cannulation. Lastly, a left thoracotomy was used for infants who underwent simple coarctation repair, with the coarctation repair being performed first followed by opening of the pericardium and exposing the main pulmonary artery.
All patients were followed through the interstage period by a dedicated Single Ventricle team. Candidacy for BCPS was determined by catheter-based hemodynamic assessment with or without cardiac MRI. For uncomplicated patients with appropriate hemodynamics, BCPS were routinely scheduled between 5 and 6 months of age.
Outcomes
The frequency of unplanned surgical or catheter-based intervention undertaken to modify pulmonary blood flow, and the transplant-free survival to BCPS were used as primary outcomes. These individual outcomes were selected to inform the effectiveness of PAB as a first stage palliation to prepare patients to continue down a traditional single ventricle pathway.
Secondary outcomes included characterization of unplanned interventions (frequency and type of surgical and catheter procedures) and measurements of somatic and pulmonary artery growth prior to BCPS. Weight, standardized as weight-for-age z-scores using WHO standards, was used the primary growth parameter [9]. Pulmonary artery growth was measured according to branch pulmonary artery diameters and presented using calculations of institutionally derived Z-scores. Measurements were extracted by a single team member (BL) from the echocardiographic, cross-sectional (CT or MRI), and catheterization imaging just prior to BCPS.
Statistical Analysis
Baseline clinical characteristics were summarized using descriptive statistics. Continuous variables were described using mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate. Dichotomous and polytomous variables were summarized by frequencies. We considered two time-to-event outcome variables in the study: intervention following PAB and prior to BCPS, and receipt of BCPS. The time to these outcomes were measured separately from the time of index PAB and analyzed using competing risk models. Post-PAB mortality and transplant were considered as competing risks. The cumulative proportion of patients undergoing reintervention receiving BCPS summarized the results of the competing risk model. The occurrence of reintervention was analyzed similarly, as was the secondary outcome of hospital discharge.
We conducted univariable and multivariable cause-specific hazard regression to assess the association of pre- and intra-operative clinical factors with the receipt of BCPS and reintervention separately. In the univariable analysis, we explored possible non-linear association between a continuous factor and an outcome variable and summarized the non-linear association graphically. In the multivariable analysis, we considered the following variables a priori in the model: prematurity, weight at PAB, systemic oxygen saturation following PAB, PAB circumference, PAB gradient, presence of genetic syndrome, and unbalanced AVSD. These variables were selected based on their association with outcomes from previous studies and clinical experience. The nonlinear associations were presented graphically, and linear associations were summarized in terms of hazard ratios (HRs); the corresponding 95% confidence intervals (CIs) and p values were evaluated using Wald’s statistics.
Results
Seventy-eight patients with single ventricle physiology underwent main PAB over 21 years, one of which underwent the surgery beyond infancy and was therefore excluded from the study. Patient demographics and cardiac diagnoses were summarized in Table 1. Patients underwent PAB at a median age of 47 (24–66) days and weight of 3.73 (3.2–4.5) kg, and preoperative oxygen saturation of 95 (91–96)%. Ten percent of patients (N = 8) demonstrated evidence of coexisting syndrome or genetic abnormality, Trisomy 21 being most common (88%, N = 7). Eighteen percent (N = 14) and 16% (N = 12) were receiving mechanical ventilation support or inotropic preoperative support, respectively. PAB was preceded by balloon atrial septostomy in 19% (N = 15).
Supplementary Table 1 summarizes the 9% (N = 7) of patients who received PAB under 3 kg, 43% (N = 3) which occurred within the first week of life and 43% (N = 3) who were premature. Indications for timing of PAB included concurrent coarctation repair, low systemic cardiac output and poor perfusion and heart failure symptoms.
