Abstract
Drought is a major constraint throughout the world, and it creates a major yield loss by changing the plant metabolic process. However, the negative effects of drought on plant growth and development were alleviated by using plant growth-promoting bacteria. With these backgrounds, the study was conducted to identify the drought-tolerant endophytic bacteria and to know their plant growth promotion (PGP) effect on sorghum plants under drought conditions. From sorghum root, Acinetobacter pittii, Bacillus lichiniformis, Bacillus sp., Pseudacidovorax intermedius, and Acinetobacter baumannii strains were isolated and identified through 16S rRNA sequencing. These strains had higher levels of proline, protein, exopolysaccharides (EPS), 1-aminocyclopropane-l-carboxylic acid (ACC) deaminase, indole-3-Acetic Acid (IAA), and gibberellic acid (GA). An experiment was carried out in the laboratory to evaluate the effects of three drought-tolerant strains, A. pittii, Bacillus sp., and P. intermedius, on the growth of sorghum seedlings. Whereas root length (RL), shoot length (SL), seedling vigor index (SVI), and total dry matter production (TDM) were more in the Bacillus sp., and P. intermedius inoculated plants in both stress and non-stress condition. Principle component analysis revealed that Bacillus sp. and P. intermedius improved the growth characteristics and protect the seedling from water stress situations. A correlation study between the variables showed a positive significant correlation between all variables except root: shoot ratio (RSR) and SL. Variable RSR was not significantly correlated with GP, GRI, and SL; SVI and TDM showed a non-significant correlation with RSR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought stress causes a major problem around the world especially in the agriculture sector and it reduces crop growth, which in turn, affects crop production. Soil water potential decides the availability of water and nutrient to the plants and microorganisms (Jacoby et al. 2017). Plant growth-promoting rhizobacteria (PGPR) play an important role in the maintenance of plant growth, nutrient management, and hormonal balance, PGPR mediates several direct and indirect mechanisms which are involved to overcome abiotic stress (Vurukonda et al. 2016). The term Induced Systemic Tolerance was coined to study the microbe-stimulated physical and chemical changes that result in enhanced tolerance of plants to abiotic stress (Yang et al. 2009, 2016). Endophyte is a Greek word “endon” means within and “phyton” means plant. Endophyte is a microorganism that includes bacteria, archaea, fungi, and protists that colonize inside the plants without causing any harmful effect on the host plant (Hardoim et al. 2015). Obligate endophytes either depend on the host plant for their growth and survival or have a stage in their life cycle, in which they exist outside the host plant (facultative endophytes) (Marella 2014). Endophytes enhancement of plant growth and alleviate biotic and abiotic stress were extensively studied in Oryza sativa L., (Ganie et al. 2021), Solanum tuberosum L., (Surette et al. 2003), Solanum lycopersicum L. (Cheplick and Faeth 2009) and Zea mays L. (Naveed et al. 2014; Vurukonda et al. 2016) has been extensively reported. Endophytes may play a role in plant growth and development by producing plant growth-promoting phytohormones like Auxin (IAA) and gibberellins (GA), ethylene-inhibiting compound 1-amino-cyclopropane-1-carboxylic acid (ACC) deaminase, compatible solute (Proline), cell wall-related compound exopolysaccharide (EPS), and protein content of bacterial cells. Pseudomonas bacteria can live even under stressful situations by producing EPS, which protects microorganisms against dehydration by increasing water potential and controlling the diffusion of organic carbon sources. (Roberson and Firestone 1992; Chenu 1993; Chenu and Roberson 1996).
Plants under adverse conditions microorganisms can activate the possible mechanisms to combat the situation. Drought is the major constraint throughout the world and it creates a major yield loss by changing the plant metabolic process. Sorghum is the fourth most important cereal followed by maize and it has been used for a variety of purposes, including grain, forage, syrup, and bioethanol production (Shoemaker and Bransby 2010). Yet, sorghum plants require more fertilizer for achieving the optimum yield, resulting in the leaching of Nitrogen (N) and phosphorous (P) fertilizer into the environment and affecting the aquatic environment (Adesemoye and Kloepper 2009). To overcome this, Plant Growth Promoting Bacteria’s (PGPB) were used. The influence of endophytic bacteria seed inoculation on sorghum plants has been studied in a small number of tests. Seed inoculation is reported to be an efficient and successful delivery technique among the various uses of beneficial microbial inoculant. We believe that sorghum seeds treated with sorghum root-associated endophytic bacteria might increase plant development in stressful situations.
The goal of this research was to isolate and describe endophytes from sorghum roots, learn about their drought tolerance mechanisms, and investigate the influence of drought-tolerant isolates on sorghum growth characters.
Materials and methods
The laboratory experiments were conducted in the Department of Crop Physiology and Department of Agricultural microbiology at Tamil Nadu Agricultural University, Coimbatore (11°N latitude and 77°E longitude). Sterile borosil grade glassware was used for media preparation, inoculation, and alternative microbiological work. AR grade chemicals from M/s.Sigma Chemicals and M/s. HiMedia, India was used for media preparation and genomic DNA isolation. All the glassware, petri plates, and other materials were completely autoclaved before use.
Bacterial endophytes isolation from sorghum root
The goal of isolating bacteria from sorghum root was achieved using the methods outlined by Mareque et al. (2015). Sorghum roots (Vellacholam and COFS 29) were collected from two different regions of Tamil Nadu [Krishnagiri (12.5266° N, 78.2150° E) and Kovilpatti (9.1727° N, 77.8715° E)], during sample collection root materials, were kept in the icebox at field level itself and brought to the lab immediately. Briefly, root materials were washed thoroughly with tap water and incubated for 5 min in 70% Ethanol then for 20 min in 4% Sodium hypochlorite, and finally rinsed 4 times with sterile deionized (DI) water. The sterile check was performed by inoculating the final rinsed cleaned water in the TSA (Tryptic Soy Agar) agar medium. Then, root materials were aseptically ground with 0.9% NaCl, supplemented with cycloheximide (100 µg µL−1). In the case of field plants, serial dilutions of the suspensions were inoculated onto agar plates using TSA medium. That culture medium was chosen to get a large number of heterotrophic bacterium isolates. Based on the characteristic of the colony such as shape, color, and margin, ten different isolates were identified (Table 1), obtained ten isolates were further cultured, purified, and stored at – 80 °C with 50% glycerol stock for future use.
