Abstract
Using different reaction conditions of photosulfochlorination of n-dodecane, two samples of anionic surfactants of sulfonate type are obtained. Their micellar behavior has been already reported and the relationship between their isomeric distribution and their chemical structures and micellar behaviors have been more thoroughly explored. In this investigation, we screened the foaming properties (foaming power and foam stability) by a standardized method very similar to the Ross–Miles foaming tests to identify which surfactants are suitable for applications requiring high foaming, or, alternatively, low foaming. The results obtained for the synthesized surfactants are compared to those obtained for an industrial sample of secondary alkanesulfonate (Hostapur 60) and to those of a commercial sample of sodium dodecylsulfate used as reference for anionic surfactants. The foam formation and foam stability of aqueous solutions of the two samples of dodecanesulfonate are compared as a function of their isomeric distribution. These compounds show good foaming power characterized in most cases by metastable or dry foams. The highest foaming power is obtained for the sample rich in primary isomers which also produces foam with a relatively high stability. For the sample rich in secondary isomers we observe under fixed conditions a comparable initial foam height but the foam stability turns out to be low. This property is interesting for applications requiring low foaming properties such as dishwashing liquid for machines. The best results are observed near and above the critical micellar concentrations and at 25 °C for both the samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because they are encountered in so many important technological areas, foams have been the subject of a significant amount of discussion in the literature [1]. Foams are systems in which a gas is dispersed in a liquid. They consist of agglomerations of gas bubbles separated by liquid films [2–6]. Absolutely pure liquids do not foam. For foaming to occur, the presence of surface-active materials is required [1, 3, 5, 7]. They are present at the interfaces and are responsible for both the tendency of a liquid to foam and the persistence of the resulting dispersion of bubbles [3]. The presence of this surface-active solute produces lamellae between the gas cells of the foam that have adsorbed monomolecular films of surfactant molecules on both sides at the liquid/gas interface. These adsorbed films provide the system with the property that distinguishes foaming from non foaming systems—the ability of the former to resist excessive localized thinning of the lamella surrounding the bubbles—while general thinning of the lamella proceeds. This property, which is generally known as film elasticity, is a necessary condition for the production of foam; however, it is not sufficient for the formation of persistent foam [5]. Foaming does not occur in pure liquids because no such mechanism for the retardation of lamellae drainage or interfacial stabilization exists. All foams are thermodynamically unstable, due to their high interfacial free energy [5, 7]. Foam bubbles and their agglomerations are never in a state of equilibrium and are usually undergoing a breakdown by liquid drainage or bursting of the foam films. They may be classified by their levels of stability [2]. In considering foam stability, there are two alternative kinds of foam systems: (1) metastable or ‘dry’ or ‘permanent’ foams with lifetimes of minutes, hours or even days, and (2) unstable or ‘wet’ or ‘transient’ foams possessing short lifetimes of seconds (less than a minute) [4–9]. Certainly, these are the limiting cases and there are transitions between these alternative systems [8]. Foaming is a property inherent to all surfactant solutions. The phenomenon of foaming is encountered and made use of in nature, in industry and in domestic situations [8–10]. Important uses for foams vary widely from familiar examples of detergents, cosmetics, ore flotation, foam separations to fire extinguishing, oil recovery, and a host of physical and chemical separation techniques [1, 3, 9]. Foams are also present in many foods (ice cream, whipped topping, breads, cakes, meringues, champagne, etc.) [11]. Although foams have wide technical importance, unwanted foams may be a significant problem in many technical processes [1]. For instance, in cosmetics, its beneficial value is lowered owing to its unfavourable effects [12]. They are detrimental in wastewater treatment, oil extraction, surface coating, and automatic dishwashing [13]. So, the presence of foam in a product or process may or may not be desirable [14]. In many industrial processes, it is often useful to add surfactants that can show certain types of surface activity without producing much foam. For example, in paper-making or textile dying processes, low-foaming or non foaming surfactants are used [5]. So, foam is an important criterion in the evaluation of detergent compositions, and since the design of a product is often centered upon foaming characteristics, it is important to be able to measure this interesting phenomenon under many conditions [15]. In conclusion, characterization of foams is important in many applications, and this makes investigation of foam an active field of research [16].
