Abstract
Aqueous film-forming foam extinguishing agent (AFFF) plays an important role in fighting pool fires, but the environmental problems caused by them are also increasingly apparent. The key component of traditional AFFF is C8 fluorocarbon surfactant, which is harmful to the ecological environment and human health. In this paper, a perfluorinated branched short-chain fluorocarbon anionic surfactant with high surface activity, simple synthesis route and easy industrial scale-up was reported. The surface activity and aggregation behavior of the mixed solution of this fluorocarbon surfactant and cetyltrimethylammonium bromide (CTAB) were studied by surface tension meter and transmission electron microscope. The results showed that CTAB and anionic fluorocarbon surfactant had the significant synergistic effect, and many vesicles could be observed to nestle together at the concentration of 0.0406 mol/L. Subsequently, three environmental friendly AFFF formulations (F-1, F-2, F-3) were designed with the mixed system of this fluorocarbon surfactant, CTAB and dodecyl betaine (BS-12) as foaming agent. The foam properties confirmed that F-1 possessed excellent foam properties, the initial foam height h0 was 115.34 mm, 25% drainage time was 182.4 s, extinguishing time was 31 s, and burn-back time was 720 s. This type of fire extinguishing agent had potential for fire protection applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluorocarbon surfactants are widely used in fire protection, detergents, wetting agents and other industrial fields because of their high surface activity, high thermal stability, high chemical stability and excellent hydrophobic, oleophobic and antifouling properties (Verdia et al. 2016; Szymczyk 2013; Sheng et al. 2019). Like conventional surfactants, fluorinated surfactants can also be divided into anionic, cationic, nonionic and amphoteric surfactants according to the different types of ions generated by hydrophilic groups. At present, the production of fluorinated surfactants is mainly concentrated in 3 M and DuPont companies in the USA, ICL companies in the UK, Atochem companies in France, etc. (Aihara 1988).
It was widely known that fluorocarbon surfactants were the core components of AFFF, and perfluorooctane sulfonate (PFOS), and its derivatives were the most commonly used. Unfortunately, PFOS was officially listed in the controlled list of persistent organic pollutants (POPs) by the United Nations Environment Programme (UNEP) due to its biological accumulation, persistence and the possibility of long-distance environmental migration (Kishi and Arai 2008; Jia et al. 2019; Zhang et al. 2019b). On November 11, 2014, Stockholm Convention on persistent organic pollutants came into force in China, which implied that China would gradually reduce and eventually stop using AFFF with PFOS as the main component (Jia 2015; Houde et al. 2006; Prescher et al. 1985; Key et al. 1997). Therefore, it was very important to develop new fluorocarbon surfactants with less environmental pollution, no bioaccumulation and biodegradation. Reducing the number of carbon atoms of perfluorinated radicals from C8–C10 to C4–C6 and branching perfluorocarbon chain were two effective methods at present (Zhang et al. 2019a; Jiang et al. 2018; Vinogradov et al. 2016; Lin et al. 2018a). Perfluorinated short-chain surfactant was a new type of fluorocarbon surfactant. The number of carbon atoms in the fluorocarbon chain was 6, while the number of carbon atoms in the straight chain was 5, and the perfluorocarbon chain was branched, which satisfied the requirements of current environmental protection (Lin et al. 2018b). In addition, a large number of studies suggested that this type of fluorocarbon surfactant possessed excellent surface properties, so it was an environmental friendly and efficient fluorocarbon surfactant (Wang et al. 2017; Howell et al. 2004). Surprisingly, these studies were mainly focused on theory, but the further application performance was very few.
High chemical stability implied excellent chemical inertness. However, fluorocarbon surfactants were compatible with other surfactants and could be used in almost all formulation systems. This made fluorocarbon surfactants played an irreplaceable role in the development of fire extinguishing agent. As a new type of extinguishing agent, it had attracted more and more attention. Typical examples included fluorine protein foam fire extinguishing agent, light water foam fire extinguishing agent and anti-resume dry powder fire extinguishing agent, etc. (Liu et al. 2008). Currently, the widely used AFFF still adopted the formulas of decades ago, which had serious ecological damage and environmental pollution problems. In view of this, the foam industry introduced fluorine-free fire foam, completely eliminating fluorocarbon surfactants and perfluorooctane sulfonate in the solution, and relying solely on hydrocarbon surfactants and other additives. However, commercial fluorine-free products did not meet the requirements of the current US MilSpec, including fire extinguishing performance requirements for ship fire protection (Hinnant et al. 2020). Sheng et al. reported the spreadability of fluorine-free foam and fluorine-containing foam and concluded that fluorine-containing foam spread faster than fluorine-free foam at the same foam flow rate (Sheng et al. 2018a). Yu et al. studied the foam stability of fluorine-free foam and fluorine-containing foam and concluded that fluorine-containing foam film was more stable than fluorine-free foam film (Yu et al. 2020). Therefore, searching for PFOS alternatives was of great significance to the development of alternative technologies for traditional AFFF.
This paper provided a synthetic route for the perfluorinated branched short-chain fluorocarbon anionic surfactant, and its structure was characterized by 1HNMR. Because the combination of fluorocarbon surfactant and proper hydrocarbon surfactant could reduce the production cost and improve the performance, the surface activity, adsorption property and aggregation behavior of the mixed solution of benzene ring side chain anionic fluorocarbon surfactant and CTAB were studied. Herein, three kinds of new environment-friendly AFFF formulations (F-1, F-2, F-3) were designed by adopting the perfluorinated branched short-chain fluorocarbon anionic surfactant, CTAB and dodecyl betaine as foaming agents. Through the study of foaming, foam stability, drainage, spreading, fire extinguishing and burn-back testing, it was known that F-1 had the potential to replace the traditional AFFF.