Isolates growth under drought stress
Drought stress was induced via two methods: PEG diffusion and broth inoculation, both at various PEG 6000 concentrations (− 0.2, − 0.4, − 0.6, − 0.8, − 1, and − 1.2 MPa) (Michel and Kaufmann 1973). In this connection, each petri plates contain 20 mL of Luria Bertani (LB) medium and 20 mL of different PEG solution on top of the plates and allow to diffuse for 24 h, after diffusion, the remaining PEG solution was decanted and kept for a dry in aseptic condition. Subsequently, cultures were streaked on plates with different concentrations of PEG 6000, incubate for 2–3 days and growth was observed. Meanwhile, LB broth was prepared by adding different concentrations of PEG 6000. Overnight growing bacterial cultures (OD600 = 0.6) were inoculated (10 µL) and kept under shaking (120 rpm at 28 °C) for 24 h; growth was calculated in terms of OD at 600 nm by using a UV spectrophotometer (Jasco V-730) (Sandhya et al. 2009). Growth and Plant Growth Promoting (PGP) activities of isolates with and without drought stress levels (− 1 MPa) were quantified. It was categorized as drought tolerant and susceptible based on bacterial growth and growth-promoting properties of bacterial isolates. The growth potential of bacterial isolates (− 1 MPa of PEG solution grown) was measured using a UV spectrophotometer (Jasco V-730) (OD at 600 nm) at a different time interval (1 h, 2 h, 4 h, 6 h, and 8 h) after the inoculation and the growth was compared with the bacterial cultures grown in normal condition (Without PEG 6000).
Plant growth promotion properties
Quantification of proline
An assay of proline quantification was done as described by Bates et al. (1973). Initially, bacterial isolates were grown in LB broth with and without PEG 6000. Cells were harvested by centrifugation at 6000 rpm for 5 min, and pellets were collected and boiled after being combined with 70–80% Ethanol for 45 min at 60˚C. After boiling, the solution was again centrifuged at 6000 rpm (15 min) and the supernatant was collected. Then, 1 mL of acid ninhydrin and glacial acidic acid were added and incubated at 100 °C for 1 h. Later, the reaction was terminated immediately by placing it into an ice bath. To the reaction mixture, 2 mL of toluene was added and mixed vigorously. The chromophore containing toluene was aspirated from the aqueous phase, warmed to room temperature, and the absorbance was measured by UV spectrophotometer (Jasco V-730) at 520 nm using toluene as a blank. The proline concentration was determined by forming a standard curve using D-Proline and the amount of proline content was expressed as µg/mL.
Quantification of protein content
Lowry’s technique was used to determine the endophytes’ protein synthesis capability (Lowry et al. 1951). 1 mL of cultured solution (200 µL cultured cell with and without PEG 6000 and 800 µL of distilled water) was prepared and then add 4.5 mL of reagent 1 which contain 48 mL of 2% Na2CO3 in 0.1 N NaOH; 1 mL of 1% C4H4O6KNa; 1 mL of 0.5% CuSO4 and incubated for 10 min. Sequentially, 0.5 mL of reagent 2 (1part Folin- Phenol: 1 part water) was added and incubated for 30 min before reading the protein concentration at 660 nm by using a UV spectrophotometer (Jasco V-730). BSA was employed as a standard for protein estimation and its concentration was stated in mg/mL.
Quantification of exopolysaccharides (EPS) production
An exopolysaccharides production was determined by the estimation of total carbohydrates content (Dubois et al. 1956), about, 1 mL of overnight grown bacterial culture (OD600 = 0.6) with and without PEG 6000 was mixed with 5% (w/v) phenol and 5 mL of concentrated sulfuric acid by keeping sample tubes in ice. The mixture was incubated at room temperature for 20 min and the color development was read by measuring absorbance at 490 nm using a UV spectrophotometer (Jasco V-730) against the concentration of glucose and the amount of EPS production was expressed as mg/mL.
IAA production
The IAA production was measured using the procedure described by Patten and Glick (2002). About, 5 mL of LB broth containing 0.2% L-tryptophan, pH 7.0, and bacterial cultures have been grown with and without PEG 6000 and incubated in the broth solution for 7 days at 125 rpm (25 °C) in a shaker. After centrifuging the culture at 11,000 rpm for 15 min, adding 1 mL of supernatant to 2 mL of Salkowski reagent (Gordon and Weber 1951), and incubating until the pink color development, it indicates the presence of IAA production. The UV spectrophotometer (Jasco V-730) was used to measure the OD at 530 nm and pure IAA was used as a standard for the IAA curve. The IAA production was denoted as µg/mL culture.
GA production
The method described by Mahadevan and Sridhar (1982) was used to analyze the GA production. The overnight grown culture was inoculated into LB broth and incubated for seven days at room temperature. Sequentially, the culture was centrifuged at 10,000 rpm for 10 min and collected the supernatant adjust the pH (2.0) using 1 N HCL. Add equal quantity of ethyl acetate in separation funnel for GA3 extraction from the supernatant, by vigorous shaking solvent phase was collected which contains GA3. 2 mL of zinc acetate (21.9 g zinc acetate in 80 mL of water containing 1 mL of glacial acetic acid, final volume made up to 100 mL) was added to the 5 mL of dissolved residue. The above mixture was centrifuged at 10,000 rpm after the addition of 2 mL potassium ferrocyanide solution (10.6 g potassium ferrocyanide dissolved in 100 mL distilled water) for 10 min. Then, 5 mL of supernatant was mixed with 5 mL of 30% HCL and incubated for 75 min at 20 °C. The absorbance was measured at 254 nm using a UV spectrophotometer (Jasco V-730). The blank was prepared with hydrochloric acid (5%). The GA production was denoted as µg/mL culture.