PAB Perioperative Considerations
Approximately half (52%, N = 40) the patients had at least one concomitant surgical procedure during PAB, including PDA ligation (35%, N = 27), atrial septectomy (16%, N = 12), balloon atrial septostomy (1%, N = 1), arch reconstruction (8%, N = 6), and coarctation repair (6%, N = 5). PAB was performed off bypass through median sternotomy in 71% (N = 53) patients and lateral thoracotomy in 9% (N = 8). Twenty-one percent (N = 16) underwent sternotomy and bypass, including 12% (N = 9) who were exposed to circulatory arrest.
The median (IQR) PAB length was 25 (24–26) mm, measuring slightly longer than the 24 (23–25) mm calculated length using the Trusler formula for biventricular hearts, but shorter than the Trusler calculations for SV. The patients’ intraoperative systemic oxygen saturation dropped to 88% (82–92) and remained unchanged at admission to the critical care unit [88 (83–94) %] and prior to hospital discharge [92 (88–96)] %. Final intraoperative peak PAB gradient was 31 (27–38) mmHg when measured directly and 40 (30–50) mmHg by echocardiogram. The echocardiographic gradient across the PAB was measured at 54 (44–64) mmHg in the echocardiogram immediately prior to hospital discharge. Placement of PAB was associated with a trend toward higher frequency of moderate or severe atrio-ventricular valve regurgitation [preoperative 19 (25%) vs postoperative 22 (29%), p = 0.062].
PAB surgery was associated with delayed chest closure in 14% (N = 11), and complicated by cardiac arrest in 9% (N = 7) and extracorporeal membrane oxygenation in 1% (N = 1). (Supplementary Table 2) Patients received 2 (1–6) days of positive pressure ventilation support, 4 days (3–9) of critical care support, and were discharged from hospital after 8 (6–22) days.
Outcomes
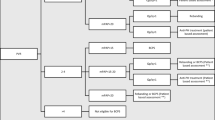
Figure 1 illustrates the modified history of the patient cohort within the 18 months following PAB.
Reintervention
21% (N = 16) of patients underwent at least one reintervention within 18 months of PAB and before BCPS, of which 13% (N = 10) were to address pulmonary blood flow (Fig. 2). Only 5% (N = 4) underwent two interventions. Table 2 lists the frequency and proportions of interventions. The PAB was readjusted in 6 patients, including dilatation in 1, tightening in 2, and reapplication in 3. PAB migration was found in 10% (N = 8) on follow up echocardiograms or cardiac catheterizations.
Univariable cause-specific hazard regression analysis suggested that demographics, cardiac anatomy, PAB length, and systemic oxygen saturation and pressure gradients following PAB application were not associated with increased hazard of reintervention (Table 3). There was a trend for patients with a proven syndrome or genetic abnormality (hazard ratio 4.62 [95% CI 0.92–23.2] p = 0.06) and lower weight at time of PAB (p = 0.057) to have higher risk of reintervention. Nevertheless, after adjusting for PAB circumeference, and intraoperative oxygen saturation, neither factor was associated with an increased risk of reintervention prior to BCPS.
Transplant-Free Survival to BCPS
Details of the comprehensive assessment prior to BCPS are presented in Supplementary Table 3. A BCPS was performed on 79% (N = 60) patients in the 18 months following PAB, the majority within the first year (Supplemental Fig. 1). Of the remainder, 12% (N = 9) patients died, 1% (N = 1) received a heart transplant, and 9% (N = 7) were alive with first-stage physiology. Over half the deaths occurred during the index hospitalization for the PAB. Of the 77 patients, 90% (N = 69) of patients were discharged alive by 6 months, while 6% (N = 5) died and 2 (3%) were alive waiting for BCPS. Among the 10 patients who required unplanned intervention for pulmonary blood flow, 60% (N = 6) were ultimately deemed eligible for a BCPS, 20% (N = 2) died, and 20% (N = 2) were alive waiting for further surgery. A BCPS was completed in 25% (N = 2) of patients with genetic anomaly or syndrome.
At least one additional surgical procedure was performed on 93% (N = 56) of those undergoing BCPS, including a DKS in 53% (N = 32). Pulmonary artery plasty was completed in 52% (N = 31), atrial septostomy 48% (N = 29), and atrio-ventricular valve repair 18% (N = 11).