Experimental
Synthesis of anionic fluorocarbon surfactant with benzene ring side chain (Sha et al. 2015)
20 g hexafluoropropylene dimer (D2), 7.6 g methyl 4-bromomethylbenzoate, 2.906 g anhydrous potassium fluoride, 0.784 g tetrabutylammonium bromide and 50.7 ml dry N-N dimethylacetamide (DMAc) were placed in a closed tube, heated to 75 °C and stirred for two days. The mixed solution was cooled to room temperature, poured into ethyl ether, washed with water and salt water, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The product was purified by column chromatography (petroleum ether/ethyl acetate = 20:1).
2.11 g of compound 1 was dissolved in 50 ml methanol, then sodium hydroxide solution was added in ice water bath, and the mixture was moved to 60 °C water bath and stirred for 4 h. After the reaction, water was added to the system, the mixed solution was washed with ether, and the pH value of the solution was adjusted to 3 with 6 mol/L HCl solution. The aqueous solution was extracted with ether, dried on anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain compound 2.
In a 50-ml round-bottomed flask, 1.00 g compound 2 and 10 ml methanol were added, stirred until completely dissolved, and then 0.1 mol/L potassium hydroxide aqueous solution was added slowly until the pH value of the system was 7. The solvent was distilled under reduced pressure to obtain a white solid, washed with acetone and then vacuum dried to obtain benzene ring side chain anionic fluorocarbon surfactant. Figure 1 shows the synthetic route of anionic fluorocarbon surfactant with benzene ring side chain.
Surface tension measurements
The surface tension was usually used to characterize the surface activity of the aqueous solution of surfactant and its mixed system. In this paper, the surface tensions of all surfactants and their mixed system aqueous solutions were measured by the platinum plate method with SFZL-A2 series automatic surface tension tester of Shanghai Yingnuo Precision Instrument Co., Ltd. at 25 °C. The average value of each concentration of aqueous solution was taken after three measurements.
Transmission electron micrographs (TEM)
In order to study the morphology of vesicles in the mixed solution of benzene ring side chain anionic fluorocarbon surfactant and CTAB, the samples were rebalanced in a controlled incubator for two weeks, then stained negatively with 2 wt% phosphotungstic acid and finally observed by JEM-1011 transmission electron microscope at 100 kV.
Optical microscope
A certain amount of blistering mixed solution was dripped onto the glass slide, and the foam coarsening process was observed under optical microscope. A foam coarsening image was taken every minute to analyze the foam size distribution and foam coarsening kinetics.
Formulation design
Selection of foaming agent
According to the relevant literature (Wang et al. 2017), the mixed system of benzene ring side chain anionic fluorocarbon surfactant/CTAB could produce significant synergistic effect. In addition, amphoteric surfactants had certain bactericidal, fungicidal, foam stability and foaming properties and could be compatible with various surfactants (Lan and Zou 2003). Therefore, the new perfluorinated branched short-chain fluorocarbon anionic surfactant, CTAB and BS-12 were selected as foaming agents.
Selection of foam stabilizer
Commonly used surfactants were foaming agents as well as foam stabilizers. But for foam extinguishing agents, the stability of foaming agents was not enough to satisfy the high stability required by fire extinguishing, so foam stabilizer was needed. This paper selected one of the conventional industrial fields of sodium carboxymethyl cellulose, xanthan gum and coconut diethanol amide (6501) as the foam stabilizer (Sheng et al. 2018b; Duanmu et al. 2011).
Selection of other components
Common cosolvents mainly included high boiling point solvents such as ethylene glycol, ethylene glycol butyl ether, diethylene glycol monobutyl ether, propylene glycol methyl ether, propylene glycol methyl ether acetate, among which ethylene glycol was an excellent antifreeze agent. In this paper, diethylene glycol monobutyl ether was selected as cosolvent and ethylene glycol as antifreeze agent. In addition, urea as a burn-back agent not only played a burn-back role, but also could further improve the stability of foam and had certain solubilizing effect.
Determination of foam extinguishing agent formulation
According to the selected components, determine the basic formula of fire extinguishing agent, as shown in Table 1.
Determination of component concentration of foam extinguishing agent
AFFF was an efficient fire extinguishing agent developed in foreign countries in 1960s. The foam solution of this foam extinguishing agent could be mixed with water and could be divided into two kinds of 3% AFFF and 6% AFFF according to their mixing ratios (Sontake and Wagh 2014). Since 3% AFFF were widely used in the market, this work focuses on 3% types. According to the volume concentration of the concentrated solution in the diluted foam solution, the concentration of each main component in the foam concentrate used in this paper could be determined by formula 1:
where CFoam concentrate solution and CFoam solution referred to the concentration of surfactant in foam concentrate before and after dilution, respectively.
The concentration of surfactants in the foam solution depended on the critical micelle concentration (CMC) of the surfactant. Most surfactants had the lowest surface tension after reaching the CMC, and their properties were gradually stable. Finally, the dosage of each surfactant should be considered to avoid waste.
In addition, the concentration of antifreeze glycol was set at 20%, the concentration of cosolvent diethylene glycol monobutyl ether was set as 5%, the concentration of burn-back agent urea was set as 5%, and the rest components were water.
The foam solution used in this study was a solution diluted with foam concentrate and water at a volume ratio of 3:97.
Test conditions for foam concentrate
To sum up, this paper designed a set of fluorocarbon foam test conditions based on hydrocarbon and fluorocarbon anionic surfactants as shown in Table 2.
