Abstract
Plant growth-promoting rhizobacteria (PGPR) have demonstrated its importance in agriculture globally including beneficial dynamics change in plant rhizosphere leading better tolerance towards abiotic stresses. Hundred and one bacterial cultures from sugarcane rhizosphere zone of > 50 years of sugarcane growing fields were isolated using standard protocols and were further subjected to in vitro screening to visualize their impact on plant growth. Of these, two cultures based on biochemical test and 16S rRNA gene sequences were classified as Bacillus subtilis (BSSC11) and Bacillus megaterium (BMSE7). Sugarcane settlings exposed to these strains exhibited more nutrient content, improved growth in terms of early sprouting, increased vigor (high shoot and root weight) and better antioxidant enzyme system ability including quantitative overexpression of superoxide dismutase (SOD) isoforms over controls. Treated cane seed (setts) with B. megaterium culture exhibited high expression of invertase genes which facilitated early and improved growth of settlings through increased inversion of sucrose to glucose and fructose. When these settlings were exposed to drought, a significant decrease in SOD enzyme activity and increase in proline content was observed especially in B. megaterium-exposed samples indicating less generation of free radicals in inoculated than those of non-inoculated samples where SOD activity increased significantly. This is apparently a first study of PGPRs isolated from continuous growing sugarcane fields on the growth and vigor of sugarcane settlings in vivo and further hypothesized that a multiple chain of events is involved in imparting better crop growth of PGPR-exposed settlings both under normal and stress conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For agricultural growth and productivity, microorganisms have been implicated in providing nutrients to plants. For sustainable agriculture growth, applications of plant growth-promoting rhizobacterias (PGPRs) have been advocated in many parts of the world. In general, these rhizobacteria promote plant growth as they colonize with root system being free-living saprophytes (Vessey 2003; Damam et al. 2014). Sharma and Archana (2016) narrated different sets of events including inhibition of soil-borne pathogens, improved nutrient solubilisation and production of phytohormones that occurred during different stages of plant growth presumably influences growth and tolerance to abiotic stresses.
The commercialized PGPRs have provided better and improved crop growth acting either as bio-protectants, biofertilizers or bio-stimulants. Bacteria in the genera Bacillus, Pseudomonas, Agrobacterium, Burkholderia and Streptomyces are predominantly studied either as bio-stimulants or biofertilizers and have been increasingly marketed (Chandra and Chandra 2016). Several authors reported PGPRs help in mitigating impact of abiotic stresses along with increased plant growth (Hamdia et al. 2004; Barnawal et al. 2012). Further, they have postulated that under salt stress selectivity of Na+, K+, and Ca++ is altered in plants and ultimately they sustain higher K+/Na+ ratio. At molecular level, a transcriptomic study has helped in identifying genes and regulatory sequences associated with promoting plant growth under the influence of plant growth-promoting rhizobacteria (Cartieaux et al. 2008; Vargas et al. 2014). However, concerted efforts are required to decipher the basic mechanism being underplayed by these rhizobacteria so as to use them in normal/routine agricultural practices to realize improved crop yield (Bharti et al. 2016). It is more important when these microorganisms impart drought tolerance in plants (Zahran 1999; Dimkpa et al. 2009; Singh et al. 2011).
Sugarcane is a C4 ratoon crop where the planted cane stays in soil for more than a year and produces profuse roots, hence the study of the rhizosphere of the sugarcane crop is challenging, as numerous microbes have found a good residing environment. In the sub-tropical part of India, sugarcane crop experienced extreme temperatures (min/max temp 1–4 °C/46–48 °C), light and water during four distinct crop cycles (germination, tillering, grand growth and maturity) (Shrivastava et al. 2015; Rai et al. 2017). In light of the long duration of the crop, isolation of PGPRs from the sugarcane rhizosphere is considered noteworthy. In sugarcane, Gluconacetobacter diazotrophicus which is a beneficial nitrogen-fixing bacterium has shown enhanced tolerance to abiotic stresses (Vargas et al. 2014). In many parts of the world, sugarcane productivity is diminishing in light of low water availability (Tammisola 2010), hence any information pertaining to the effective use of PGPRs in imparting drought tolerance shall be acceptable so that the area under sugarcane is not reduced.
In drought, several physio-biochemical and molecular events are regulated through complex signal transduction mechanisms leading to variable plant responses (Bhargava and Sawant 2013). Enzymes belonging to antioxidant enzyme system and the level of proline have been correlated with drought tolerance (Chandra and Dubey 2010; Muscolo et al. 2015). Proline is usually involved in maintaining the osmotic adjustment of the cell as well as reducing the ROS under drought conditions by an unknown mechanism (Wutipraditkul et al. 2015). Further, Sharma et al. (2011) postulated that increased catabolism of proline lowered ROS during drought stress. Therefore, the increased level of proline as well as catabolism can be easily explained especially where proline levels are not positively correlated with stress tolerance (Montesinos-Pereira et al. 2014). In the present investigation, sugarcane rhizosphere was used to isolate potential PGPR which has the ability to induce plant growth. Attempts were also made to molecularly characterize the isolated bacteria and examine the role of these bacteria on growth, expression of sucrose-hydrolyzing genes and other associated attributes that supports its importance in sugarcane physiology and agronomy including tolerance to abiotic stresses. Enzymes belonging to antioxidant system and the level of proline indicated isolated bacteria having a positive response towards drought tolerance at an early stage of sugarcane growth.
Materials and methods
Collection of soil sample and isolation of bacteria
The root-free rhizospheric soil was collected from different research operational fields of ICAR-Indian Institute of Sugarcane Research, Lucknow, India (26°56′N, 80°52′E and 111 m above sea level), where sugarcane has been growing > 50 years. Soils of these fields are largely sandy loam (13.3% clay, 24.5% silt and 62.2% sand) of Indo-Gangetic alluvial origin, classified as non-calcareous mixed with hyperthermic udic ustochrept. Bacteria were isolated by employing the serial dilution plate technique using nutrient agar at 37 °C for 24–48 h. Colonies exhibiting prolific growth were isolated by streak plate method. Isolates were kept in nutrient agar slants at 4 °C and used for screening their plant growth-promoting properties.