1-aminocyclopropane-l-carboxylic acid (ACC) deaminase activity
The ability of bacterial isolates to utilize ACC as a single nitrogen source was used to assess their ACC deaminase activity. Isolates were inoculated into Dworkin and Foster (DF) salt minimal broth (DF salts per liter: 4.0 g KH2PO4, 6.0 g Na2HPO4, 0.2 g MgSO4.7H2O, 2.0 g glucose, 2.0 g gluconic acid, and 2.0 g citric acid with trace elements: 1 mg FeSO4.7H2O, 10 mg H3BO3, 11.19 mg MnSO4.H2O, 124.6 mg ZnSO4.7H2O, 78.22 mg CuSO4.5H2O, 10 mg MoO3, pH 7.2) (Dworkin and Foster. 1958) supplemented with 3 mM ACC as the main source of nitrogen. To screen ACC deaminase activity under stressed conditions (Supplemented with PEG 6000), selected isolates were grown individually in liquid DF minimal medium (DF + ACC) and their activity was measured in terms of α-ketobutyrate (α‐KB) production that is generated by the cleavage of ACC-by-ACC deaminase. After determining the protein and α‐KB content, the enzyme activity was measured spectrophotometrically (Jasco V-730) at 600 nm (Honma and Shimomura 1978; Penrose and Glick 2003). The amount of ACC deaminase enzyme activity was expressed as the amount of α-ketobutyrate liberated in nmol per milligram of cellular protein per hour.
16S rRNA gene amplification, sequencing, and phylogenetic analysis
The CTAB (cetyltrimethylammonium bromide) technique was used to extract whole genomic DNA from chosen isolates, which was validated using a 1% agarose gel electrophoresis. For the amplification of the 16S rRNA region, 27F (5’ AGA GTT TGA TCM TGG CTC AG 3’) and 1492R (5’ CGG TTA CTT TGT TAC GAC TT 3’) primers were used (Weisburg et al. 1991). About 10 µL of PCR reaction contain 5 µL of master mix (MgCl2, buffer, and Taq), each 1 µL of reverse and forward primer, 2 µL of cell lysate template, and 1 µL of DI water. PCR was performed in the following condition: an initial denaturation 94 °C for 5 min; 40 cycles at 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 2 min and a final cycle at 72 °C for 10 min. The amplification of the final product was analyzed by 1% agarose gel electrophoresis in TAE buffer and gel documented under UV light.
16S rRNA gene sequencing was done by synergy scientific service Pvt Ltd (Bangalore) for their specific strain identification. The tree was created using MEGA 7.0 software and the maximum likelihood technique, with 100 resamplings used in the bootstrap analysis. Then, to corroborate the discovered species, NCBI sequences of closely related organisms were downloaded and compared. As a result, the sequences were submitted to NCBI and an accession number was created for each organism.
Growth-promoting activities of endophytes on sorghum under drought conditions
Screening of sorghum for drought stress
Sorghum CO 30 and K 12 seeds were kindly collected from the Department of millets, Tamil Nadu Agricultural University. A preliminary laboratory experiment was carried out to fix the PEG 6000 concentrations using the petri plate method, with various concentrations of PEG 6000 (− 0.1 to − 1 MPa) being employed to produce drought stress. Literally, − 0.6 MPa shows the 50% drought stress, drought effect was measured in terms of germination percentage, root and shoot length (Data not shown); above mentioned PEG 6000 concentration (− 0.6) was used for further study.
The effect of bacterization on sorghum growth characters
To investigate the influence of isolates on the growth and development of sorghum, a laboratory experiment was conducted using a completely randomized design with four replications. Two different types of sorghum varieties viz., CO 30 and K 12 were used. Bacterial cultures were grown in LB broth for 16 h (OD600 = 0.6) then centrifuged at 10,000 rpm for 20 min and the cell pellet was collected and applied on disinfected, overnight soaked sorghum seeds, which were then left to shade dry. Petri plates were disinfected with 3% sodium hypochlorite and then washed three times with sterile water, germination sheet was used as a bed material for seed germination. Treatment details were following, T1 Absolute control (non-stress) T2 Control (Stress without inoculation) T3 Seed treated with Acinetobacter pittii (non-stress) T4 Seed treated with Bacillus sp. (non-stress) T5 Seed treated with Pseudacidovorax intermedius (non-stress) T6 Drought + Seed treated with A. pittii T7 Drought + Seed treated with Bacillus sp. T8 Drought + Seed treated with P. intermedius.
The seeds of each variety were placed in petri plates, which were then moistened with 5 mL of distilled water. After 24 h, each treatment received 10 mL of PEG 6000 (− 0.6 MPa) solution. Germination count was taken from each day up to 7 days after those with radicle lengths of at least 2 mm. On the 8th day, germinated seedlings were taken out from the petri plate to assess their germination components according to ISTA (2018). Germination plates were maintained at room temperature (24–30 °C).
Germination percentage (GP) was calculated as the total number of germinated seeds by the total number of seeds used in 100 and expressed as a percentage
Germination rate index (GI) was calculated by the formula given by Maguire (1962) and expressed as (%/day)
The Seedling vigor index (SVI) was calculated as shoot and root length into germination percentage divided by 100.
Growth characters
Root: shoot ratio (RSR) calculated as root length divided by shoot length into 100. Root length (RL) and shoot length (SL) were measured and expressed in cm, root and shoot dry weight was evaluated for total dry matter production (TDM) and expressed as g/plant. Root and shoot dry weight were obtained after drying at 70 °C for 48 h.