It should be pointed out that in the table, "CTAB" referred to cetyltrimethylammonium bromide; "BS-12" referred to dodecyl betaine; the anionic fluorocarbon surfactant − 0.8%"referred to the benzene ring side chain anion fluorocarbon surfactant, and the mass concentration in the foam concentrate was 0.8%; "DB" referred to diethylene glycol monobutyl ether; "6501-3%" referred to the type of hydrocarbon surfactant was coconut diethanol amide, the mass concentration in the foam concentrate was 3%; all concentrations in the table referred to the mass concentration, and the unit was "%".
Determination of foaming properties
There are many ways to generate foam: one is the traditional air inhalation, that is, the foam production point is located at the sprinkler head. The sprinkler is specially designed, which can inhale the surrounding air and mix with foam liquid. The other is compressed air, that is, a certain proportion of compressed air is mixed into the foam mixture, and foam is produced after impact and mixing. In this paper, Ross–Miles method was used to evaluate the foaming performance of the solution. Then, the foaming properties were characterized by initial foam height h0 (Sheng et al. 2016a).The specific operation steps were as follows: 10 ml solution was poured into 100-ml measuring cylinder with stopper and shook for 1 min, and then the foaming height of different solutions was recorded, and each experiment was repeated at least three times (Xiao et al. 2008).
Determination of foam stability
The stability of foam is the main performance of foam fluid. Foam fluid is a bubble dispersing system composed of gas. It has high surface free energy and is thermodynamically unstable. However, some measures can be taken to change the conditions and enhance the stability of foam to meet the application requirements. It is generally accepted that the mechanism of foam decay is the loss of liquid in the foam and the diffusion of gas through the liquid membrane.
The method of determining foam stability was to record the initial foam height h0 after the foam solution was foamed and let it stand for 5 min and then recorded the foam height h5 (mm) again. According to formula 2, R5 was used to characterize the foam stability of surfactant (Azira et al. 2008; Lunkenheimer and Malysa 2003a; Lunkenheimer and Malysa 2003b; Sebastiani et al. 2019; Tamura et al. 1995):
Determination of drainage performance
The characteristic parameters of foam drainage, such as the drainage rate parameter, the maximum drainage rate and the 50% drainage time, are all related to the mass fraction of the foam solution. The change in the mass fraction of the foam solution has a great influence on the foam drainage process (Lin et al. 2007). Essentially, foaming is the flow of liquid through the voids between bubbles. The flow is driven by capillary action and gravity and is resisted by viscous damping (Bikerman 2013; Weaire et al. 1997; Bhakta and Ruckenstein 1997). Usually, a foam is composed of films (between bubbles), channels (also known as the platform boundary at the intersection of three layers of films) and vertices (also known as vertices or nodes at the intersection of four layers of films) (Sheng et al. 2018c).
The test method of drainage performance was as follows: after the above foaming process was completed, started the timer and recorded the time of 2.5 ml liquid discharge at the bottom as the 25% drainage time (Xiao et al. 2008).
Determination of spreading performance
Theoretically, whether the aqueous foam solution (above) can spread on the surface of the fuel (below) is determined by three forces, namely the surface tension of the aqueous foam solution, the surface tension of the fuel and the interfacial tension between the aqueous foam solution and the fuel. The spreading coefficient can be calculated by formula 3:
where S is the spreading coefficient; γU is the surface tension of the foam aqueous solution; γL is the surface tension of the fuel; γLU is the interfacial tension between the foam aqueous solution and the fuel.
When S is greater than zero, the foam solution (above) can be spread on the fuel (below) surface; when S is less than or equal to zero, the foam solution (above) cannot be spread on the fuel (below) surface (Woodman et al. 1978; Harkins and Films 1922; He et al. 2019).
As shown in Fig. 2, the spreading performance of foam solution was tested by the following methods: The foam solution spread to the surface of cyclohexane through a foam release tube, and then a camera was used to record the spreading of the foam solution on the surface of the cyclohexane at different times. Finally, the spreading area of the foam solution was calculated using the MATLAB image processing technology (Sheng et al. 2018d).
Determination of fire extinguishing performance
The extinguishing mechanism of AFFF is mainly due to its good cooling, covering and suffocating effects. AFFF can quickly form a aqueous film on the surface of combustible liquid to isolate the heat feedback of flame and inhibit the generation of combustible steam, thus effectively preventing the further combustion of fuel (Xu et al. 2020).
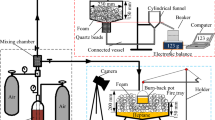
Figure 3 is a device for testing foam extinguishing performance. In brief, the following methods were tested: n-heptane (50 mL) was poured into a glass and ignited. After 60 s of pre-burning, supplying foam was started until the flame was completely extinguished. The fire extinguishing time was recorded, and the fire extinguishing performance of the foam was analyzed by the fire extinguishing time (Sheng et al. 2016b).
Determination of burn-back performance
The burn-back time refers to the time from the ignition of the burn-back tank to the ignition of the fuel surface. The burn-back time of foam depends not only on the components of the fire extinguishing agent, but also on the percentage of gases in the foam.
Figure 4 is a device used to study the foam burn-back test. After the abovementioned bubble supply was finished for 1 min, the 20 mL n-heptane could be placed in the positive center of the oil pool to adjust the position of the burn-back tank. Then ignite the burn-back tank, and adjust the position of the burn-back tank continuously during this period. Finally, the burn-back time of different stages was recorded until the entire fuel surface was completely burned (Yu et al. 2020).