Presence of a syndrome or genetic abnormality (hazard ratio 0.07 [95% CI = 0.01– 0.56], p = 0.013) and cardiac diagnosis of unbalanced AVSD (hazard ratio 0.43 [95% CI = 0.21−0.87], p = 0.019) were associated on univariable regression analysis with a decreased odds of undergoing a BCPS. Only the presence of a syndrome (hazard ratio 0.08 [95% CI = 0.01−0.68], p = 0.021) remained significant in multivariable modeling accounting for prematurity, initial PAB gradient, and patient weight and oxygen saturation at PAB.
Discussion
This study demonstrated that while PAB was an effective first stage palliation that allowed 78% of SV patients with unrestrictive pulmonary blood flow to reach a BCPS, nearly 13% required reintervention to modify pulmonary blood flow, 12% died, and 1% was transplanted. Contrary to our initial hypothesis, the study failed to identify modifiable factors that would reduce the risk of unexpected interventions and optimize support down a single ventricle pathway as only the presence of a syndrome or genetic abnormality was identified risk factor for lower transplant-free survival to BCPS.
Timing of surgery has often been thought to influence outcomes following PAB. PAB was routinely scheduled at our institution after the onset of heart failure symptoms, indicating a drop in pulmonary vascular resistance, but prior to emergence of pulmonary vascular changes from sustained exposure to elevated pressures. The “sweet spot” often fell at approximately 4–6 weeks of age. Earlier PAB was only undertaken in the setting of concomitant aortic arch repair or if a rapid fall in pulmonary vascular resistance was observed. In line with this practice, most study patients were older than 3 weeks at PAB and had a median systemic oxygen saturation of 95%, reflecting a state of high pulmonary blood flow. The data to support this common practice of timing PAB within a narrow physiological window is extremely limited. Interestingly, neither younger age at PAB nor lower systemic oxygenation levels were associated with either primary outcome in this study. Similarly, recent studies demonstrate good outcomes with both early and late surgery [10, 11].
Low weight at SV palliation has been associated with increased mortality, longer intervals of hospital care, and lower likelihood to reach BCPS, particularly in patients undergoing PAB [12]. Smaller patient size introduces added surgical complexity and technical challenges, particularly fine-tuning PAB circumference to achieve a balanced parallel circulation. Miniscule adjustments may lead to a slightly looser band, ongoing excessive pulmonary blood flow and failure to thrive, and a perfectly balanced circulation at time of surgery may lead to early desaturation following somatic growth. Moreover, lower patient weight is often a surrogate to poor health status and a reflection of low physiologic reserve, both associated with a complicated postoperative recovery. Weight at time of PAB was not as a strong predictor of either primary outcome in this study; a weak association between operative weight and reintervention was suggested by the univariate analysis but was not supported by the multivariable models. No relationship was found with achieving candidacy for BCPS surgery in this study. It is noteworthy that the limited study sample size may have reduced the power to detect a small association. Even if a positive association was found, individual review of the smallest patients indicated that operative weight may not be modifiable as all patients underwent PAB following failure of medical management, the majority far earlier than our expected practice.