Statistical analysis
All statistical data analysis was carried out using SPSS statistical software (version 16.0). The PGPR characters and growth promotion of endophytes on sorghum seedlings were analyzed by the analysis of variance (ANOVA) and compared by Duncan’s Multiple-Range Test (DMRT) with four replicates. All statistical tests were performed at the p ≤ 0.05 level. Graph pad Prism 8 (USA) was used for graphical presentation. Principle component analysis was used to perform variables and treatments with XLSTAT (2020.5.1 USA).
Results
Isolation and drought screening of bacterial endophytes associated with sorghum root
Endophytic bacteria were isolated from surface-sterilized root materials under in vitro conditions, using Tryptic Soy Agar (TSA) media. Colonies were chosen and purified based on morphological characteristics such as form, color, and fluorescence, only a few colonies were abandoned. The drought screening phase included a total of 10 isolates (Table 2). A total of all ten isolates showed normal growth at − 0.5 MPa (− 5 bars) but in the case when we increased osmotic potential to − 1 MPa (− 10 bars) isolates, VR1, VR3, VR5, and SR4 isolates recorded poor growth. Even though, VR2 (A. pittii), VR4, SR1 (Bacillus lichiniformis), SR2 (Bacillus sp.), SR3 (P. intermedius), and SR5 (Acinetobacter baumannii) isolates showed normal growth even under water-stressed conditions (− 1 MPa). Finally, there was no bacterial growth was observed in − 1.2 MPa (− 12 bars) of increased osmotic potential.
Proline, Protein, and EPS production by bacterial isolates with and without PEG 6000
The amount of proline produced in different endophytes was analyzed and indicated in Fig. 1a. Isolates SR3 (P. intermedius) and VR2 (A. pittii) had maximum proline accumulation in both non stress (32.30±0.49 μg/mL) (21.95±0.09 μg/mL) and stressed (46.86±0.71 μg/mL), (38.18±0.16 μg/mL) circumstances, respectively. The VR1 (without PEG 4.68±0.04, with PEG 7.49±0.71 μg/mL) isolate had the least level of proline content of any of the others. The protein concentration of the selected isolates in normal and drought situations was shown in Fig. 1b. When compared to non-stressed situations, the concentration of protein content was considerably lower under stressed conditions (− 1 MPa). Under normal situations, VR2 (A. pittii) synthesis more amount of protein (56.90±0.23 μg/mL) than other isolates followed by VR5 (43.39±0.21 μg/mL). In drought-induced conditions (− 1 MPa), the greatest protein concentrations were reported by strains VR2 (A. pittii) (42.47±0.23 μg/mL) and SR3 (P. intermedius) (23.12±0.62 μg/mL).
The synthesis of exopolysaccharide (EPS) was greater in drought-stressed endophytic isolates than in normal circumstances. Root-associated endophytes produced a significant amount of EPS, which was measured and shown in Fig. 2a. Without PEG, SR3 (P. intermedius) strain produced a considerable quantity of EPS (0.50±0.008 mg/mL) than other isolates. PEG imposition significantly increased the EPS production of isolates. Among them, isolate SR3 (P. intermedius) produced the highest amount of EPS content (0.80±0.012 mg/mL) followed by SR2 (Bacillus sp.) (0.66±0.003 mg/mL) and SR1 (B. lichiniformis) (0.46±0.004 mg/mL) when compared with all other isolates under osmotically stressed condition (− 1 MPa).
Bacterial isolates from sorghum roots producing plant growth-promoting substances under stress and non-stress condition. a Exopolysaccharide production of bacterial isolates; b 1-aminocyclopropane-1-carboxylic acid deaminase (ACCd) production of bacterial isolates under PEG imposed conditions. Values are the mean of four replications ± S.E. Values with different letters are significantly different at p ≤ 0.05
Plant growth promoting (PGP) activities of bacterial isolates
The amount of IAA production in selected isolates was detected and explored in Supplementary Fig. 1a. During the exponential growth stage, the IAA content was measured. In the absence of PEG 6000, isolates yielded IAA ranges of 19.07 ± 0.09 to 69.61 ± 0.34 μg/mL. While under stressed conditions (− 1 MPa) SR3 (P. intermedius) produced more amount of IAA (56.39 ± 0.86 μg/mL), whereas VR1 produced less amount of IAA (33.15 ± 0.30 μg/mL) than the other isolates.
Gibberellic acid (GA) production ability result was depicted in Supplementary Fig. 1b. Based on the results, it was observed that SR3 (P. intermedius) produced a higher amount of GA (45.62±0.69 μg/mL) followed by VR2 (A. pittii) (42.32±0.17 μg/mL) under non-stressed (without PEG 6000) conditions. However, GA production was much greater in the VR2 (A. pittii) (104.87±0.43 μg/mL) and VR4 (71.95±0.12 μg/mL) strains under the increased osmotic potential (− 1 MPa) condition.
Based on quantifying the amount of α‐KB production ACC deaminase enzyme activity was assayed in the presence of PEG 6000 (− 1 MPa), chosen endophytes may generate 1-aminocyclopropane-l-carboxylic acid (ACC)-deaminase activity, as shown in Fig. 2b. SR3 (P. intermedius) had the highest level of ACC deaminase activity (1.16-fold) among the bacterial strains, followed by SR2 (Bacillus sp.) (1.02-fold) and VR2 (A. pittii) (1.01-fold). VR1 exhibited the minimum (97.86±0.87 mg–1 protein h-1) level of ACC deaminase activity.
The growth potential of isolates with and without PEG 6000
The spectrophotometric growth potential of the ten isolates was determined, and the optical density (O.D) values are listed in Table 3. Growth was monitored in both stressed (− 1 MPa) and non-stressed (without PEG 6000) conditions at intervals of 1, 2, 4, 6, and 8 h, respectively. The growth of the isolates rose up to 6 h, following which the majority of the isolates' growth was fairly decreased after the 6-h time interval. Isolate growth was significant in the absence of PEG 6000, but it was severely inhibited in the presence of PEG imposed environment (− 1 MPa). The growth of isolates gets a peak at the time of 4 and 6 h time intervals. Under PEG enforced (− 1 MPa) circumstances, SR1 (B. lichiniformis) and SR3 (P. intermedius) had the greatest OD values at 4 to 6 h time intervals and the growth of the isolates also get increased (16% and 17%).