In vitro screening of isolates and their plant growth-promoting properties
Phosphate solubilizing property
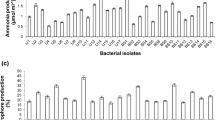
Bacterial strains were screened for phosphate solubilization on Pikovskaya’s agar medium (PAM). Bacterial culture was inoculated on PAM at the centre of plate under standard condition and kept at 30 ± 2 °C for 5 days. A clear zone as developed around the colonies showed phosphate solubilization property. Results were represented as diameter of the zone in millimeters (Tokala et al. 2002).
Siderophore production
Siderophore production was assayed following Tokala et al. (2002). Blue/green agar (1 l) was prepared by mixing 60.5 mg of Chrome Azural S (CAS) with 10 ml of iron (III) solution (1 mM FeCl3·6H2O in 10 mM HCl). This solution was mixed with another solution (72.9 mg of hexadecyltrimethyl ammonium bromide dissolved in 40 ml of distilled water) with constant stirring. Finally, this dark blue/green mixture was added in nutrient agar to get CAS mixture. Inoculation of bacterial isolates was done on CAS agar and kept at 30 °C for 2–3 days. Yellow–orange hallow developed around the growth which indicated production of siderophore. Diameter of the resultant zone was measured.
Production of IAA
Indole acetic acid (IAA) was estimated following the protocol of Yadav et al. (2010) with minor modifications. 50 ml of nutrient broth having 0.1% dl-tryptophan was kept with 500 µl of 1-day-old bacterial cultures and kept at 28 ± 2 °C for 72 h. Culture was centrifuged (10,000 rpm) at 4 °C for 10 min. In the supernatant (2 ml), two drops of ortho-phosphoric acid and 4 ml of Salkowski reagent (50 ml of 35% perchloric acid, 1 ml 0.5 M FeCl3 solution) was added. Pink color indicated IAA production. The Spectrophotometer was used to record the optical density at 530 nm. Concentration of IAA was expressed in µg/ml.
Production of NH3 and HCN
Peptone water was used to visualize the production of ammonia by bacterial isolates. In 10 ml peptone water, freshly grown culture was added and then kept at 30 °C for 48–72 h. In the same mixture, 0.5 ml Nessler’s reagent was added. Faint yellow to dark brown color indicated the production of ammonia (Yadav et al. 2010). Production of HCN was checked following the method of Bakker and Schippers (1987). Bacterial culture was mixed with nutrient agar having glycine as supplement. Soaked Whatman filter paper [solution of 2% (w/v) sodium carbonate in 0.5% (w/v) picric acid] was placed inside the upper part of petri plates. After sealing, plates were incubated at 30 °C for 48 h. HCN production was confirmed with color change from yellow to reddish brown.
Zinc solubilisation
Isolated bacteria were checked for their zinc-solubilising ability based on hallow formation on solid basal medium containing 0.1% ZnO (Saravanan et al. (2003). Diameter of the resultant zone was measured and recorded.
Identification and molecular characterization of selected PGPRs
Phenol chloroform method was used to isolate the genomic DNA using 2 ml of overnight grown pure culture (Mauti et al. 2013). 0.8% agarose gel was used to visualize the quality and quantity of isolated DNA. To molecularly characterize the bacteria, 16S rRNA gene-based primers (F5′CMGSCVTDACACAWGCHAGYC3′/R 5′GCGSMTGWGTNCAAGSV3′) were used to amplify the genomic DNA of isolated bacteria. The resultant PCR product (~ 1.3 kb) was sequenced bi-directionally. Based on sequence alignment, the isolated bacteria were deciphered using NCBI database. With the help of Weighbor with alphabet size of 4 and length of 1000, the phylogenetic tree was created.
Efficiency of selected PGPR on growth of sugarcane
Preparation of bud chips and exposure of settlings to bacterial culture
For the present study, sugarcane variety CoLk94184 (high sugar and early maturing) was used. Nursery was raised in plastic trays nearly 15 days before actual transplanting. Bud chips were scooped out using hand bud chippers. These were soaked in 100 ppm ethrel for 2 h. These pre-treated bud chips were planted in an upright position in trays filled with a soil mixture having sand, organic matter and soil (1:1:1). These nursery beds were irrigated adequately. Bud chips were sprouted into settlings with 3–4 leaves in 15–20 days. The selected bacterial cultures were grown for 24 h at 37 °C in nutrient broth. The broth obtained was used to expose the 15-day-old settlings of sugarcane. Settlings of almost same length were kept in a flask containing bacterial culture so that complete root part was exposed to bacterial culture and were left for 2 h. Then, they were transplanted in 2 × 5 m2 plots covered with soil leaving about 5 cm of the shoot above the ground level. A distance of 10 cm was kept for plant to plant and 90 cm for row to row. After 2 months, three plants were randomly selected to record the length of root and the shoot as well as the leaves were used for all biochemical and molecular studies. Hot air oven was used to dry the fresh plant materials (80 °C for 24 h).
Chlorophyll content
Hiscox and Israelstam (1978) procedure was used to measure the Chl content in leaf. 50 mg of leaf sample, cut into small pieces, was dipped in 5 ml dimethyl sulphoxide (DMSO) and kept overnight in dark. The optical density of the solution was recorded at 645 nm and 663 nm. Using Arnon’s formula (Arnon 1949), the amount of chlorophyll was calculated.