Surgical technique and optimal PAB circumference have varied widely across institutions [6,7,8, 13,14,15]. In Trusler’s original publication, the application of a PAB measuring 24 mm plus 1 mm per patient weight (kilograms) achieved the targeted physiologic goals for those with SV: 69% showed immediate improvement and 71% had acceptable pulmonary pressure on late catherization. A tighter PAB adjusted to physiologic parameters has since been advocated [6, 15]. In one small case series, the PAB was initially placed according to the Trusler equation for biventricular heart disease (20 mm + 1 mm/kg) and adjusted to achieve a Qp:Qs 0.3. The tighter Trusler equation still resulted in excessive pulmonary blood flow with a calculated Qp:Qs of 1.4–3.2; further tightening of the PAB to achieve the physiologic target resulted in a PAB gradient of 55-90 mmHg and a lower Qp:Qs of 0.21–0.32, while maintaining systemic oxygenation at 76–79%. All patients reached BCPS without need for reintervention [6]. In a larger case series, the initial PAB circumference of 18 mm + 1 mm/kg was reduced further to 16.1 ± 3.8 mm + body weight. Systemic oxygen saturation at hospital discharge was 80% and echocardiographic gradient measured 52 (range 31–85) mmHg. Approximately 95% (18/19) reached BCPS, one patient required conversion to a BTT shunt due to PAB migration, and one patient died [15]. Variability in initial PAB sizing existed within our surgical practice, with most surgeons starting with the Trusler SV equation and others starting with the tighter biventricular length, however modifying band tightness based on physiologic principles leads to similar final diameter. The postoperative systemic oxygen saturation of 88% appeared to strike the appropriate balance of controlling pulmonary blood flow and avoiding cyanosis with somatic growth. Placing tighter bands may have decreased the need for PAB tightening but increased rate of reintervention for pulmonary blood flow augmentation (1/77) and conversion to DKS and BTT shunt (4/77) and BT shunt (1/77) prior to BCPS. Comparable rates of reinterventions have been reported in other institutions [1].
Although recurrent systemic outflow tract obstruction is known to complicate PAB in setting of ventriculo-arterial discordance and aortic arch anomalies (29–72%) [16, 17], the impact on reintervention and survival is unclear. The frequency of concomitant aortic arch repair during PAB in patients with Double inlet left ventricle was previously reported as high as 14% [18] While the need for reintervention was not reported, all patients survived through BCPS. In our study, one patient developed isolated systemic ventricular outflow obstruction requiring previously unplanned DKS and BTT; another three patients underwent conversion DKS and BTT shunt for potential systemic ventricular outflow obstruction that was identified and addressed during a reintervention for pulmonary blood flow. BCPS was achieved in 75% (n = 3) of the study patients in whom an interstage DKS was completed. Patients identified as higher risk in the neonatal period may have undergone alternative surgical approach as the initial palliation. Furthermore, although not included as a reintervention, a DKS was also completed in half the patients undergoing BCPS. The value of deferring DKS surgery beyond the neonatal period and up to the second stage palliation is unclear. Avoiding cardiopulmonary bypass or circulatory arrest may avoid injury to a susceptible immature neonatal brain but may expose the heart to loading conditions that promote ventricular hypertrophy and atrio-ventricular valve regurgitation.
The presence of a genetic syndrome, mainly Trisomy 21 (T21), was the primary predictor of inability to proceed with BCPS. T21 has been previously associated with poor outcomes in SV patients, and although improved outcomes are seen if pulmonary vascular resistance remains low in the first year of life [19], pulmonary hypertension is common and often precludes advancement down SV palliation [20]. Obtaining an optimal band tightness is particularly difficult with coexisting pulmonary hypertension, tightening the PAB is limited by cyanosis and looser bands are associated with higher pulmonary pressure. Importantly, the presence of an unbalanced AVSD, a common finding in patients with T21, was not associated with poor outcomes. The result of this study raises the question whether this specific subgroup would benefit from an alternative treatment strategy, such as an early conversion to BTT shunt or heart transplantation [21].
The use of a BTT shunt with main pulmonary artery ligation has been considered by some as a preferable strategy over PAB to provide this SV population with reliable and controlled pulmonary blood flow [22]. Direct comparison of these divergent surgical approaches is limited to a single-institution study of PAB and Norwood repair across two surgical eras, which reported comparable rates of reintervention and reaching BCPS, although both occurring later in the PAB group [17]. Patients’ characteristics were similar aside from a higher frequency of arch obstruction in the Norwood subgroup. These finding were reinforced by retrospective reviews of SV patients with heterotaxy and atrioventricular septal defects which showed similar mortality rates between PAB and BTT shunts, but a trend for more frequent reinterventions in PAB group [23, 24].