This paper presents the evaluation of foaming characteristics (foaming power or foam ability and foam stability) for aqueous solutions of two samples of sodium dodecanesulfonate obtained by photosulfochlorination using sulfuryl chloride and a catalyst. The height of the foam formed and its decay with time are determined. The results obtained are compared to those obtained with a commercial sample of secondary alkanesulfonate (SAS), Hostapur 60, and to those of a well known commercial foaming surfactant, sodium dodecylsulfate (SDS). The temperature effects on the foaming power and stability are also been studied.
Experimental Procedures
The photosulfochlorination as well as the separation of the sulfochlorinated compounds from the reaction mixtures in the case of n-dodecane have already been described in detail [17–19]. The sodium dodecanesulfonates were prepared by photosulfochlorination with sulfuryl chloride. First, the n-dodecane (>99% pure; Fluka, Buchs, Switzerland) was converted to the pure phase and in the presence of solvent (chlorobenzene) at fixed conversion rates into the corresponding n-dodecanesulfonyl chlorides. The isomeric distribution of different samples was determined by gas chromatography, and the different isomers were analyzed by gas chromatography coupled to mass spectrometry with electronic impact mode [20]. The resulting sulfonyl chlorides were reacted with sodium hydroxide to give sodium dodecanesulfonates [17]. The resulting sulfonates were purified by recrystallization with ethanol (95%) and checked by IR. The purity was confirmed by active matter analysis and found to be about 98%. Surface active matter analyses were performed with Hyamine 1622 as a chemical reagent by two-phase titration [21]. The commercial SAS (Hostapur 60) was a kind gift from Clariant (France) and was used as received. Sodium dodecylsulfate (99% pure) was purchased from Sigma Chemical (USA). Distilled water was used. The influence of isomeric distribution of the synthesized anionic surface-active agents of sulfonate type on micellar behavour was studied [22]. Surface tensions of the aqueous solutions of sodium dodecanesulfonates and the Hostapur 60 were measured with a Prolabo tensiometer equipped with a platinum Wilhelmy plate [22]. The foaming properties of surfactant solutions were characterized through their foam formation (foamability) and stability. The Bartsh (shaking) and the Ross Miles (pouring test) methods are the most commonly applied simple tests for the comparison of the foamability of solutions [23]. For the determination of the foaming power of our samples and commercial surfactants solutions, the French standardized procedure NFT 73-404 [24] was used. This procedure is very similar to the Ross and Miles’ test [25]. A total of 500 mL of a solution of surfactant contained in a separating funnel was allowed to fall 45 cm through a tube of specified dimensions with a 1.9-mm-i.d. orifice onto 50 mL of the same solution contained in a cylindrical vessel maintained at a given temperature. The height of the foam produced in the cylindrical vessel was read immediately after the last drop had fallen into the graduated cylinder (initial foam height) and then again after a given amount of time (generally, 5 min). The foam height and its decay with time were determined. Each experiment was repeated at least twice. For estimating foam stability, some workers have measured the height of foam produced immediately after the mechanical agitation has stopped and after 1 min [26]. Others have expressed the foam stability as the time (t 1/2 or half life) required for the foam volume or height to decay to one half of the initial height [23, 27]. To avoid long-lasting measurements of decay of the foam height, the R 5 parameter was proposed. It represents the quotient of the foam height after 5 min to the initial foam [8, 23, 28, 29]. So the initial foam height h 0 and residual foam height, h 5, after 5 min were measured for all surfactants. The residual foam ratio R 5% was calculated as follows:
Results and Discussion