Nitrate reductase activity
Using Srivastava (1975) procedure, in vivo nitrate reductase (NR) activity in leaves was measured. 200 mg of leaves were cut into small pieces and incubated with 5 ml of phosphate buffer (0.1 M, pH 7.2) solution containing KNO3 (0.1 M) and propanol (0.1%), under dark and anaerobic conditions. To estimate nitrite content in the assay solution, 2 ml of color development reagent containing 1 ml each of 1% sulfanilamide in HCl and 0.02% N-(1-naphthyl)-ethylene diamine hydrochloride (NDD) was added to 100 µl of the assay solution. The optical density of each sample was measured at 540 nm and enzyme activity was expressed as µmole min−1 g fresh wt−1. Using the standard curve developed with KNO2, the activity of NR was calculated.
Estimation of total soluble proteins and soluble acid invertase (SAI) activity
Leaf samples (200 mg) were homogenized in 2 ml 0.1 M citrate buffer (pH 5.2). After centrifugation at 4 °C (10,000 rpm for 10 min), the resultant supernatant (enzyme extract) was used to estimate the protein content and the activity of soluble acid invertase enzyme. For protein estimation, 1 ml of distilled water and 20 µl of extract of each plant were added to respective test tubes. 5 ml of reagent C was added and an incubation time of 10 min was provided. 0.5 ml of FC reagent was added to each tube. After 30 min of incubation at room temperature in dark, the optical density was recorded at 660 nm. Lowry et al’s. (1951) procedure was used to determine the concentration of protein. BSA was used as standard protein. For soluble acid invertase enzyme activity, two sets of reactions as control and experiment were made. In both sets, 3 ml citrate buffer and 250 µl enzyme extract were taken. The control set was kept for boiling for 10 min, while 1 ml 2% sucrose was added in the experimental set. Both the sets were placed in a water bath (37 °C) for 2 h, then the reaction of experimental tubes was stopped by boiling for 2 min. Nelson’s method was used to measure the hexoses in both control and experimental samples. Optical density was recorded at 540 nm and the differences of control and experiment samples depicted invertase activity.
Estimation of total proline
Total proline was measured following the procedure of Bates et al. (1973) with minor modifications. Fresh leaf tissues (500 mg) were homogenized in 10 ml of 3% sulphosalicylic acid and the extract was filtered with the help of Whatman filter paper. To the 2 ml of filtrate, 2 ml of glacial acetic acid and 2 ml of freshly made acid ninhydrin solution were added and shaked vigorously. The test tubes were kept in a water bath at 100 °C for 1 h. Reaction was stopped keeping the tubes on ice for 10 min. 4 ml of toluene was added in the tubes and stirred for 20–30 s. With the help of separating funnel, the toluene layer was separated. Red color intensity was measured at 520 nm with the help of UV–visible spectrophotometer. Toluene was taken as blank. A similar procedure was followed to prepare a standard curve using pure proline and this was further used to determine the proline content.
Level of lipid peroxidation
Heath and Packer (1968) procedure was used to estimate the level of lipid peroxidation in terms of malondialdehyde (MDA). 250 mg fresh sugarcane leaves both of control and treated plants (exposed to microbes) were homogenized in 2 ml of 5% TCA. After centrifugation of homogenate at 9000 rpm for 15 min, the supernatant was used to measure the MDA content. In 1 ml supernatant, 2 ml of TBA reagent was added. The test tubes were boiled in a water bath for 15 min. Reaction was stopped by keeping the tubes in ice for 10 min. At 532 nm, absorbance of the mixture was recorded. Absorbance was also recorded at 600 nm to correct the non-specific absorbance by subtracting these two readings. The level of MDA was calculated using its molar extinction coefficient (155 mM−1 cm−1).
Leaf nutrient content
Analysis of leaf nutrient content namely nitrogen (N), phosphorus (P) and potassium (K) was made from an acid digestion of dried leaf tissues. Standard colorimetric and flame photometric techniques for plant tissues were used.
Total RNA isolation, cDNA synthesis, semi-quantitative RT-PCR (qRT-PCR) and real-time PCR (RT-PCR) analyses
Trizol was used to isolate the total RNA from untreated and treated LTM/3rd fully expanded leaf samples, ground in liquid nitrogen. Quality and quantity of isolated RNA was visualized using Quawell UV–visible spectrophotometer. The concentration of the RNA samples was adjusted based on nanodrop readings. DNase I treatment was performed to remove any DNA contamination. The RNA normalization was re-checked using 25S rRNA as internal control (Iskandar et al. 2004). cDNA synthesis and qRT-PCR were performed on the samples using one-step RT-PCR kit (Qiagen, India) using soluble acid invertase (SAI) gene-based primers. The reaction conditions for qRT-PCR were as follows: 50 °C for 30 min (cDNA synthesis), 95 °C for 15 min (initial denaturation), 40 cycles consisting of 94 °C for 1 min, annealing temperature of 60° (25S rRNA)/58 °C (gene specific primer) for 1 min and 72 °C for 1 min. In the last, a reaction of 10 min at 72 °C was carried out as extension step. 1.6% agarose gel was used to separate the amplified products and was seen using the gel documentation system (Alpha Innotech, USA). For real-time PCR, cDNA was synthesized using 2 µg of total RNA in the presence of oligo-dT and RevertAid H minus Reverse Transcriptase enzyme (Thermo Scientific). Primers for SAI (F5′-CAGAGGAACTGGATGAACGA-3′/R5′-CCGCTTGAAATGTCAATGTC-3′) and CWI (F5′-TCTGTACAAGCCAACCTTCG-3′/R5′-CCGCTTGAAATGTCAATGTC-3′) were designed as described earlier (Chandra et al. 2015) and utilized to determine the expression of genes through real-time PCR. This was performed in 48-well plates Step One Real-Time PCR system (Applied Biosystems, USA) using SYBR Green. The reaction was performed at 50 °C for 2 min and 95 °C for 10 min as holding stage, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The 25S rRNA gene-encoding primers (F5′-GCAGCCAAGCGTTCATAGC-3′/R5′-CCTATTGGTGGGTGAACAATCC-3′) were used as reference (i.e., for calibration) in all reactions. RT-PCR was performed through relative quantification method and expression ratios were calculated from cycle threshold values to get \({2^{ - \Delta \Delta {C_{\text{T}}}}}\) or fold change (RQ values).