Results and discussion
Characterization of anionic fluorocarbon surfactant with benzene ring side chain
The anionic fluorocarbon surfactant with benzene ring side chain was synthesized from compound 2 and potassium hydroxide solution by acid–base neutralization reaction. The structure of anionic fluorocarbon surfactant and compound 2 were characterized by 1HNMR. The 1HNMR spectra of the anionic fluorocarbon surfactant and compound 2 are given in Fig. 5. The signal at δ = 13.07 ppm was the characteristic absorption peak of –COOH, and the signal at δ = 3.66 ppm was the characteristic absorption peak of –CH2c, the signal at δ = 7.29 ppm was the characteristic absorption peak of –CHb. Due to the influence of adjacent groups, the signal at δ = 7.84 ppm was the characteristic absorption peak of –CHa. By analyzing the signal at δ = 13.07 ppm, it could be confirmed that the functional group –COOH had disappeared, that was to say –COOH and KOH were well neutralized and anionic fluorocarbon surfactant was prepared.
1HNMR(CD3OD, 300 MHz)δ:3.66(s, C6H4CH2C6F13, 2H), 7.29(d, J = 8.1 Hz, Ar–H, 2H), 7.84(d, J = 8.1 Hz, Ar–H, 2H); 19FNMR(CD3OD, 282 MHz)δ: − 63.68 to − 63.90(m,6F), − 82.05(t, J = 13.7 Hz,3F), 106.90 to − 107.25(m, 2F), − 124.10 to − 124.35(m, 2F).
Surface tension, foaming, foam stability and critical micelle concentration of the mixed system
Through the measurement of surface tension meter, we could observe that the surface tension of the benzene ring side chain anionic surfactant was 20.4 mN/m.
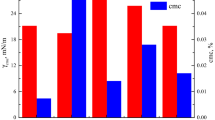
Figure 6 shows the relationship between surface tension and concentration of benzene ring side chain anionic fluorocarbon surfactant/CTAB with different mole fraction at 25 °C. The surface tension and CMC of benzene ring side chain anionic fluorocarbon surfactant/CTAB with different mole fraction at 25 °C are listed in Table 3. It could be seen from the figure that the surface tension of the anionic fluorocarbon surfactant with benzene ring side chain was lower than that of CTAB, but the CMC value was higher, which indicated that the ability of reducing the surface tension of aqueous solution by the anionic fluorocarbon surfactant with benzene ring side chain was stronger than that of CTAB, and the efficiency was lower. For the study of the mixed system, we were surprised to find that the CMC was 0.00991211 mmol/L when the mixed ratio of benzene ring side chain anionic fluorocarbon surfactant and CTAB reached 1:10, which was 103 times lower than that of benzene ring side chain anionic fluorocarbon surfactant and 10 times lower than that of CTAB. It was due to the strong interaction between anionic and cationic surfactants. The interaction included electrostatic attraction between heterogenous ions and hydrophobic interaction between hydrocarbon groups, which made the surfactant molecules in the solution easier to gather and form micelles, and the surfactant molecules in the surface adsorption layer were arranged more closely and the surface energy was lower (Ghosh et al. 2011). In addition, fluorine was the most negative electronic element. The negative charge around fluorine atom and the electron cloud densely covered in the fluorine chain made the hydrophobicity of CF–CH chain stronger than that of CH–CH chain. When the mixed ratio was 1:2, the CMC was lower than that of the benzene ring side chain anionic fluorocarbon surfactant, close to that of CTAB, but its surface tension was higher than that of benzene side chain anionic fluorocarbon surfactant. According to the viewpoint of molecular motion, the possibility (probability) of free movement of the longer hydrocarbon chain in the system was greater, because there was a "blank" around the top, which allowed the single bond to rotate freely. And then the longer hydrocarbon chain might bend and cover the shorter fluorocarbon chain, which made the density of the outermost fluorocarbon chain relatively decrease and eventually led to the increase of surface tension (Wang et al. 2017).
Figure 7 shows the foaming and foam stability of aqueous solutions of CTAB and benzene ring side chain anionic fluorocarbon surfactant at the optimal compound ratio. Figure 8 shows the initial foam height of solutions with different concentrations of CTAB/benzene ring side chain anionic fluorocarbon surfactant at the optimal mixed ratio. Figure 9 shows the foam height of solutions with different concentrations of CTAB/benzene ring side chain anionic fluorocarbon surfactant at the optimal compound ratio after five minutes. It could be seen from the figure that the h0 value of the benzene ring side chain anionic fluorocarbon surfactant/CTAB had no obvious change in the CTAB with higher concentration, which indicated that the molecular number at the liquid gas interface was saturated when the concentration of CTAB was high. For most surfactants, the surface tension almost did not change with the increase of the concentration after the concentration reached CMC, but the foaming property of them was not completely consistent with the concentration after reaching CMC. The foaming performance of the benzene ring side chain anionic fluorocarbon surfactant/CTAB still improved with the increase of the concentration after reaching CMC. It should be noted that the difference between h0 and h5 curves was very small, especially for CTAB with high concentration. Besides, with the increase of CTAB concentration, R5 tended to stabilize after a small floating. The reason was that the CTAB molecules were arranged more tightly at the liquid air interface as the concentration of solution increased. This change improved the foaming property and the foam stability of surfactant solution.
Aggregation behavior of aqueous solution in the mixed system
According to Gibbs adsorption equation (Zhou and Rosen 2003; Rosen 2004) (formulas 4, 5), the saturated adsorption capacity (\(\Gamma_{\max }\)) and the minimum area (Amin) of single surfactant and binary surfactant on air/water interface were calculated.