As reported in previous papers, aqueous solutions of sodium dodecanesulfonates synthesized by this new process exhibit good surface activity [17, 18, 22]. It is also well known that this kind of surfactant (SAS) is widely used in liquid laundry detergents, dishwashing liquids, shampoos, and other personal care products [9]. Contrary to end-use properties, the foaming power of aqueous solutions of surfactants (such as maximum foam height and foam stability under fixed conditions) depends on the ability of amphiphiles to be adsorbed as monolayers at gas–liquid interfaces [13]. Foaming is an important aspect of detergent products which are widely used in manufacturing and processing of various products [30]. The process of foaming could lead to favourable or detrimental results based upon the way of application and the conditions. Therefore, it is essential to evaluate the foaming properties of the synthesized sulfonates. Some studies bring out that samples of sulfonates show different isomeric distributions according to the reaction conditions of photosulfochlorination [17–20, 22]. Therefore, it was interesting to check if the isomeric distribution had or had no influence on the foaming properties. The sample A (obtained in pure phase) was richer in primary isomers than sample B (obtained in the presence of solvent) (Table 1). The foaming power of the aqueous solutions of the two samples of sodium dodecanesulfonate was measured and compared to that obtained for a commercial sample of SAS (Hostapur 60) and SDS (Table 1). Foam studies of all these surfactants were carried out at surfactant concentrations below, at, and above the critical micellar concentration (CMC) at 25 °C and either at 45 or 60 °C. The foam evolution versus the time was studied for all surfactants over a concentration range and foam formation and foam stability were determined as a function of concentration and temperature.
Foaming Power
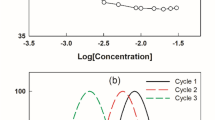
It seems reasonable to associate to the initial foam height to the foam formation, because foam generation of the Ross–Miles technique is a dynamic phenomenon involving rapid entrainment of air [29]. The evolution of foam height formed versus the concentration of sodium dodecanesulfonate (samples A and B), Hostapur 60 and SDS samples is illustrated in Fig. 1.
Figure 1 shows that foam height for all surfactants increased gradually as a function of concentration. At the CMC, there is a sudden change of the slope. This is in agreement with literature data where it is reported that in most custom foams, the surfactant concentration in the base liquid is near the CMC [3, 7, 9]. For all the studied surfactants, the foaming power increased with the concentration, even beyond the CMCs. This fact has been observed by other authors, [13] contrary to the usually found statement that the amount of foam produced by a surfactant under a given set of circumstances will increase with its bulk concentration up to a maximum, which occurs somewhere near the CMC [1, 5, 14]. In fact, it seems to be interesting to use foaming agents at concentrations higher than their CMC [13]. The lower values of initial foam height below CMCs were more pronounced for the synthesized sulfonates, and this can be explained by the fact that the air water interfaces do not contain sufficient amounts of surfactant to stabilize the foam [26]. The results obtained show that the two samples A and B possess foaming properties which compare well to those of Hostapur 60 having good foaming power which is especially pronounced in soft water. SDS was used under the same conditions as a reference. As expected, the highest foamability was observed for SDS, corroborating that SDS has a good foaming power [31] and forms metastable foams [23]. Compared to the SDS case, the foam height formed by samples A and B was slightly lower, but still quite good. Near and above the respective CMC, sample A (rich in primary isomers) showed a better foaming power than sample B (rich in secondary isomers). It is reported that foam formation is influenced by the adsorption of the foaming agent at the air–water interface [12], to produce foam; the required minimal concentration of a surfactant must correspond to the formation of a saturated monolayer at the bubbles surface. As well known, the equilibrium adsorption of surfactant may be estimated from the experimental surface tension isotherm with the help of the Gibbs equation [1].
The adsorption amount of surfactant reaches it maximal value Гm at some concentration C m. The value of C m may be identified with the CMC of the surfactant and serves to quantify the potential foaming ability of surfactants [31]. Thus, the CMC of a surfactant is a good measurement of its efficiency as a foaming agent; the lower the CMC, the more efficient the surfactant as a foamer [1, 5, 14, 16]. In the case of sample A, the surfactant had a lower CMC and was packed more efficiently at the interface according to the Г value which was greater than that of sample B (3,04 × 10−10 mol/cm2 and 2,83 10−10 mol/cm2, respectively) [22]. An increase in adsorption (number of molecules per unit surface Г) increased the foamability of the surfactant [32]. Thus, sample A exhibited a better foaming power than sample B.