Isozyme analysis of super oxide dismutase (SOD)
Five hundred milligrams of leaf samples (LTM/3rd fully expanded) were extracted in 1 ml phosphate buffer (0.1 M, pH 7.5) having 0.5 mM EDTA. After centrifugation (10,000 rpm for 10 min at 4 °C), the supernatant was stored at 4 °C till further use. Isozyme analysis of super oxide dismutase enzyme was carried out using polyacrylamide native gel (10%). The staining of gel for SOD isozyme was carried out keeping the gel initially in dark in 100 ml 0.05 M Tris buffer (pH 8.0) containing 2 mg riboflavin, 1 mg EDTA and 10 mg nitro blue tetrazolium salt for 30 min. After this, the gel was placed in bright intense light for 30 min and finally rinsed with distilled water. The SOD isoforms were observed in the form of negative bands with the background of dark blue color.
Imposition of drought stress and estimation of RWC and SOD enzyme assay
Two-month-old settlings were used for imposition of drought stress. Irrigation was withheld for 2 weeks in both inoculated (with Bacillus megaterium, BMSE7 strain) as well as non-inoculated settlings. Control settlings were irrigated regularly (twice a week). Relative water content (RWC) of LTM leaf was determined using Barrs and Weatherley (1962) formula: RWC = (FW − DW)/(TW − DW) × 100, where FW = leaf fresh weight, DW = leaf dry weight and TW = turgid weight of leaf (soaked in water for 6 h). The activity of superoxide dismutase (SOD; EC 1.15.1.1) was assayed following Giannopolitis and Ries (1977). The assay mixture contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 75 mM NBT, 13 mM methionine, 2 mM riboflavin and 50 µl enzyme extract (as mentioned in SOD isozyme head). SOD activity was presented as the enzyme unit which was defined as the amount of enzyme needed to inhibit 50% reduction of NBT. This was measured at 560 nm and presented in units min−1 mg−1.
Statistical analysis
Statistical analyses were performed using SPSS software ver 16.0 (SPSS Inc, Chicago). Duncan’s Multiple Range Test (DMRT) was used to compare the means (Snedecor and Cochran 1967). Three replications encompassing three independent plants were used to generate all experimental data as means.
Results
Screening of isolates and plant growth-promoting attributes
Following the procedures as described above, a total of 101 bacterial cultures were isolated from soils samples collected from various research fields of ICAR-Indian Institute of Sugarcane Research, Lucknow, India, where primarily sugarcane is regularly cultivated since > 50 years. These 101 bacterial cultures were subjected to six plant growth-promoting attributes namely phosphate and zinc solubilisation, production of ammonia, IAA, siderophore and HCN. As depicted in Table 1, 34 cultures showed positive response towards at least 4 attributes and of these 26 cultures towards 5 attributes indicating their usefulness towards induced plant growth. Rest of the cultures have not shown measurable responses towards these attributes, hence they were not taken into study. When these 26 cultures were subjected to zinc solubilisation, only 10 cultures (A6, B6, B10, B19, B20, C6, C11, D7, E7 and F8) showed positive response towards it (Table 1). Based on the intensities towards these attributes and overall performances at in vitro level, seven cultures namely B6, B20, C6, C11, D7, E7 and F8 were selected for the qualitative study (Table 2). Among these, E7 and C11 have shown better phosphate solubilisation (Table 2) over other strains. These two strains were also found to be good in production of siderophore, IAA, ammonia, HCN and in solubilisation of zinc (Table 2). On the basis of these primary qualitative indicators, two strains namely C11 and E7 were found to be superior over others, hence they were selected to identify molecular traits and were further used for the plant growth-promoting activities.
Identification and molecular characterization of selected plant growth-promoting rhizobacteria
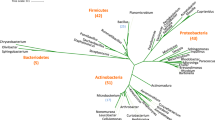
Genomic DNA of C11 and E7 cultures was amplified using 16S rRNA gene specific primers. The amplified products were sequenced. The aligned data of E7 was > 1255 bp, whereas C11 was 1256 bp. Sequencing and homology data showed that isolates of these two cultures (C11 and E7) belong to genus Bacillus. Sequence alignment of 16S rDNA (homology tree) of bacterial isolates provided input for rapid phylogenetic analysis which indicated C11 and E7 strains as Bacillus subtilis and Bacillus megaterium, respectively (BSSC11 and BMSE7) (Fig. 1a, b). Sequences were submitted to NCBI Gene Bank (KY604948 and KY604952). The E7culture was most similar to Bacillus megaterium strain GP2, 16S rRNA gene partial sequence (Acc. no. KJ_854404.1), whereas C11 was found to be most similar to Bacillus subtilis strain APFSG3isox, 16S rRNA gene partial sequence (Acc. no. KX_129778.1). To visualize the stability of Bacillus spp. entities, RNA secondary structure prediction was performed using Vienna RNA Web Services. The free energy of secondary structure of Bacillus subtilis rRNA and Bacillus megaterium was (−) 475.40 and of (−) 455.90 kcal/mol, respectively. Mountain plot was also created by the RNAfold web server which helps in predicting the hierarchical organization of RNA secondary structure.