Identification of bacterial strains
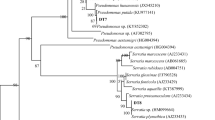
Based on the results, only five prospective drought-tolerant bacterial isolates were chosen from the ten isolates for ~1500 bp DNA amplification, and DNA sequence data were analyzed using the BLAST search tool and the non-redundant database. The identity of the tested isolates was validated by phylogenetic analysis of the 16S rRNA gene sequences of all five bacterial isolates from related bacterial species, and clustering at the genus level was detected (Fig. 3). The sequences of identified bacterial isolates were submitted to the NCBI database and the accession numbers for the respective isolates were assigned (Table 4).
Phylogenetic analysis of 16S rRNA gene sequences of the bacterial endophyte isolated from sorghum root. Sequences were aligned through “Clustal W” using MEGA 7 software. A phylogenetic tree was constructed using the maximum likelihood method. Bootstrap values are shown as percentages of 100 replicates; values below 50% are not indicated
The effect of endophytic bacterial inoculation of sorghum seeds under osmotic stress
Acinetobacter pittii, Bacillus sp., and P. intermedius isolates were chosen for laboratory research based on their in-vitro PGP properties. Germination percentage results of both varieties such as CO 30 and K 12, were given in Fig. 4a, respectively. Drought stress drastically reduces the germination percentage (61.5% and 39.4%) in the control (T2) plant than in absolute control (T1). Sorghum seeds bacterized with selected isolates increase the germination percentage in both drought stress and non-stress conditions, respectively. Seeds treated with A. pittii significantly increased germination percentage in T6 (Drought + Seed treated with A. pittii) (45% and 18.8%) treatment when compared to T2 (uninoculated control). Similarly, when CO 30 (29%) and K 12 (13%) variety seeds were treated with A. pittii strains, the germination rate index (germination percent per day) of T6 also get increased (Fig. 4b).
Growth promotion of seed inoculated endophytes on sorghum seedlings under stress and non-stress condition. CO 30 and K12-Sorghum varieties. a Germination percentage (%); b Germination Rate Index (GRI). T1 Absolute control (non-stress), T2 Control (Stress without inoculation), T3 Seed treated with A. pittii (non-stress), T4 Seed treated with Bacillus sp. (non-stress), T5 Seed treated with P. intermedius (non-stress), T6 Drought + Seed treated with A. pittii, T7 Drought + Seed treated with Bacillus sp., T8 Drought + Seed treated with P. intermedius. Values are the mean of four replications ± S.E. Values with different letters are significantly different at p ≤ 0.05
There is no significant difference was observed in root length development of bacterized plants under non-stress conditions of T3, T4, and T5 respectively (Supplementary Fig. 2a). Interestingly, under drought conditions there was a substantial difference; all three isolates increased total root and shoot length. There was no significant difference in shoot length across isolates, although seed inoculation with isolates enhances shoot length in both non-stress and drought stress situations as compared to uninoculated control (Supplementary Fig. 2b). There was no positive effect of inoculation on Root: Shoot ratio of variety CO 30 in between the treatments whereas in T8 of K 12 variety treated with P. intermedius recorded significantly highest root shoot ratio (91.1a) followed by T7 of K 12 variety than other isolates under drought condition (Supplementary Fig. 2c).
Supplementary Fig. 3a shows the results of the Seedling Vigour Index (SVI). Under drought, all three isolates demonstrate a substantial variation in SVI in both varieties. T7 of the K12 variety had a higher SVI (39.5%) than the other treatments, followed by T6 of the CO 30 and K 12 varieties (56.4% and 35.2%) respectively. Under the nonstress situation, bacterization of A. pittii and Bacillus sp. produced a substantial variation in CO 30 variety, but P. intermedius isolate did not create the highest change in SVI. None of the isolates showed a significant difference in total dry matter production in drought situations except T7 and T8 (Bacillus sp. and P. intermedius) which were achieved increased dry matter production (57.1% and 33.3%) in K 12 and CO 30 varieties, respectively (Supplementary Fig. 3b).
Principle compound analysis of sorghum growth promotion variables of eight treatments was plotted into four quarters (Fig. 5a). In the scoring plot, T6, T7, and T2 observations were positioned in the fourth quarter of the plot (− PC1 and − PC2) in both varieties. While in the T7 of K12 variety and T8 observations were located in the third quarter of the plot (− PC1 and + PC2). In the case of the first-quarter T3 of K12 variety and T1 were positioned (+ PC1 and + PC2) (Fig. 5a). In the loading plot, inoculants have positively influenced the variables positioned orthogonally to their respective samples (Fig. 5b). Based on the PCA result, seeds treated with strain P. intermedius and Bacillus sp. have positively influenced the growth of sorghum seedlings in drought stress conditions.
Scoring plot of samples (a) and loading plot of variables (b) in Principal component analysis of variables in sorghum seedlings growth promotion by seed inoculation. CO 30 and K12- Sorghum varieties. T1 Absolute control (non-stress) T2 Control (PEG imposed without inoculation) T3 Seed treated with A. pittii (non-stress) T4 Seed treated with Bacillus sp. (non-stress) T5 Seed treated with P. intermedius (non-stress) T6 Drought + Seed treated with A. pittii T7 Drought + Seed treated with Bacillus sp. T8 Drought + Seed treated with P. intermedius. The % of principle component (PC1 & PC2) variance was given in axes. Description of variables was given in the materials and methods
Except for RSR and SL, all of the variables in the correlation analysis were positively associated at the 5% level (p ≤ 0.05). Non-significant correlations were found between variable RSR and GP (r2 = 0.209), GRI (r2 = 0.284), and SL (r2 = − 0.123). SVI and TDM had no significant relationship with RSR (r2 = 0.096 and 0.265, respectively). Finally, the remaining factors were shown to be strongly associated at a 5% level. (Table 5).