Foam Stability
Foams are thermodynamically unstable, and their relative stability is affected by factors such as drainage, disproportionation and /or coalescence. It is the property of the two air/water interfaces of the thin films which makes or breaks a foam. However, foam stabilization requires different surface properties [11]. Control of foam stability is important in all applications, whether degradation of custom foam is to be minimized or whether excessive foaming is to be prevented. In all cases, the time evolution of the foam structure provides a natural quantifying foam stability [3]. The changes in foam height (h) as a function of time (t) are shown in Figs. 2, 3, 4 and 5 for the aqueous solutions of sodium dodecanesulfonates (samples A and B), Hostapur 60 and sodium dodecyl sulfate samples at concentrations below, at and above the CMC range for each surfactant
As shown on these figures, the general aspect was similar to that obtained for SDS surfactant with other methods [23] indicating a good foam stability in some cases. As was suggested by some workers, dry or metastable foams seem to show two different regimes of foam decay, one during the initial stage, immediately after foam formation, followed by a second one of comparatively slow drainage [8]. For samples A and B and for SDS, substantial differences in foam height variation with time could be seen in the case of low surfactant concentration below CMCs (see Figs. 2, 3 and 4). Conversely a very small change was seen at higher concentrations above CMCs due to the high foam stability at these concentrations [16]. The lower values of initial foam height below the CMC have been explained by the fact that the air water interfaces do not contain sufficient amounts of surfactant to stabilize the foam. For the same reason, the foam stability is low for these surfactants [26]. The foam stability of the solutions can be more reflected by the residual foam height ratio R 5. Foams with R 5 50% can be considered as metastable; whereas lower values of R 5 indicate foams of low stability [8, 23]. In Fig. 6, R 5 values of aqueous solutions of sodium dodecanesulfonates (samples A and B), the Hostapur 60 and SDS were plotted as a function of their concentrations below CMCs (CMC/2), at CMCs, and above CMCs (2 × CMC, except for Hostapur, it is 5 × CMC).
The R 5 parameter depended strongly on surfactant concentrations for the sample A, sample B and SDS where they exhibited higher foam stability at CMC. It has been reported that, the adsorption amount of surfactant reaches it maximum value Гm at CMC [31]. For Hostapur 60 the foam stability is independent of concentration. High foam stability was obtained for SDS (R 5 > 80% even below CMCs), and this is in agreement with literature data [23]. Sample A had not only a higher foam height than sample B, but it also exhibited the most stable foam. It is reported that the initial foam height and stability do not have necessarily the same trend [13]. Indeed, for sample B (sulfonates rich in secondary isomers), a comparatively high initial foam volume was observed (Fig. 1), but the foam stability was low, as indicated by the quick decrease in the foam height. This is probably due to the fact that the hydrophilic group shifts to a more central position in the molecule which causes an increase in the CMC of the surfactant with a resulting decrease in its efficiency as a foaming agent [5]. Indeed, the CMC value of sample B was higher [22]. It appears that surfactant CMC can be used as a guide in predicting the foaming ability of a substance, but not necessarily the persistence of the associated foam [1, 5, 14]. It is also reported that surfactant with a large area/molecule at the liquid–air interface, forms a loosely packed noncoherent film with weak cohesive forces that produce an unstable foam [5]. However, as reported previously, the area/molecule was larger for sample B (poor in primary isomer) [22]. The increase in area/molecule and therefore the production of a weaker cohesive force at the surface, caused a lower foamabilty [12]. According to the criterion that foams showing values of the R 5 parameter higher than 50% can be considered as metastable, while lower R 5 values indicate that the foams are of low stability [8, 23], it is interesting to note from Fig. 6, that for both samples A and B, at concentrations near or above CMC, the values were roughly 82 and 54%, respectively. Consequently, formed foams are stable (metastable or dry) in this case, whereas at lower concentration (below CMC) low stability foams (transient or temporary wet or humid) are formed. Hence, dodecanesulfonates exhibit a better foaming performance at concentrations equal to or higher than the CMC.