Impact of selected PGPRs on growth and physio-biochemical attributes of sugarcane
As indicated in Table 3, the enhancement in root length, shoot length, and total weight was found in treated plants in comparison to the control. Values of these were highest in 2-month-old plants raised from settlings treated with BMSE7, followed by BSSC11 and control (Table 3; Fig. 2a–c). Both shoot and root length of the plants that emerged from the BMSE7 treated settlings was almost double to that of control plants. Activity of nitrate reductase enzyme in plants provides nitrogen status of the plant and is usually correlated with growth and yield. The present results indicated higher NR activity in treated plants (54.3 and 68.9 µg g−1 h−1) as compared to untreated plants (40.4 µg g−1 h−1). The chlorophyll, protein and activity of soluble acid invertase (SAI) enzyme were also found higher in treated plants (Table 3). These changes were significant to highly significant. Concentration of proline and level of lipid peroxidation (represented as the level of malondialdehyde) was less in treated plants over untreated plants (Table 3). In comparison to control untreated samples, values of proline and malondialdehyde were lower in E7 than those of C11 treated plants. As NR activity, nitrogen content (N) was observed significantly higher in plants raised from settlings treated with BMSE7 and BSSC11 than those of non-treated plants. However, BMSE7-treated plants only showed a significant increase in content of both phosphorus and potassium over control (Table 3).
Effect of microbial isolates on growth of root and shoot as visualized in 2-month-old plants raised using treated settlings (15 days old) with E7 and C11 bacterial stains. a Two-month-old settlings, b root growth of 2-month-old settlings and c individual settling obtained from treated and untreated control (C) sugarcane settlings raised from bud chips
Quantitative reverse transcriptase and real-time PCR analysis
Soluble acid invertase (SAI) and cell wall invertase (CWI), catalyzing the hydrolysis of sucrose into hexose sugars is considered a key sucrose-hydrolysing enzyme needed for early sprouting and crop growth. Using total RNA from control and treated samples, quantitative reverse transcriptase data indicated higher expression of SAI gene in samples treated with bacterial strains, whereas expression of CWI was not significantly different among these samples (Fig. 3a). With respect to SAI gene, the level of expression was significantly higher in BMSE7-treated samples than those of BSSC11 strain. Expression of 25S rRNA gene was used as internal reference to calibrate the level of expression. To further decipher and validate the qRT-PCR results, gene expression of SAI and CWI was exactly quantitated at a given time by employing real-time PCR. The expression of genes among control and treated samples was interpreted in terms of log2 RQ, where \({\text{RQ}}\,=\,{2^{ - \Delta \Delta {C_{\text{T}}}}}\) value, depicting fold change. As depicted in Fig. 3b, RQ values (fold change over control) for SAI gene was higher in BMSE7 (1.7)-treated samples than those of BSSC11 (1.5)-treated samples indicating higher hydrolysis of sucrose to hexoses leading to better crop growth. The RQ value for CWI was not significantly different between control and E7-treated samples. The expression of CWI and fold change was even lower than those of control in C11-treated sample (Fig. 3b).
Gene expression analysis. a Quantitative reverse transcriptase PCR using soluble acid invertase (SAI) gene using RNA isolated from leaf of 2-month-old plants raised from BMSE7 and BSSC11 bacterial strain-treated settlings. b Real-time PCR using SAI and cell wall invertase (CWI) gene using same set of samples. Vertical bars represent ± SE of mean values of three replicates. Means followed by same superscript in each parameter do not differ significantly at p = 0.05 by Duncan’s Multiple Range Test (DMRT). RQ = relative quantification over untreated control, M = 100 bp DNA ladder as a molecular weight marker
Drought stress and antioxidant enzyme and proline content
Under normal irrigated conditions, the superoxide dismutase enzyme isoform showed no qualitative change; however, quantitative expression of each isoform was observed more in inoculated settlings than those of non-inoculated. No distinct differences in expression of isoforms were noticed among the settlings inoculated with two strains namely Bacillus subtilis (BSSC11) and Bacillus megaterium (BMSE7) strains (Fig. 4a). When settlings were subjected to drought, the relative water content of non-inoculated samples decreased from 84.29% (control) to 69.8%, whereas this decrease was only from 86.8 to 82.1% in Bacillus megaterium-inoculated settlings. A significant decrease in superoxide dismutase (SOD) activity was observed when inoculated settlings were exposed to water stress; however, non-inoculated samples showed a significant increase in SOD activity. Contrary to this, both inoculated and non-inoculated samples showed a very high increase in proline content under stress condition (Fig. 4b, c).
a Superoxide dismutase (SOD) isozyme pattern (Replication 1 and Replication 2) of 2-month-old settlings grown under normal irrigated condition. Lanes 1, 2: non-inoculated settlings; lanes 3, 4: inoculated with BSSC11; lanes 5, 6: inoculated with BMSE7 bacterial strain. b SOD enzyme activity under control and drought conditions with and without inoculation with BMSE7 strain. c Proline content measured in control and drought settlings having inoculation and no inoculation with BMSE7 strain. Vertical bars represent ± SE of mean values of three replicates. Means followed by same superscript in each parameter do not differ significantly at p = 0.05 by Duncan’s Multiple Range Test (DMRT)
Discussion
Keeping in view that sugarcane is a ratoon perennial crop that harbors numerous beneficial bacteria in its rhizosphere, any effective growth-promoting microbes isolated from such rhizosphere will presumably enhance early sprouting of cane seed with high vigor when utilized optimally. In sub-tropical India, late germination along with initial slow growth of settlings hinders total productivity of the sugarcane, as the total growth period (grand growth phase) is shortened by extreme high temperature with dry climate (Rai et al. 2017). Initial emergence of sett roots from root primordia above the leaf scar on the nodes of the setts and later on the shoot roots from the base of the shoots in sugarcane covers a significant soil area which exudes numerous bio-chemicals that supports growth of bacteria. In the present study, a total of 101 bacterial cultures were isolated where sugarcane is regularly grown since > 50 years. When these bacterial cultures were further analyzed for growth promoting as well as zinc and phosphate-solubilising activities, two cultures namely C11 and E7 were found to be promising PGPRs. Generally, plant growth-promoting rhizobacteria enhances growth of the plant directly/indirectly. Direct impact which is well-reported is usually influenced through nitrogen fixation, phosphorus solubilisation, production of HCN and phytohormones (Lugtenberg and Kamilova 2009). Indirect modes of actions mostly included biological controls (Vessey 2003).