where R was 8.314 J/mol−1 K−1; T was the thermodynamic temperature (K); \({{\partial_{\gamma } } \mathord{\left/ {\vphantom {{\partial_{\gamma } } {\partial_{\lg c} }}} \right. \kern-\nulldelimiterspace} {\partial_{\lg c} }}\) was the maximum slope; NA was Avogadro constant, n = 2 for pure surfactant and 1 for cation/anion mixed system (Zhao and Xiao 1995; Rosen 1989); higher \(\Gamma_{\max }\) or lower Amin value meant that the surfactant molecules were more densely arranged at the air/water interface. The most direct result of surfactant adsorption on the solution surface was to reduce the surface tension of aqueous solution. Generally speaking, the greater the surface adsorption capacity, the more the surface tension decreased. It could be seen from Table 3 that compared with single surfactant, the \(\Gamma_{\max }\) of binary surfactant mixture was higher, while Amin was lower, which indicated that the molecules of the mixed system were more densely arranged at the air/water interface. By comparing the \(\Gamma_{\max }\), Amin, CMC and \(\gamma_{{{\text{CMC}}}}\) at the mixed ratios of 1:2 and 1:10, although the molecular arrangement of the mixed system was more compact when the molar ratio was 1:2, its CMC and \(\gamma_{{{\text{CMC}}}}\) were higher than that of 1:10. The reason was that the ability of surfactant to reduce the surface tension of the aqueous solution was not only related to the amount of surface adsorption, but also mainly depended on the outermost atom or clusters in the state of saturated adsorption on the surface of the aqueous solution.
Figure 10 shows the TEM negative staining images of vesicles in the mixed system of benzene ring side chain anionic fluorocarbon surfactant/CTAB at the optimum mixture ratio. It could be seen that the change trend of vesicles was consistent with that observed in TEM images. Generally speaking, the change of concentration would affect the number of vesicles, but almost had no effect on the size of vesicles (Zhai et al. 2005). The reason for this phenomenon was that with the increased of concentration, the aggregation of vesicles led to the change of vesicle diameter. Obviously, many vesicles nestled together to form a beaded distribution at the concentration of 0.0406 mol/L.
Optical microscope pictures of the foam coarsening process of the mixed system aqueous solution
Figure 11 shows the optical microscope images of the foam coarsening process of aqueous solutions of different concentrations at the optimum mixture ratio. From the diagram, we could see that the morphology of low-concentration foam was much larger than that of high concentration at the beginning (1 min). Due to the pressure difference between bubbles of different sizes, bubbles grew and merged with time. However, there were many phenomena in the bubble morphology at different concentrations. From 1 to 20 min, the foam morphology of aqueous solution of high-concentration mixed system changed little, while 20 min still had a large amount of liquid trapped in the bubble, and the bubbles were round. With the passage of time, the amount of foam in aqueous solution of low-concentration mixed system was slightly reduced and was looser than that in high concentration. The dispersion of foam was related to the stability of foam. Under the action of the background light, some of the liquid in the foam film and the platform glass evaporated into steam. The evaporated vapor accumulated on the slide and aggregated into droplets (Sheng et al. 2020). To sum up, the high-concentration mixed system aqueous solution was more suitable for AFFF than the low-concentration mixed system aqueous solution.
Surface tension, foaming property and foam stability of the formula
Table 4 shows the surface tension of three AFFF formulations. Table 5 shows the h0, h5 and R5 of the three AFFF formulations. Figure 12 is a histogram of three foam extinguishing agent formulations h0, h5 and R5. It could be seen from Table 4 that the surface tension of F-2 was the highest and the surface tension of F-1 was the lowest. According to the definition and calculation of the spreading coefficient S, when the S was greater than zero, the foam solution could be spread on the fuel surface. The fuel used in this paper was cyclohexane. The surface tension test showed that the surface tension of cyclohexane was 25.24 mN/m, while the surface tension of F-2 was 27.10 mN/m. Therefore, F-2 could not be spread on the surface of cyclohexane. From Table 5 and Fig. 12, we could see that F-1 and F-3 possessed better foaming properties, and F-2 had poor foaming capacity. In terms of foam stability, R5 of F-1 and F-3 was close to 100%, while R5 of F-2 was still poor, only 86.67%, so F-2 was not suitable for AFFF.
Drainage performance
The extinguishing effect of AFFF was shared by the aqueous film and foam layer floating on the oil surface. Proper drainage time could provide a stable foam layer, and could continuously form an aqueous film to isolate oxygen and achieve efficient extinguishment of flammable liquid fires. Table 6 shows the 25% drainage time of the three AFFF formulations. Figure 13 is the histogram of the 25% drainage time of three foam extinguishing agent formulations. It could be seen from Table 6 and Fig. 13 that the 25% drainage time of F-1 was the longest, followed by F-3, and the 25% drainage time of F-2 was the shortest. According to the standard technical requirements of GB15308-2006 foam extinguishing agent, the drainage time of the foam extinguishing agent should be greater than 2.5 min, and the 25% drainage time for F-1 was 182.4 s, while the 25% drainage time of the other two foam extinguishing agents was lower than that of the national standard (GB15308-2006), so only F-1 was considered after performance evaluation.
Spreading performance
Figure 14 is the numerical simulation of the foam spreading process (the red circle represents the culture dish boundary). Figure 15 is the binary image after digitizing the foam spreading process using MATLAB image processing technology. Figure 16 is the curve of the foam spreading area of foam extinguishing agent F-1 over time. In Fig. 15, it could be seen that there was no rapid spreading stage in the foam spreading process. From the beginning, the foam slowly spread on the surface of cyclohexane in a noncircular manner until the boundary of the foam layer was exposed to the edge of the dish and the foam spread was blocked. According to the definition and calculation method of spreading coefficient, foam solution could form aqueous film on the surface of fuel when foam coefficient was positive (Sheng et al. 2018d). By analyzing the surface tension of the formula, it was known that the surface tension of F-1 was only 19.14 mN/m. Therefore, there was a clear aqueous film formed on the surface of the cyclohexane. It could be seen from Fig. 16 that the foam spreading area raised with the increase of time, and the foam spreading rate decreased with the increase of time. This was due to the different viscosity of foam solution resulting in different sizes of resistance encountered during foam spreading. The greater the resistance encountered during foam spreading, the greater the vertical height of foam required to spread in the horizontal direction.