Temperature Effect
For all surfactants studied, we also determined the foaming power (Figs. 7, 8 and 9) and foam stability (Figs. 10, 11 and 12)] at three temperatures 25, 45 and 60 °C at concentrations below, at, and above CMC.
As reported in the literature [13], the variations as a function of temperature in most cases are almost monotonous for all surfactants. As shown by Figs. 7, 8, 9, 10, 11, 12, the foaming power and foam stability were lower at 45 and 60 °C than at 25 °C for all surfactants below the CMCs. It is also shown that for SDS below the CMC, the foam staility was poor (Fig. 10), the R 5 value was 48%, and according to Lunkenheimer and Malysa criterion [23], the foam may be considered unstable.
At and above CMCs, we observed a decrease in the foaming properties with an increase in temperature for samples A and B, while for Hostapur 60 there was a maximum at 45 °C. It can be concluded from the Figs. 7, 8, 9, 10, 11 12, that both the samples A and B generally had the same behavior, and the best foaming properties for the two samples were at 25 °C; their efficiency as foaming agents decreased with temperature increase. This is the case for SDS. The best values of foaming power and foam stability for the Hostapur 60 were at 45 °C. These results are in good agreement with literature data, where it is reported that in distilled water at room temperature, the best foaming anionic surfactants agents are those having a C12–C14 alkyl chain, whereas at 40 and 90 °C, the anionic surfactants with hydrophobic group in C16 and C18 respectively exhibit the best foaming properties [5, 32]. Finally, the studied sulfonates (samples A and B) showed good foaming power comparable to that of commercial surfactants. However, the sample A rich in primary isomers showed more stable foams than the sample rich in secondary isomers. The best results for the two samples were obtained at concentrations above the CMC and at 25 °C.
Abbreviations
- SAS:
-
Secondary alkanesulfonates
- SDS:
-
Sodium dodecylsulfate
- CMC:
-
Critical micellar concentration
- IR:
-
Infrared
- CR:
-
Conversion rate of n-dodecane
- R1SO2Cl:
-
Primary isomer
- R2SO2Cl:
-
Secondary isomers
- exp:
-
Experimental data
References
Myers D (2006) Surfactant science and technology, 3rd edn. Wiley, New Jersey
Sosis P (1975) Detergency: theory and test methods. In: Culler WG, Davis RC (eds) Surfactant sciences series, vol 5. Marcel Dekker, New York
Kroschwitz JI (1994) Kirk Othmer’ encyclopedia of chemical technology, 4th edn. Wiley, New York
Holmberg K, Johnson B, Kronberg B, Lindmann B (2003) Surfactants and polymers in aqueous solution, 2nd edn. Wiley, UK
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley, New Jersey
Tadros ThF (2005) Applied surfactants principles and applications. Wiley, VCH Verlag GmbH, Weinheim
Pugh RJ (1996) Foaming, foam films, antifoaming and defoaming. Adv Colloid Interface Sci 64:67–142
Lunkenheimer K, Malysa K (2003) A simple automated method of quantitative characterization of foam behaviour. Polymer Int 52:536–541
Holmberg K, Shah DO, Schwuger MJ (2002) Handbook of applied surface and colloid chemistry, vol 2. Wiley, UK
Domingo X, Fiquet L, Meijer H (1992) Foam ability/stability of surfactants. Tenside Surf Det 29(1):16–22
Rodríguez Patino JM, Conde JM, Linares HM, Pedroche Jiménez JJ, Carrera Sánchez C, Pizones V, Millán Rodríguez F (2007) Interfacial and foaming properties of enzyme-induced hydrolysis of sunflower protein isolate. Food Hydrocoll 21(5–6):682–693