Discussion
Drought, being one of the serious problems causing a negative impact on agricultural productivity, and scientists have devised a number of techniques to combat it. As a result, microorganisms that promote plant development are a recent improved and cost-effective solution for dealing with drought stress. The involvement of a section of the endophytic bacterial population in sorghum root in drought tolerance was examined in this research. Based on the findings of this study, there is some indication that these bacteria aid sorghum growth in drought environments. About, ten isolates were obtained from vellaicholam and COFS29 sorghum roots, respectively. Six strains among the isolates were able to withstand drought stress up to − 1 MPa. Based on the plant growth-promoting activity analysis, five promising isolates were characterized. Authors reported that endophytes belonging to the Acinetobacter genera had a potential mechanism to tolerate drought stress up to − 1.02 MPa (Sandhya et al. 2017). The results from the present study revealed that SR3 (P. intermedius) (40%) and VR2 (A. pittii) (70%) strains to produce the supplementary amount of proline during drought imposed (− 1 MPa) condition over the absence of PEG 6000 in their growth medium. Under the water-stressed condition, the bacteria may accumulate some amount of osmolytes i.e., glutamate, glutamine, proline, alanine, etc., for improving the cell growth (Sandhya et al. 2010). When we expose the bacteria to the drought condition it secretes osmolytes in response to drought stress, which acts synergistically with plant-produced osmolytes and stimulates plant growth by mitigating stress (Paul et al. 2008). Bacteria accumulate proline via up-regulation of proline biosynthesis which serves as an osmolyte. Cellular energy and NADP+/NADPH balance, signaling pathway activation, and contribution to other pathways such as the tricarboxylic acid cycle (TCA) and Glutathione (GSH) biosynthesis were maintained by the proline metabolic flux (Liang et al. 2013). Under stressed situations proteins are probably used for polysaccharide production this may as a reason for reducing the protein content during drought imposition (Roberson and Firestone 1992). Sandhya et al. (2010) revealed that PEG imposition may significantly reduce the protein production and increase the EPS accumulation under drought conditions in Bacillus sp. in the same way, SR3 (P. intermedius) bacterial isolate was incorporated into our current experiment (Fig. 1b).
Analyzing of exopolysaccharide (EPS) production of cultures was carried out when grown at both PEG induced and normal growth medium. Results showed that PEG-imposed growth cultures could be able to produce a higher amount of EPS than normally grown cultures. Among them, SR3 produced a higher amount of EPS in both PEG imposed and normal conditions respectively. Whereas, the production was increased up to 1.6-fold in PEG imposed conditions than normally grown ones. The present study result was admitted with the previous finding (Ali et al. 2014) who articulated that under drought (− 0.30 MPa) SunfP12 isolate was the best producer of EPS. The result indicated that EPS production happens as a response to stress and it helps the bacteria from a desiccated environment and dried out slowly when compared to surrounding microorganism (Wilkinson 1958; Hepper 1975), which not only protect bacteria against desiccation but also protect host plants from drought stress through improved soil structure (Sandhya et al. 2009) and improving water holding capacity.
IAA is an important plant growth hormone, plays a major role in lateral root formation and root elongation. Similarly, endophytic bacteria such as Bacillus sp., Bacillus subtilis, Pseudomonas putida, Ochrobactrum sp. EB-165, Microbacterium sp. EB-65, Enterobacter sp. EB-14 and Enterobacter cloacae strain EB-48 isolates were able to produce IAA (Khan and Doty. 2009, He et al. 2013 and Govindasamy et al. 2020). According to the results of the present research, each of the selected bacteria had different abilities to produce IAA and GA content. Without PEG inoculation bacterial strain SR5 produced more IAA content among other bacterial strains. However, under PEG imposed stress conditions SR3 produced more amount of IAA when compared to other bacterial isolates. The result was directly correlated with proline accumulation, from this point of view, increasing osmotic stress in the growing medium might increase the proline and IAA production. The earlier study was also reported that plant growth-promoting bacteria Delftia sp. TSAC2 can able to produce the maximum amount of IAA in the presence of PEG 6000, whereas in the same experiment Janibacter melonis R2AA1 isolates produce more amount of IAA in the absence of PEG 6000 (Nivitha et al. 2019). After IAA another important plant growth hormone is GA. Results revealed that GA production was high in the osmotically stressed axenic cultures than in the non-stressed grown culture. The result was contrary to the previously observed finding (Kumar et al. 2019) who reported a gradual decrease in GA production with an increase in − 0.73 MPa osmotic stress in PB3 and PB 46 isolates.
Under the condition of drought stress, the role of the ACC deaminase enzyme was assayed by quantifying the amount of α-ketobutyrate released during the deamination of ACC by the enzyme ACC deaminase. SR3 registered a maximum level of ACC deaminase enzyme activity (1.16-fold) compared with SR2 (1.02-fold), VR2 (1.01-fold), and SR1 (1.01-fold) strains. By reducing the level of the stress hormone ethylene through the action of the enzyme ACC deaminase, which hydrolyzes ACC into α-ketobutyrate and ammonia instead of ethylene, ACC deaminase activity allows plants to endure stress (Glick et al. 1998).
The growth of bacterial endophytes was analyzed in both stress and non-stress condition respectively. Based on the OD value, the growth of the isolate was calculated and most of the endophyte’s growth was noticed gradually increasing at the time interval of 6 h incubation, subsequentially linear reduction in the growth was observed. However, B. lichiniformis and P. intermedius strains showed an increased growth rate in terms of OD value under stressed conditions. The growth of bacterial culture defines the number of bacterial cells in a population rather than size. PEG imposition strongly reduces the bacterial cell growth, the growth reduction could have occurred by changes in the lipid composition of the bacterial cell and this lipid composition change could affect the activation of some proteins in the bacteria (Halverson and Firestone 2000). In the case of PEG degradation, either “new” enzymes or enzymes similar to alcohol/α -hydroxy acid dehydrogenases and diol dehydrogenases may be involved, the latter being perhaps in the case (Kawai 2002).