Fire extinguishing performance
Figure 17 shows the fire extinguishing process of F-1, and Table 7 shows the extinguishing time of F-1. It could be seen from the table that the fire extinguishing time of F-1 was only 31 s. According to the graph, the fire extinguishing process of foam could be divided into three stages: pre-combustion stage, foam spreading stage and flame extinction stage. In the pre-combustion stage, the flame spread rapidly to the whole fuel area and formed a relatively stable flame after 60 s; In the foam spreading stage, with the increased of foam, the foam gradually covered the surface of n-heptane, and the foam's inhibitory effect on fuel vapor was enhanced. However, the increased of total vapor pressure on the liquid surface aggravated the evaporation of fuel, resulting in the accumulation of a large number of flammable gases in the middle of the flame, which made the flame still strong. In the stage of flame extinction, the foam had completely covered the surface of n-heptane, but the foam was still unable to inhibit the fuel vapor, and continued to increase the amount of foam, so that the foam layer was thickened, the flame could not cover the entire fuel surface, and the fire gradually decreased and eventually extinguished.
Burn-back performance
Figure 18 shows the burn-back process of F-1, and Table 8 revealed the burn-back time of F-1. It could be seen from the table that the foam expansion phase of F-1 lasted for 370 s. At the 550 s, the upper foam completely disappeared and the foam entered a relatively stable phase. Moreover, the oil surface was completely burned at the 720 s. It could be seen from the diagram that the burn-back process of foam could also be divided into three stages: foam expansion stage, foam layer attenuation stage and flame reburning stage. The foam expansion stage was due to the heat radiation of the flame to the foam, which caused the gas inside the foam to expand due to heat. Meanwhile, the volume of the foam layer increased and expanded outward. In the decay stage of the foam layer, fuel vapor began to overflow from the vicinity of the foam layer and was ignited by the burn-back tank. Because the overflowing fuel vapor was directly exposed to the flame, the upper layer foam rapidly decayed. In the stage of flame reburning, there were many pores on the foam surface, and the fuel vapor spilt from the cracked pores and then ignited. Due to the decreased of foam content, the foam layer could not completely inhibit the overflow of fuel vapor, and the continuous flame started to burn on the surface of the foam layer, resulting in complete combustion of the entire oil surface.
Conclusions
In this paper, a perfluorinated branched short-chain fluorocarbon anionic surfactant with high surface activity was prepared by adopting industrialized D2 as the starting material, and then three kinds of new environment-friendly AFFF formulations (F-1, F-2, F-3) were designed by using the mixed system of the perfluorinated branched short-chain fluorocarbon anionic surfactant and CTAB as the foaming agent. The performances of foam extinguishing agents were also studied. The conclusions were as follows:
-
1.
The above synthetic route possessed the advantages of simple operation, mild reaction conditions and low cost.
-
2.
The surface tension of the benzene ring side chain anionic fluorocarbon surfactant was 20.4 mN/m and the CMC was 0.01 mol/L. Besides, the mixed system of benzene ring side chain anionic fluorocarbon surfactant and CTAB would produce synergistic effect. The results showed that the addition of CTAB could significantly reduce the amount of fluorocarbon surfactant and further decrease its surface tension. The optimum mixed ratio of this fluorocarbon surfactant and CTAB was 1:10. Meanwhile, there were many vesicles nestling together to form a bead-like distribution in the TEM images of the optimum mixed solution with the concentration of 0.0406 mol/L.
-
3.
The amount of foam in aqueous solution of low-concentration mixed system was slightly reduced and was looser than that in high concentration. Accordingly, the mixed solution with a concentration of 0.0406 mol/L was more suitable for AFFF than that with a concentration of 0.0203 mol/L. Moreover, compared with the other two foam stabilizers, sodium carboxymethyl cellulose had better foam stability and film-forming properties in this system.
-
4.
The foam performance of F-1 was better than that of F-2 or F-3. Its foam concentrate formula was CTAB 5.92%, BS-12 56%, anionic fluorocarbon surfactant with benzene ring side chain 0.8%, ethylene glycol 20%, diethylene glycol monobutyl ether 5%, urea 5%, sodium carboxymethyl cellulose 4%, and water 3.28%. The initial foam height of F-1 was 115.34 mm; the extinguishing time was 31 s; the burn-back time was 720 s; the 25% drainage time was 182.4 s. Obviously, the property of F-1 was consistent with the standard technical requirements of GB15308-2006 foam extinguishing agent and was superior to that of the traditional AFFF. Therefore, it has the broad application prospect in oil pool fire extinguishing.
References
Aihara T. Manufacture of fluorine-containing aqueous dispersions for coatings[P]. JP63314202, 1988-12-22.
Azira H, Tazerouti A, Canselier JP (2008) Study of foaming properties and effect of the isomeric distribution of some anionic surfactants. J Surfactants Deterg 11(4):279–286. https://doi.org/10.1007/s11743-008-1093-3
Bhakta A, Ruckenstein E (1997) Decay of standing foams: drainage, coalescence and collapse. Adv Colloid Interfac 70:1–124. https://doi.org/10.1016/S0001-8686(97)00031-6
Bikerman J, J. Foams (2013). Springer Science & Business Media, Russia.