The phosphate solubilizing activity is the in-built potential of many microbes to secrete organic acids. Normally, the hydroxyl and carboxyl groups of these acids quench cations and convert them to soluble forms. Therefore, the use of these microorganisms is a potential approach for phosphate solubilization. These two strains namely E7 and C11 have shown better phosphate solubilisation ability (Table 2) over other strains. Being that these two strains were found to be good in production of siderophore which is generally of low molecular weight, iron-binding ligands bind to ferric ion and make it available to the producer microorganism, indicating their function as a good bio-control agent by depriving the pathogens from iron nutrition, resulting in increased crop yield (Srivastava et al. 2013). Since E7 and C11 strains were better IAA-producing strains, they were considered to have better growth-promoting capability. This will have better root growth which not only provides increased nutrients but also helps in anchoring of a tall crop like sugarcane with the soil. Vacheron et al. (2013) have reported better and early root growth causes better anchoring of these with soil, enhances more and easy uptake of water and nutrients form the rhizosphere which in turn increases the chance of their survival. Bacteria found in rhizosphere of various plants synthesize and release auxin (secondary metabolites) due to rich supplies of substrate released from the roots compared to non-rhizospheric soils. PGPR promote crop growth indirectly as they produce ammonia causing alkaline condition of soil and suppresses the growth of certain pathogenic fungi, nitrobacteria and inhibits germination of spores of some pathogenic fungi (Chandra and Chandra 2016). Both E7 and C11 were observed to be the highest ammonia-producing strains justifying their suitability as PGPR. Normally, HCN inhibits the electron transport chain and therefore the energy supply to the cell is discontinued causing death of the organism. It also hinders normal working of enzymes and natural receptors including action of cytochrome oxidase. HCN is released by many rhizobacteria and thus controls pathogens biologically (Santiago et al. 2015). Isolated bacteria in this study also showed HCN production which indicated that these strains are potent PGPRs. Zinc-solubilising microorganisms can solubilise zinc from both organic and inorganic pools so as to make the zinc available to plants to enhance their growth (Saravanan et al. (2003). Strains isolated from sugarcane rhizosphere were observed to be capable of solubilising zinc. On the basis of these primary qualitative indicators, C11 and E7 strains were found to be superior over others.
The systematic bacteriology has revolutionized proteobacterial classification in the recent past and the resultant phylogenetic relationships developed based on 16S rRNA gene sequences have defined bacterial isolates into distinct species. This is being regularly used to identify bacteria being novel or non-culturable (Matsumoto and Sugano 2013). Based on the molecular data, C11 and E7 strains were identified as Bacillus subtilis and Bacillus megaterium, respectively (BSSC11 and BMSE7). The free energy of secondary structure of Bacillus subtilis rRNA and Bacillus megaterium was found well within the range. Mountain plot was also drawn by the RNA fold web server which has also helped in predicting the secondary structure of RNA (data not shown).
As indicated in Table 3, the enhancement in the length of root, shoot, and total weight was found in treated plants in comparison to the control. Both shoot and root length of the plants emerged from the BMSE7-treated settlings as almost double to that of control plants. As reported earlier (Vacheron et al. 2013), the present study results also revealed that the shoot roots of 2-month-old plants exhibited increased root surface area and root weight from the BSSE7 and BMSC11-treated settlings. The positive effect of microbes may be due to an increase in the supply of available P and Zn, directly/indirectly causing changes in the growth and metabolic activities of the settling. These have led to more root exudates making a favorable habitat for the microorganisms to grow. It is seen possibly that the inoculated PGPR strains have affected the root hormone levels by producing enhanced IAA and/or other plant hormones in the rhizosphere, which were then absorbed by the root. Positive relationships between growth and the level of bacterial hormones reported earlier are also observed in the present study. Vacheron et al. (2013) and Shakeel et al. (2015) have reported an yield increase in many major crops like rice, wheat, barley, tomato and maize when these crops rhizosphere were associated with plant growth-promoting Bacillus species.
Normally, plant growth-promoting rhizobacteria influences physiological properties like photosynthesis of the host plants. Vacheron et al. (2013) reported increased growth of maize when inoculated with Azospirillum and Pseudomonas. This was observed due to better N2 fixation and enhanced uptake of mineral nutrients namely N, P, K, Ca and Mg. Leaf tissues of plants raised with settlings treated with BSSE7 strain exhibited a significant higher N, P and K which supported increased uptake causing better plant growth. Activity of nitrate reductase (NR) enzyme has been correlated with nitrogen status of the plant and thus the crop growth and yield. Higher NR activity and content of chlorophyll, protein and soluble acid invertase (SAI) enzyme activity in treated plants as compared to control plants supported that the isolated strains were good in promoting the growth of the crop. Mia et al. (2010) reported that high nitrogen content enhances the formation of chlorophyll and increased photosynthetic activity.
Apart from breeding for stress tolerance or genetic engineering, application of plant growth-promoting microbes have been advocated to improve stress tolerance in crops (Nautiyal et al. 2013). Apart from environmental and ethical issues associated with genetically modified crops, both breeding and genetic engineering approaches are time consuming and labor intensive (Tiwari et al. 2016). Apart from improving plant growth, these rhizobacteria are known to trigger physio-biochemical changes including enhancement of the level of osmoprotectants, heat shock proteins and antioxidant enzymes making the crop tolerant to abiotic stresses (Yang et al. 2009; Ahemad and Khan 2012; Tiwari et al. 2016). The present study’s results also showed decreased concentrations of proline and malondialdehyde (lipid peroxidation) and high quantitative expression of some SOD isoforms in treated plants over untreated indicating overexpression of antioxidant enzymes in treated plants so as to maneuver any impact of abiotic stresses. Interestingly, inoculated settlings showed decreased SOD activity when exposed to drought. At the same time, proline content increased 17-fold over control indicating less generation of free radical with increased osmoprotectant ability due to increased proline content, Pseudomonas putida, a PGPR has been reported to be promoting growth and better drought tolerance (Tiwari et al. 2016).