Study Limitations
This study has several important limitations. Although no major changes to surgical practice were implemented during the study and no era effect was identified, minor, yet clinically important, adjustments may have been introduced over the 21-year span. Despite covering over two decades, the sample size was limited to under 80 patients, and fewer than 20 patients underwent reintervention or failed to reach BCPS. This reduced power associated with this sample size limits the statistical analyses and increases the risk of Type II errors. Lastly, patient data collection for this study terminated at the BCPS with the assumption that the clinical impact of the PAB did not translate past the second stage palliation. It is theoretically possible that risk of reinterventions may extend beyond BCPS and could be investigated further.
Conclusions
PAB is a reasonable first stage palliation for SV patients with unobstructed systemic blood flow, but multicentre collaborations are needed to identify risk factors for reinterventions, which remain common. Patients with genetic anomalies have poor prognosis and may benefit from future studies to identify an alternative treatment strategy.
Abbreviations
- PAB:
-
Pulmonary artery banding
- SV:
-
Single ventricular
- S2P:
-
Stage 2 palliation
- AVSD:
-
Atrio-ventricular septal defects
- BCPS:
-
Bidirectional cavopulmonary shunt
- BTT:
-
Blalock–Taussig–Thomas
- DKS:
-
Damus–Kaye–Stansel
- T21:
-
Trisomy 21
- ASD:
-
Atrial septal defect
- DILV:
-
Double inlet left ventricle
- VA:
-
Ventricular-arterial
- VSD:
-
Ventricular septal defect
- AVVR:
-
Atrioventricular valve regurgitation
- Echo:
-
Echocardiographic
- PDA:
-
Patent ductus arteriosus
- PBF:
-
Pulmonary blood flow
References
Alsoufi B, Manlhiot C, Ehrlich A, Oster M, Kogon B, Mahle WT et al (2015) Results of palliation with an initial pulmonary artery band in patients with single ventricle associated with unrestricted pulmonary blood flow. J Thorac Cardiovasc Surg 149:213–220
Rodefeld MD, Ruzmetov M, Schamberger MS, Girod DA, Turrentine MW, Brown JW (2005) Staged surgical repair of functional single ventricle in infants with unobstructed pulmonary blood flow. Eur J Cardiothorac Surg 27:949–955
Ergun S, Cilsal E, Genc SB, Yildiz O, Tanidir IC, Onan IS et al (2021) Univentricular pulmonary artery banding: how tight is tight enough for successful progress? Pediatr Cardiol 42:840–848
Alsoufi B, Gillespie S, Kogon B, Schlosser B, Sachdeva R, Kim D et al (2015) Results of palliation with an initial modified Blalock-Taussig shunt in neonates with single ventricle anomalies associated with restrictive pulmonary blood flow. Ann Thorac Surg 99:1639–1646 (discussion 46-7)
Dirks V, Pretre R, Knirsch W, Valsangiacomo Buechel ER, Seifert B, Schweiger M et al (2013) Modified Blalock Taussig shunt: a not-so-simple palliative procedure. Eur J Cardiothorac Surg 44:1096–1102
Baslaim G (2009) Modification of Trusler’s formula for the pulmonary artery banding. Heart Lung Circ 18:353–357
Trusler GA, Mustard WT (1972) A method of banding the pulmonary artery for large isolated ventricular septal defect with and without transposition of the great arteries. Ann Thorac Surg 13:351–355
Albus RA, Trusler GA, Izukawa T, Williams WG (1984) Pulmonary artery banding. J Thorac Cardiovasc Surg 88:645–653
Group WHOMGRS (2006) WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl 450:76–85
Ramakrishnan K, Alfares FA, Hammond-Jack K, Endicott K, Nettleton M, Zurakowski D et al (2016) Optimal timing of pulmonary banding for newborns with single ventricle physiology and unrestricted pulmonary blood flow. Pediatr Cardiol 37:606–609