Duanmu T, Wang J, Liang L et al (2011) Preparation a new high-concentration of no-PFOA type of water film extinguisher and the performance tests. Fire Technol 30(9):825–829. https://doi.org/10.3969/j.issn.1009-0029.2011.09.020
Ghosh S, Khatua D, Dey J (2011) Interaction between zwitterionic and anionic surfactants: spontaneous formation of zwitanionic vesicles. Langmuir 27(9):5184–5192. https://doi.org/10.1021/la1040147
Harkins WD, Films FA (1922) The spreading of liquids and the spreading coefficient. J Am Chem Soc 44(12):2665–2685. https://doi.org/10.1021/ja01433a001
He YH, Sun Q, Xing H et al (2019) Cationic–anionic fluorinated surfactant mixtures based on short fluorocarbon chains as potential aqueous film-forming foam. J Disper Sci Technol 40(3):319–331. https://doi.org/10.1080/01932691.2018.1468262
Hinnant KM, Giles SL, Smith EP et al (2020) Characterizing the role of fluorocarbon and hydrocarbon surfactants in firefighting-foam formulations for fire-suppression. Fire Technol 56:1413–1441. https://doi.org/10.1007/s10694-019-00932-7
Houde M, Martin JW, Letcher RJ et al (2006) Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40(11):3463–3473. https://doi.org/10.1021/es052580b
Howell JL, Lu N, Perez EW et al (2004) The preparation of primary poly-hexafluoropropylene oxide halides (poly-HFPO-CF2X where X= I, Br, Cl and F). J Fluorine Chem 125(10):1513–1518. https://doi.org/10.1016/j.jfluchem.2004.06.002
Jia X (2015) Reseach progess of aqueous film-forming foam extinguishing agent. Guangzhou Chemical Industry 24:10. https://doi.org/10.3969/j.issn.1001-9677.2015.24.009
Jia X, Bo H, He Y (2019) Synthesis and characterization of a novel surfactant used for aqueous film-forming foam extinguishing agent. Chem Pap 73(7):1777–1784. https://doi.org/10.1007/s11696-019-00730-z
Jiang N, Sheng Y, Li C et al (2018) Surface activity, foam properties and aggregation behavior of mixtures of short-chain fluorocarbon and hydrocarbon surfactants. J Mol Liq 268:249–255. https://doi.org/10.1016/j.molliq.2018.07.055
Key BD, Howell RD, Criddle CS (1997) Fluorinated organics in the biosphere. Environ Sci Technol 31(9):2445–2454. https://doi.org/10.1021/es961007c
Kishi T, Arai M (2008) Study on the generation of perfluorooctane sulfonate from the aqueous film-forming foam. J Hazard Mater 159(1):81–86. https://doi.org/10.1016/j.jhazmat.2007.09.122
Lan Y, Zou X, Gu X (2003) Synthesis and application of amphoteric phosphated surfactant. China Leather 32(15):37–40. https://doi.org/10.3969/j.issn.1001-6813.2003.15.008
Lin L, Weng T, Fang Y et al (2007) (2007) Study on liquid drainage process of compressed air foam. J Univ Sci Technol China 37(1):11. https://doi.org/10.3969/j.issn.0253-2778.2007.01.012
Lin C, Pan R, Xing P et al (2018a) Synthesis and surface activity study of novel branched zwitterionic heterogemini fluorosurfactants with CF3CF2CF2C(CF3)2 group. J Fluorine Chem 214:35–41. https://doi.org/10.1016/j.jfluchem.2018.07.015
Lin C, Pan R, Xing P et al (2018b) Study on the synthesis and properties of novel branched fluorinated surfactants. Chinese J Org Chem 38(12):3260–3269. https://doi.org/10.6023/cjoc201806014
Liu J, Xu B, Cheng J et al (2008) Special surfactants and functional surfactants (I)-Progress and future development of fluorocarbon surfactants. China Surfactant Deterg Cosmet 38(5):331–335. https://doi.org/10.13218/j.cnki.csdc.2008.05.009
Lunkenheimer K, Malysa K (2003a) Simple and generally applicable method of determination and evaluation of foam properties. J Surfactants Deterg 6(1):69–74. https://doi.org/10.1007/s11743-003-0251-8
Lunkenheimer K, Malysa K (2003b) A simple automated method of quantitative characterization of foam behaviour. Polym Int 52(4):536–541. https://doi.org/10.1002/pi.1105
Prescher D, Gross U, Wotzka J et al (1985) Zum umweltverhalten von fluortensiden. Teil 2. Untersuchung der biochemischen Abbaubarkeit. Acta Hydroch Hydrob 13(1):17–24. https://doi.org/10.1002/aheh.19850130103
Rosen MJ (1989) Surfactants and Interfacial Phenomena. J. Wiley, New York
Rosen MJ (2004) Foaming and Antifoaming by Aqueous Solutions of Surfactants. John Wiley & Sons Ltd, New York
Sebastiani D, Vilardi G, Bavasso I et al (2019) Classification of foam and foaming products for EPB mechanized tunnelling based on half-life time. Tunn Undergr Sp Tech 92:103044.1-103044.6. https://doi.org/10.1016/j.tust.2019.103044
Sha M, Pan R, Xing P et al (2015) Synthesis and surface activity study of branched fluorinated cationic (FCS), gemini (FGS) and amphoteric (FAS) surfactants with CF3CF2CF2C(CF3)2 group. J Fluorine Chem 169:61–65. https://doi.org/10.1016/j.jfluchem.2014.11.005
Sheng Y, Wu X, Lu S et al (2016a) Experimental study on foam properties of mixed systems of silicone and hydrocarbon surfactants. J Surfactants Deterg 19(4):823–831. https://doi.org/10.1007/s11743-016-1822-y
Sheng Y, Lu S, Xu M et al (2016b) Effect of Xanthan gum on the performance of aqueous film-forming foam. J Disper Sci Technol 37(11):1664–1670. https://doi.org/10.1080/01932691.2015.1124341