Drought stress affected the seedling’s health characters such as germination, speed of germination, seedling length, and seedling weight (biomass). To end this, plants were treated with plant growth promoting (PGP) bacteria which could alleviate the droughts negative impact and raise the plant development process. The initial preliminary in vitro experiment was conducted to evaluate the endophyte effect on seedling characters. In the experiment, man-made drought stress was created using PEG compound and drought stress strongly reduced the germination percentage. However, seeds bacterized with A. pittii strain could be enhanced germination percentage. Various mechanisms are involved in the seed germination process by PGPB treatment, which included increasing seed germination and vigour index thereby inhibiting the incidence of seed microflora (Begum et al. 2012). Another possible mechanism was described by Duarah et al. (2011) that the starch to sugar conversion by amylase which provides energy to germinating seeds for root and shoot growth and increased the speed of germination too. Inoculation of GA producing Bacillus idriensis M50 and Bacillus vietnamensis KNUC511 strains can able to increase seed germination (Gholamalizadeh et al. 2017). Based on our findings, P. intermedius strain was able to produce IAA and GA under stress conditions so this might be a reason for the increasing germination of sorghum seed.
Endophyte’s treatment beneficially increased the seedling length (root and shoot) of sorghum but there was no significant difference between treatments under normal conditions, but bacterial treatments showed significant difference during PEG imposed conditions (Drought stress). P. intermedius strain increases the root: shoot ratio of sorghum under drought-imposed conditions. Based on the seedling length, seed vigor is mainly decided. The vigor index represents the fitness, establishment, and condition of the plant's final productivity (McDonald and Copeland 2012). The present findings concluded that inoculation of bacterial endophytes during PEG imposed drought conditions could enhance the seedling characters such as seedling length and root: shoot ratio. Bacterial-producing PGP traits might have promoted the plant growth mechanism (Lucy et al. 2004). Inoculated bacterial isolates produced plant growth hormones, such as GA and IAA which can indirectly give strength to plant growth (Nadeem et al. 2014). Another possible mechanism is stimulating plant growth by reducing ethylene levels through the synthesis of ACC deaminase enzyme, which plays a major role in inhibiting ethylene biosynthesis by bacteria used ACC (ethylene precursor) as the sole nitrogen source thereby reducing ethylene level in plants during drought and can control the negative effect on root and shoot growth (Glick et al. 2007). A similar conclusion was reported in rice and wheat on the beneficial effect of PGPB inoculation on root and shoot growth (Rana et al. 2015) who could observe stimulating seedling growth in their study.
In the present study, drought stress drastically reduced seedling root and shoot biomass. However, Bacillus sp. and P. intermedius strain treatments significantly increased seedling biomass in terms of total dry weight (57 to 33 % increased dry matter); these results were observed in CO 30 and K 12 varieties respectively. Under PEG imposed conditions all the three endophytes significantly produce higher TDM than uninoculated control. The current result was substantiated by the previous finding, which already reported that the inoculation of S. pseudovenezuelae and A. arilaitensis in maize plants significantly increased the root and shoot dry weight when compared to uninoculated treatment (Chukwuneme et al. 2020). The reason for this increase might be by bacterial producing siderophores which increased the absorption of micro and macronutrient and thus absorption effectively increased the plant biomass production and can contribute to the plant for better growth and development. The results from this study were in concurred with the study of Gholizadeh et al. (2017), who reported O1R1 and MR4 isolates had the most effect on the enhancement of root and shoot biomass in rice seedlings.
Conclusion
In both drought (− 1 MPa) and non-stress circumstances, ten distinct sorghum root-associated endophytes were isolated and screened to determine their drought-tolerant level, proline, protein, IAA, GA, EPS, ACC deaminase, and growth potential activities. Among all the ten isolates, SR3 (P. intermedius) and VR2 (A. pittii) performed well in most of the PGP traits in both drought and non-stress environments. Only five potential isolates were chosen for the 16S rRNA gene sequencing based on the drought-tolerant capacity and PGP findings. The study concluded that inoculation of P. intermedius, Bacillus sp. and A. pittii strains act as plant growth-promoting bacteria and mitigate the drought effect in sorghum plants possibly through their plant growth-promoting substances and can improve the plant growth activities such as germination, root, shoot length, and dry matter production. This work provides basic knowledge about the impact of potential endophytes on plant growth promotion and abiotic stress alleviation in sorghum plants.