Generally, relative water content (RWC) reports the water balance of plants (Lata et al. 2011; Tiwari et al. 2016). As observed in the present study, plant water status was maintained when droughted plants were exposed to Bacillus megaterium (BMSE7), which was supported by earlier studies (Figueiredo et al. 2008; Kang et al. 2014; Tiwari et al. 2016). Increased proline helped plants to overcome drought stress (Grover et al. 2014; Lata et al. 2011). Increased level of proline is also observed in the present study under drought stress. Oxidative damage via production of toxic reactive oxygen species is reported under drought stress in plants that needs to be minimized (Lata et al. 2011; Grover et al. 2014). SOD is a defense enzyme that dismutases superoxide (O2−) radical into harmless O2 or H2O2. After this, H2O2 is taken care by an catalase enzyme (Lata et al. 2011). Comparatively low SOD activity as observed in Bacillus megaterium-inoculated samples over control indicated less oxidative stress which is perceived by Bacillus megaterium-inoculated sugarcane settlings. Kang et al. (2014) reported that PGPR reduces negative effect of osmotic stress by controlling plant growth hormones and antioxidants in cucumber.
Soluble acid invertase (SAI), that catalyzes the hydrolysis of sucrose into hexose sugars, is considered a key sucrose -hydrolysing enzyme needed for early sprouting and crop growth. In sugarcane, expression of SAI has been largely associated with the accumulation of sucrose in maturing internodes and their expression have been reported to be variable in early and late maturing sugarcane varieties (Jain et al. 2013; Chandra et al. 2015). Shrivastava et al. (2015) reported an increased level of SAI activity at early stage of the crop facilitates better crop growth. Expression of both SAI and CWI genes was higher in BMSE7-treated samples than those of BSSC11-treated samples indicating higher hydrolysis of sucrose to hexoses leading to better crop growth.
Conclusions
Two promising bacterial strains (BSSC11 and BMSE7) having better plant growth-promoting ability was isolated and characterized from sugarcane rhizosphere. 16S rRNA gene-based sequence homology confirmed that these are B. subtilis and B. megaterium, respectively. Both growth parameters and biochemical estimates including higher leaf nutrient content (N, P and K) which indicated that isolated strains especially B. megaterium possessed better abilities to induce early growth and improve the vigor of the sugarcane settlings. This was further substantiated as expression of both soluble acid invertase and cell wall invertase genes were higher in treated samples over control helping higher availability of hexoses for growth. Various facets of drought-induced changes such as lowering of RWC, accumulation of osmoprotectants and antioxidative enzyme activities have directed that in the presence of B. megaterium, the impact of drought reduced as proposed in model wherein the overall impact of drought stress was minimized apart from the improved plant growth under normal irrigated condition observed with PGPR inoculation (Fig. 5). Currently, we are in process of developing the consortium of beneficial bacterial strains with press mud, a sugarcane byproduct, that interact synergistically to visualize sustainable and improved crop production through such interventions per se.
Author contribution statement
The manuscript was written through contributions of all authors. PC, AC and PT: conceived and designed the experiments. PC and PT: performed the experiments. AC and PC: analyzed the data. All authors have given approval to the final version of the manuscript.
References
Ahemad M, Khan MS (2012) Productivity of greengram in tebuconazole-stressed soil, by using a tolerant and plant growth-promoting Bradyrhizobium sp. MRM6 strain. Acta Physiol Plant 34:245–255
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere and relation to potato yield reduction and Pseudomonas spp.-mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2012) 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol Biochem 58:227–235
Barrs HD, Weatherley PF (1962) A re-examination of relative turgidity technique for estimating water deficit in leaves. Aust J Biol Sci 15:413–428
Bates LS, Waldren RD, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bhargava S, Sawant K (2013) Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breed 132:21–32
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6:34768
Cartieaux F, Contesto C, Gallou A, Desbrosses G, Kopka J, Taconnat L, Jean-Pierre R, Touraine B (2008) Simultaneous interaction of Arabidopsis thaliana with Bradyrhizobium sp. strain ORS278 and Pseudomonas syringae pv. tomato DC3000 leads to complex transcriptome changes. Mol Plant Microbe Int 21:244–259
Chandra P, Chandra A (2016) Elucidation of rRNA secondary structure and phylogenetic analysis of plant growth promoting Streptomyces sp. based on 16S rRNA. J Wheat Res 8:61–65
Chandra A, Dubey A (2010) Effect of ploidy levels on the activities of ▲1-pyrroline-5-carboxylate synthetase, superoxide dismutase and peroxidase in Cenchrus species grown under water stress. Plant Physiol Biochem 48:27–34
Chandra A, Verma PK, Islam MN, Grisham MP, Jain R, Sharma A, Roopendra K, Singh K, Singh P, Verma I, Solomon S (2015) Expression analysis of genes associated with sucrose accumulation in sugarcane (Saccharum spp. hybrids) varieties differing in content and time of peak sucrose storage. Plant Biol 17:608–617
Damam M, Gaddam B, Kausar R (2014) Effect of plant growth promoting rhizobacteria (PGPR) on Coleus forskohlii. Int J Curr Microbiol Appl Sci 3:266–274
Dimkpa C, Weinand T, Asch F (2009) Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32:1682–1694
Figueiredo MVB, Burity HA, Martínez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40:182–188
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 59:309–314