Hong-Rong Wang, Keng-Ming Chen (2006) Preparation and surface active properties of biodegradable dextrin derivative surfactants. Colloids Surf A 281:190–193
Unda Carbott de Escobar T, Canselier JP (2000) Foaming power of anionic-nonionic surfactant mixtures in aqueous solution. Cahiers de Formulation 9:139–153
Myers D (1999) Surfaces, interfaces, and colloids: principles and applications, 2nd edn. Wiley, New York
Spangler W (1964) Dynamic foam test. J Am Oil Chem Soc 41:300–306
Powale RS, Andheria AP, Maghrabi SS, Bhagwat SS (2005) A novel method for evaluating foam properties. J Dispersion Sci Technol 26:597–603
Tazerouti A, Azira H (2000) Synthesis and Surface Activity of Dodecanesulfonates. In: Comité Espagnol de la Detergencia, Tensioactivos y Afines (CED) (eds) Communicaciones Presentadas XXX Jornadas Del Comité de la Detergencia, Barcelona, Spain, pp 257–263
Azira H, Assassi N, Tazerouti A (2003) Synthesis of Long-Chain Alkanesulfonates by Photosulfochlorination using Sulfuryl Chloride. J Surfactant Deterg 6:55–59
Assassi N, Azira H, Tazerouti A (2006) Recent Progress in the Manufacture of Alkanesulfonates: Photosulfochlorination of single-chain Length (C12–C16) n-Alkanes Using SO2Cl2 at high conversion rates in pure phase and in the presence of solvent. J Surfactant Deterg 9:249–257
Assassi N, Tazerouti A (2005) Analysis of chlorinated, sulfochlorinated and sulfonamide derivatives of n-tetradecane by gas chromatography/mass spectrometry. J Chromatogr A 1071:71–75
Longman GF (1975) The analysis of detergents and detergent products. Wiley, London
Azira H, Tazerouti A (2007) Micellar behavior of anionic surfactants with sulfonate function in aqueous solutions. J Surfactant Deterg 10:185–190
Lunkenheimer K, Malysa K (2003) Simple and generally applicable method of determination and evaluation of foam properties. J Surfactant Deterg 6:69–74
Recueil des normes françaises (1986) agents de surface, détergents et savons, AFNOR, Paris-la défense
Ross J, Miles GD ASTM Standard Method D 1173–53, Philadelphia Pa., 1953, reapproved 1970
Piispanen PS, Persson M, Claesson P, Norin T (2004) Surface properties of surfactants derived from natural products. Part 2: structure/property relationships—foaming, dispersion, and wetting. J Surfactant Deterg 7:161–167
Schick MJ, Beyer EA (1963) Foaming properties of nonionic detergents. J Am Oil Chem Soc 4:66–68
Rosen MJ, Zhu ZH (1988) Synergism in binary mixtures of surfactants. 7. Synergism in foaming and its relation to other types of synergism. J Am Oil Chem Soc 65:663–668
Tamura T, Kaneko Y, Ohyama M (1995) Dynamic surface tension and foaming properties of aqueous polyoxyethylene n-dodecyl ether solutions. J Colloid Interf Sci 173:493–499
Goon P, Bhirud RG, Kumar VV (1999) Detergency and foam studies on linear alkylbenzene sulfonate and secondary alkyl sulfonates. J Surfactant Deterg 2:489–493
Babak V (2000) Foams and colloid aspects of formulation and properties. Cahiers de Formulation 9:18–57
Salager JL, Andérez JM, Forgiarini A Influence de la formulation sur les mousses, L’actualité chimique, Avril 1999: 10–21
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Azira, H., Tazerouti, A. & Canselier, J.P. Study of Foaming Properties and Effect of the Isomeric Distribution of Some Anionic Surfactants. J Surfact Deterg 11, 279–286 (2008). https://doi.org/10.1007/s11743-008-1093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-008-1093-3