Li G, Zhang H, Fan X, Su J (2021) Pulmonary artery banding in patients with functional single ventricle associated with pulmonary hypertension. Clin Exp Hypertens 43:328–333
Alsoufi B, McCracken C, Ehrlich A, Mahle WT, Kogon B, Border W et al (2015) Single ventricle palliation in low weight patients is associated with worse early and midterm outcomes. Ann Thorac Surg 99:668–676
Valente AS, Mesquita F, Mejia JA, Maia IC, Maior MS, Branco KC et al (2009) Pulmonary artery banding: a simple procedure? A critical analysis at a tertiary center. Rev Bras Cir Cardiovasc 24:327–333
Kajihara N, Asou T, Takeda Y, Kosaka Y, Onakatomi Y, Nagafuchi H et al (2010) Pulmonary artery banding for functionally single ventricles: impact of tighter banding in staged Fontan era. Ann Thorac Surg 89:174–179
Kajihara N, Asou T, Takeda Y, Kosaka Y, Nagafuchi H, Oyama R et al (2010) Staged surgical approach in neonates with a functionally single ventricle and arch obstruction: pulmonary artery banding and aortic arch reconstruction before placement of a bidirectional cavopulmonary shunt in infants. Pediatr Cardiol 31:33–39
Freedom RM, Benson LN, Smallhorn JF, Williams WG, Trusler GA, Rowe RD (1986) Subaortic stenosis, the univentricular heart, and banding of the pulmonary artery: an analysis of the courses of 43 patients with univentricular heart palliated by pulmonary artery banding. Circulation 73:758–764
Ruzmetov M, Geiss DM, Fortuna RS (2013) Outcomes of double inlet left ventricle and similar morphologies: a single center comparison of initial pulmonary artery banding versus a norwood-type reconstruction. J Card Surg 28:569–575
Alsoufi B, McCracken C, Kanter K, Shashidharan S, Kogon B (2017) Current results of single ventricle palliation of patients with double inlet left ventricle. Ann Thorac Surg 104:2064–2071
Colquitt JL, Morris SA, Denfield SW, Fraser CD, Wang Y, Kyle WB (2016) Survival in children with down syndrome undergoing single-ventricle palliation. Ann Thorac Surg 101:1834–1841
Peterson JK, Setty SP, Knight JH, Thomas AS, Moller JH, Kochilas LK (2019) Postoperative and long-term outcomes in children with Trisomy 21 and single ventricle palliation. Congenit Heart Dis 14:854–863
Godown J, Fountain D, Bansal N, Ameduri R, Anderson S, Beasley G et al (2022) Heart transplantation in children with down syndrome. J Am Heart Assoc 11:e024883
Bradley SM, Simsic JM, Atz AM, Dorman BH (2002) The infant with single ventricle and excessive pulmonary blood flow: results of a strategy of pulmonary artery division and shunt. Ann Thorac Surg 74:805–810
Alsoufi B, McCracken C, Schlosser B, Sachdeva R, Well A, Kogon B et al (2016) Outcomes of multistage palliation of infants with functional single ventricle and heterotaxy syndrome. J Thorac Cardiovasc Surg 151:1369–77 e2
Alsoufi B, McCracken C, Kanter K, Shashidharan S, Border W, Kogon B (2020) Outcomes of multistage palliation of infants with single ventricle and atrioventricular septal defect. World J Pediatr Congenit Heart Surg 11:39–48
Funding
None.
Author information
Authors and Affiliations
Contributions
SK and AAF: Conceptualize the study. SK and BDL : Data curation CSF: Data analysis and interpretation BL, SK, SMS, OH, KLT and AAF : Methodology and original draft SMS, OH, KT, MS, AD and AAF: Review and editing of the original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Langanecha, B.D., Kesavan, S., Schwartz, S.M. et al. Reintervention Before Bidirectional Cavopulmonary Shunt and Intermediate Outcomes in Children with Single Ventricle Who Underwent Main Pulmonary Artery Banding. Pediatr Cardiol 44, 1839–1846 (2023). https://doi.org/10.1007/s00246-023-03242-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03242-6