Grover M, Madhubala R, Ali SZ, Yadav SK, Venkatsewarlu B (2014) Influence of Bacillus spp. strains on seedling growth and physiological parameters of sorghum under moisture stress conditions. J Basic Microbiol 54:951–961
Hamdia ABE, Shaddad MAK, Doaa MM (2004) Mechanisms of salt tolerance and interactive effects of Azospirillum brasiliense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul 44:165–174
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hiscox JD, Israelstam GM (1978) Method for extraction of chlorophyll from leaf tissues without maceration. Can J Bot 57:1332–1334
Iskandar HM, Simpson RS, Casu RE, Bonnett GD, Maclean DJ, Manners JM (2004) Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression in sugarcane. Plant Mol Biol Rep 22:325–337
Jain R, Chandra A, Solomon S (2013) Impact of exogenously applied enzymes effectors on sucrose metabolizing enzymes (SPS, SS and SAI) and sucrose content in sugarcane. SugarTech 15:370–378
Kang SM, Khan AL, Waqas M, You YH, Kim JH, Kim JG, Hamayun M, Lee IJ (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Int 9:673–682
Lata C, Jha S, Dixit V, Sreenivasulu N, Prasad M (2011) Differential antioxidative responses to dehydration-induced oxidative stress in core set of foxtail millet cultivars (Setaria italica L.). Protoplasma 248:817–828
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann Rev Microbiol 63:541–556
Matsumoto T, Sugano M (2013) 16S rRNA gene sequence analysis for bacterial identification in the clinical laboratory. Rinsho Byori 61:1107–1115
Mauti EM, Mauti GO, Ouno GA, Mabeya BM, Kiprono SJ (2013) Molecular identification of soil bacteria by 16S rDNA sequence. J Nat Sci Res 3:1–4
Mia MAB, Shamsuddin ZH, Wahab Z, Marziah M (2010) Effect of plant growth promoting rhizobacterial (PGPR) inoculation on growth and nitrogen incorporation of tissue-cultured Musa plantlets under nitrogen-free hydroponics condition. Aust J Crop Sci 4:85–90
Montesinos-Pereira D, Barrameda-Medina Y, Romero L, Ruiz JM, Sanchez-Rodriguez E (2014) Genotype differences in the metabolism of proline and polyamines under moderate drought in tomato plants. Plant Biol 16:1050–1057
Muscolo A, Junker A, Klukas C, Weigelt-Fischer K, Riewe D, Altmann T (2015) Phenotypic and metabolic responses to drought and salinity of four contrasting lentil accessions. J Exp Bot 66:5467–5480
Nautiyal CS, Srivastava S, Chauhan PS, Seem K, Mishra A, Sopory SK (2013) Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol Biochem 66:1–9
Rai RK, Tripathi N, Gautam D, Singh P (2017) Exogenous application of ethrel and gibberellic acid stimulates physiological growth of late planted sugarcane with short growth period in sub-tropical India. J Plant Growth Regul 36:472–486
Santiago TR, Grabowski C, Rossato M, Romeiro RS, Mizubuti ESG (2015) Biological control of eucalyptus bacterial wilt with rhizobacteria. Biol Cont 80:14–22
Saravanan VS, Subramonium SR, Raj SA (2003) Assessing in-vitro solubilization potential of different zinc solubilizing bacterial (Zsb) isolates. Braz J Microbiol 34:121–125
Shakeel M, Rais A, Hassan MN, Hafeez FY (2015) Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) Varieties. Front Microbiol 6::1286
Sharma RK, Archana G (2016) Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl Soil Ecol 107:66–78
Sharma S, Villamor JG, Verslues PE (2011) Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol 157:292–304
Shrivastava AK, Solomon S, Rai RK, Jain R, Singh P, Shukla SP, Chandra A (2015) Enhancing sugarcane and sugar productivity: physiological constraints and interventions. SugarTech 7:215–226
Singh L, Gill S, Tuteja N (2011) Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal Behav 6:175–191
Snedecor GW, Cochran WG (1967) Statistical methods. Oxford and IBH Publishing, New Delhi, p 593
Srivastava HS (1975) Distribution of nitrate reductase in aging bean seedlings. Plant Cell Physiol 16:995–999
Srivastava MP, Tiwari R, Sharma N (2013) Effect of different cultural variables on siderophores produced by Trichoderma spp. Int J Adv Res 7:1–6
Tammisola J (2010) Towards much more efficient biofuel crops—can sugarcane pave the way? GM Crops 1:181–198
Tiwari S, Lata C, Chauhan PS, Nautiyal CS (2016) Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem 99:108–117
Tokala RK, Strap JL, Jung CM, Crawford DL, Salove MH, Deobald LA, Bailey JF, Morra MJ (2002) Novel plant-microbe rhizosphere interaction involving Streptomyces lyrics WYEC108 and the pea plant (Pisum sativum). Appl Environ Microbiol 68:108–113
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356
Vargas L, Santa-Brígida AB, Mota-Filho JP, de Carvalho TG, Rojas CA, Vaneechoutte D, Van Bel M, Farrinelli L, Ferreira PCG, Vandepoele K, Hemerly AS (2014) Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: a transcriptomic view of hormone pathways. PLoS ONE 9:e114744. https://doi.org/10.1371/journal.pone.0114744
Vessey JK (2003) Plant growth-promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Wutipraditkul N, Wongwean P, Buaboocha T (2015) Alleviation of salt-induced oxidative stress in rice seedlings by proline and/or glycine betaine. Biol Plant 59:547–553
Yadav J, Verma JP, Tiwari KN (2010) Effect of plant growth promoting Rhizobacteria on seed germination and plant growth Chickpea (Cicer arietinum L.) under in-vitro conditions. Biol Forum Int J 2:15–18
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–3
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Acknowledgements
Authors are thankful to the Director ICAR-Indian Institute of Sugarcane Research, Lucknow, India for providing necessary facilities to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by P. Wojtaszek.
Rights and permissions
About this article
Cite this article
Chandra, P., Tripathi, P. & Chandra, A. Isolation and molecular characterization of plant growth-promoting Bacillus spp. and their impact on sugarcane (Saccharum spp. hybrids) growth and tolerance towards drought stress. Acta Physiol Plant 40, 199 (2018). https://doi.org/10.1007/s11738-018-2770-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2770-0