References
Adesemoye AO, Kloepper JW (2009) Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol 85:1–12
Ali SZ, Sandhya V, Rao LV (2014) Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann Microbiol 64(2):493–502
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Begum M, Rai VR, Lokesh S (2012) Effect of plant growth promoting rhizobacteria on seed borne fungal pathogens in okra. J Indian Phytopathol 56(2):156–158
Chenu C (1993) Clay or sand polysaccharide associations as models for the interface between microorganisms and soil: water-related properties and microstructure. Geoderma 56:143–156
Chenu C, Roberson EB (1996) Diffusion of glucose in microbial extracellular polysaccharide as affected by water potential. Soil Biol Biochem 28:877–884
Cheplick GP, Faeth S (2009) Ecology and evolution of the grass-endophyte symbiosis. Oxford University Press, New York. https://doi.org/10.1093/acprof:oso/9780195308082.001.0001
Chukwuneme CF, Babalola OO, Kutu FR, Ojuederie OB (2020) Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J Plant Interact 15(1):93–105
Duarah I, Deka M, Saikia N, Boruah HD (2011) Phosphate solubilizers enhance NPK fertilizer use efficiency in rice and legume cultivation. 3 Biotech 1(4):227–238
Dubois M, Gilles KA, Hamilton JK, Pt R, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Ganie SA, Bhat JA, Devoto A (2021) The influence of endophytes on rice fitness under environmental stresses. Plant Mol Biol. https://doi.org/10.1007/s11103-021-01219-8
Gholamalizadeh R, Khodakaramian G, Ebadi AA (2017) Assessment of rice-associated bacterial ability to enhance rice seed germination and rice growth promotion. Braz Arch Biol Technol 60:1–13
Gholizadeh A, Saberioon M, Borůvka L, Wayayok A, Soom MAM (2017) Leaf chlorophyll and nitrogen dynamics and their relationship to lowland rice yield for site-specific paddy management. Inf Process Agric 4(4):259–268
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theoretical Biol 190(1):63–68
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26(5–6):227–242
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192
Govindasamy V, George P, Kumar M, Aher L, Raina SK, Rane J, Minhas PS (2020) Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum [Sorghum bicolor (L.) Moench]. 3 Biotech 10(1):1–14
Halverson LJ, Firestone MK (2000) Differential effects of permeating and nonpermeating solutes on the fatty acid composition of Pseudomonas putida. J Appl Environ Microbiol 66(6):2414–2421
Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320
He H, Ye Z, Yang D, Yan J, Xiao L, Zhong T et al (2013) Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb Zn uptake by Brassica napus. Chemosphere 90(6):1960–196
Hepper CM (1975) Extracellular polysaccharides of soil bacteria. In: Walkered N (ed) Soil microbiology. Butterworths, London, pp 93–110
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42:1825–1831. https://doi.org/10.1080/00021369.1978.10863261
International Seed Testing Association (2018) International rules for seed testing. International Seed Testing Association, Bassersdorf
Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front Plant Sci 8:1617
Kawai F (2002) Microbial degradation of polyethers. J Appl Microbiol Biotech 58(1):30–38
Khan Z, Doty SL (2009) Characterization of bacterial endophytes of sweet potato plants. Plant Soil 322(1):197–207
Kumar DA, Sabarinathan KG, Kannan R, Balachandar D, Gomathy M (2019) Isolation and characterization of drought tolerant bacteria from rice phyllosphere. Int J Cur Microbiol App Sci 8(6):2655–2664
Liang X, Zhang L, Natarajan SK, Becker DF (2013) Proline mechanisms of stress survival. Antioxid Redox Signal 19(9):998–1011. https://doi.org/10.1089/ars.2012.5074 (Epub 2013 May 23. PMID: 23581681; PMCID: PMC3763223)
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lucy M, Reed E, Glick BR (2004) Applications of free-living plant growth promoting rhizobacteria. Antonie Van Leeuwenhoek 86(1):1–25
Maguire JD (1962) Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci 2:176–177. https://doi.org/10.2135/cropsci1962.0011183X000200020033x
Mahadevan A, Sridhar R (1982) Methods of physiological plant pathology. Sivakasi Publication, India, pp 131–132
Marella S (2014) Bacterial endophytes in sustainable crop production: applications, recent developments and challenges ahead. Int J Life Sci Res 2(2):46–56
Mareque C, Taulé C, Beracochea M, Battistoni F (2015) Isolation, characterization and plant growth promotion effects of putative bacterial endophytes associated with sweet sorghum (Sorghum bicolor (L.) Moench). Ann of Microbiol 65(2):1057–1067
McDonald MF, Copeland LO (2012) Seed production: principles and practices. Springer Science and Business Media
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiol 51:914–916
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32(2):429–448
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 97:30–39
Nivitha G, Bowya T, Kalaiselvi T, Sivakumar U (2019) Screening of rice apoplast associated endophytic bacterial isolates for moisture stress tolerance and plant growth promoting traits. Madras Agric J. https://doi.org/10.29321/MAJ.2019.000214
Patten CL, Glick BR (2002) Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can J of Microbiol 48(7):635–642
Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism andsignaling. Annu Rev Plant Biol 59:417–441
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15. https://doi.org/10.1034/j.1399-3054.2003.00086.x
Rana A, Kabi SR, Verma S, Adak A, Pal M, Shivay YS, Prasanna R, Nain L (2015) Prospecting plant growth promoting bacteria and cyanobacteria as options for enrichment of macro-and micronutrients in grains in rice–wheat cropping sequence. Cogent Food Agric 1(1):1037379
Roberson EB, Firestone MK (1992) Relationship between desiccation and exopolysaccharide production in soil Pseudomonas sp. Appl Environ Microbiol 58:1284–1291
Sandhya V, SkZ A, Grover M, Reddy G, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by exopolysaccharides producing Pseudomonas putida strain P45. Biol Fertil Soil 46:17–26
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62:21–30
Sandhya V, Shrivastava M, Ali SZ, Prasad VSSK (2017) Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ Agric Sci 43(1):22–34
Shoemaker CE, Bransby DI (2010) The role of sorghum as a bioenergy feedstock. In Sustainable Alternative Fuel Feedstock Opportunities, Challenges and Roadmaps for Six US Regions. Proceedings of the Sustainable Feedstocks for Advanced Biofuel Workshop pp. 149–159
Surette MA, Sturz AV, Lada RR, Nowak J (2003) Bacterial endophytes in processing carrots (Daucuscarota L. var. sativus): their localization, population density, biodiversity and their effects on plant growth. Plant Soil 253(2):381–390
Vurukonda SS, Vardharajula S, Shrivastava M, SkZ A (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res 84:13–24
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Wilkinson JF (1958) The extracellular polysaccharides of bacteria. Bacteriol Rev 22:46–73
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Yang L, Hong X, Wen XX, Liao YC (2016) Effect of polyamine on seed germination of wheat under drought stress is related to changes in hormones and carbohydrates. J Integr Agric 15(12):2759–2774
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Umapathi, M., Chandrasekhar, C.N., Senthil, A. et al. Isolation, characterization and plant growth-promoting effects of sorghum [Sorghum bicolor (L.) moench] root-associated rhizobacteria and their potential role in drought mitigation. Arch Microbiol 204, 354 (2022). https://doi.org/10.1007/s00203-022-02939-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02939-1