Sheng Y, Jiang N, Lu S et al (2018a) Fluorinated and fluorine-free firefighting foams spread on heptane surface. Colloid Surface A 552:1–8. https://doi.org/10.1016/j.colsurfa.2018.05.004
Sheng Y, Jiang N, Sun X et al (2018b) Experimental study on effect of foam stabilizers on aqueous film-forming foam. Fire Technol 54(1):211–228. https://doi.org/10.1007/s10694-017-0681-z
Sheng Y, Lu S, Jiang N et al (2018c) Drainage of aqueous film-forming foam stabilized by different foam stabilizers. J Disper Sci Technol 39(9):1266–1273. https://doi.org/10.1080/01932691.2017.1393432
Sheng Y, Jiang N, Sun X et al (2018d) Experimental study on effect of foam stabilizers on aqueous film-forming foam. Fire Technol 54(1):211–228. https://doi.org/10.1007/s10694-017-0681-z
Sheng Y, Jiang N, Lu S et al (2019) Molecular interaction and foaming property of the mixtures of hydrocarbon, fluorocarbon and silicone surfactants. J Mol Liq 296:111836. https://doi.org/10.1016/j.molliq.2019.111836
Sheng Y, Xue M, Zhang S et al (2020) Role of nanoparticles in the performance of foam stabilized by a mixture of hydrocarbon and fluorocarbon surfactants. Chem Eng Sci 228:115977. https://doi.org/10.1016/j.ces.2020.115977
Sontake AR, Wagh SM (2014) The phase-out of perfluorooctane sulfonate (PFOS) and the global future of aqueous film forming foam (AFFF), innovations in firefighting foam. J Fire Prot Eng 39(3):19–23. https://doi.org/10.12691/ces-2-1-3
Szymczyk K (2013) Properties of the ternary mixtures of fluorocarbon and hydrocarbon nonionic surfactants at the water–air interface. J Fluorine Chem 149:1–7. https://doi.org/10.1016/j.jfluchem.2013.01.036
Tamura T, Kaneko Y, Ohyama M (1995) Dynamic surface tension and foaming properties of aqueous polyoxyethylene n-dodecyl ether solutions. J Colloid Interf Sci 173(2):493–499. https://doi.org/10.1006/jcis.1995.1351
Verdia P, Gunaratne HQN, Goh TY et al (2016) A class of efficient short-chain fluorinated catanionic surfactants. Green Chem 18(5):1234–1239. https://doi.org/10.1039/C5GC02790J
Vinogradov AV, Kuprin DS, Abduragimov IM et al (2016) Silica foams for fire prevention and firefighting. ACS Appl Mater Inter 8(1):294–301. https://doi.org/10.1021/acsami.5b08653
Wang G, Yin Q, Shen J et al (2017) Surface activities and aggregation behaviors of cationic-anionic fluorocarbon-hydrocarbon surfactants in dilute solutions. J Mol Liq 234:142–148. https://doi.org/10.1016/j.molliq.2017.03.059
Weaire D, Hutzler S, Verbist G et al (1997) A review of foam drainage. Adv Chem Phys 102:315–374. https://doi.org/10.1002/9780470141618.ch5
Woodman AL, Richter HP, Adicoff A et al (1978) AFFF spreading properties at elevated temperatures. Fire Technol 14(4):265–272. https://doi.org/10.1007/BF01998385
Xiao J, Gao Z, Wang M et al (2008) Laboratory method for evaluating the performance of aqueous film-forming foam fire-extinguishing agent. Chem Res Appl 20(5):569–572. https://doi.org/10.3969/j.issn.1004-1656.2008.05.013
Xu Z, Guo X, Yan L et al (2020) Fire-extinguishing performance and mechanism of aqueous film-forming foam in diesel pool fire. Case Stud Therm Eng 17:100578. https://doi.org/10.1016/j.csite.2019.100578
Yu X, Jiang N, Miao X et al (2020) Comparative studies on foam stability, oil-film interaction and fire extinguishing performance for fluorine-free and fluorinated foams. Process Saf Environ 133:201–215. https://doi.org/10.1016/j.psep.2019.11.016
Zhai L, Zhang J, Shi Q et al (2005) Transition from micelle to vesicle in aqueous mixtures of anionic/zwitterionic surfactants studied by fluorescence, conductivity, and turbidity methods. J Colloid Interf Sci 284(2):698–703. https://doi.org/10.1016/j.jcis.2004.10.026
Zhang D, Sha M, Pan R et al (2019a) Synthesis and properties study of novel fluorinated surfactants with perfluorinated branched ether chain. J Fluorine Chem 219:62–69. https://doi.org/10.1016/j.jfluchem.2018.11.001
Zhang D, Sha M, Pan R et al (2019b) CF3CF2CF2C (CF3)2-based fluorinated surfactants with high surface activity. Chem Pap 73(6):1499–1508. https://doi.org/10.1007/s11696-019-00701-4
Zhao G, Xiao J (1995) Surface activity of dodecyltrialkylammonium bromide and its mixture with sodium dodecylsulfate. Acta Phys-Chim Sin 9:785. https://doi.org/10.3866/PKU.WHXB19950905
Zhou Q, Rosen MJ (2003) Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: the regular solution approach. Langmuir 19(11):4555–4562. https://doi.org/10.1021/la020789m
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.21908109), the Open Project of Key Laboratory of Fire Emergency Rescue Equipment of Ministry of Emergency Management of People’s Republic of China (No.2020XFZB05), the Fundamental Research Funds for the Central Universities (No.30919011272). And the properties characterization process was supported by Analysis and Testing Center of Nanjing University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, YW., Fang, JQ., Sha, M. et al. Study on foam extinguishing agents based on hydrocarbon and perfluorinated branched short-chain fluorocarbon surfactants mixed system. Chem. Pap. 75, 6241–6255 (2021). https://doi.org/10.1007/s11696-021-01788-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01788-4