Abstract
The application of conventional aqueous film-forming foam (AFFF) has been severely restricted due to the serious environmental hazard caused by the key component, fluorocarbon surfactants. Environmental-friendly fluorine-free firefighting foams need to be developed urgently. In this study, five silicone surfactants are chosen as key component to prepare fluorine-free firefighting foams. The aqueous solution properties of the fluorine-free firefighting foams are studied in details, including surface tension, interfacial tension, spreading property, viscosity and foaming ability. Foam drainage and foam spread on heptane surface are analyzed. Fire extinguishing and burn-back performance of fluorine-free foams is evaluated based on a small-scale standard method. Particularly, fire extinguishing and burn-back performance of a commercial AFFF is also evaluated as a comparison. Results show that fluorine-free foams cannot form aqueous film on cyclohexane surface, no matter whether spreading coefficient is greater than zero or not. Fluorine-free foams exhibit much better foam stability but worse foam spread property than commercial AFFF. Not all the fluorine-free foams containing silicone surfactant performed as well as AFFF containing fluorocarbon surfactant. Only fluorine-free foam containing silicone surfactant of OFX-5211 shows better fire extinguishing and burn-back performance than AFFF. The higher efficiency of fluorine-free foam in fire extinguishing and burn-back should be attributed to the stronger foam stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Liquid fuel (diesel, gasoline, aviation kerosene, crude oil, etc.) has been extensively used in both military and civilian areas. In the process of production, transportation, storage, and application of these liquid fuels, the accidental leakage can lead to liquid fire upon the ignition sources [1,2,3,4]. Liquid fuel fire has the characteristics of faster burning rate, longer duration and stronger heat radiation hazard, which possibly leads to serious damage to the surrounding environment [5,6,7,8,9,10]. Besides, liquid fuel fire can produce a lot of harmful materials such as sulfur oxides (SOx), nitrogen oxides (NOx), carbon monoxide (CO) and some hydrocarbons, which can lead to serious environmental pollution. Therefore, the study of clean and efficient fire extinguishing agent for liquid fuel fire is significant to decrease loss caused by liquid fuel fire and promote the safe application of energy.
Aqueous film-forming foam (AFFF), a kind of firefighting foams, is a concentrate of fluorocarbon surfactants, hydrocarbon surfactants, polymers, organic solvents, and water. It was supposed to be the most efficient fire extinguishing agent used to fight liquid fuel fire. Its high effectiveness in fire extinguishment is provided by not only foam layer but also an aqueous film layer on the surface of liquid fuel upon AFFF application. Particularly, the formation of aqueous film depends on the high surface activity of fluorocarbon surfactants, the key component in AFFF. The traditional AFFF has been widely used to fight liquid fuel fire for both military and civilian uses efficiently so far. Many properties of traditional AFFFs have been studied to understand their contribution to fire suppression, including film-forming property [11], rheological property [12], foam drainage [17, 18], spreading property [13], and fire extinguishing and burn-back performance [14,15,16]. However, the application of traditional AFFF has been severely restricted due to the serious environmental hazard caused by the key component, fluorocarbon surfactants [19,20,21]. Fluorine-free firefighting foams suitable for liquid fuel fire are being developed. Up till now, no commercially available formulation which can be used to substitute for conventional AFFF has been reported.
Silicone surfactants exhibit excellent wettability, leveling, spreading property, and higher surface activity than hydrocarbon surfactants [22]. Some studies on environmental-friendly fluorine-free firefighting foam based on the mixture of hydrocarbon and silicone surfactants have been conducted recently [23,24,25]. Several properties of fluorine-free firefighting foams have been characterized [11, 13, 26,27,28]. These studies indicated that it is great potential to develop environmental-friendly firefighting foam formulations based on the mixture of hydrocarbon and silicone surfactants. However, these studies just focused on dynamic surface and interfacial tension, seal-ability properties, foam degradation and bubble coarsening of fluorine-free firefighting foams. The key property, fire extinguishing and burn-back performance of fluorine-free firefighting foams, is still not focused. The difference between fluorine-free foams containing silicone surfactants and conventional AFFF in fire extinguishing performance remains uncertain.
In the present study, fluorine-free firefighting foam formulations based on the mixture of hydrocarbon and silicone surfactants are prepared for study on the substitute for conventional AFFF. The key properties, including film-forming, foam drainage, foam spread, fire extinguishing and burn-back performance, are deeply analyzed and compared with a kind of commercial fluorinated AFFF. The results obtained from this study can provide guidance for the development and application of experimental-friendly fluorine-free firefighting foam suitable for liquid fuel fire.
2 Experimental Section
2.1 Materials
Five silicone surfactants, OFX-5211, OFX-0193, Silok-2235, Silok-2232 and Tegopren-6950, are chosen to prepare fluorine-free firefighting foam. OFX-5211 and OFX-0193 were purchased from DOW CORNING. Silok-2235 and Silok-2232 were purchased from Guangzhou Silok Polymer Co. Ltd. Tegopren-6950 was purchased from Shanghai Honestever Co. Ltd. The critical micelle concentration (cmc) of aqueous solutions and the corresponding surface tensions at cmc of the five silicone surfactants were shown in Fig. 1. OFX-5211 shows the minimum cmc, and Silok-2235 shows the best ability to lower the surface tension of water.
Five fluorine-free firefighting foam concentrates containing OFX-5211, Silok-2235, OFX-0193, Tegopren-6950 and Silok-2232 are prepared, denoted as FF-1#, FF-2#, FF-3#, FF-4#, and FF-5#, respectively. The components of the five foam concentrates and their concentration are listed in Table 1. Besides, a commercial fluorinated foam (AFFF) was purchased as a comparison from Yangzhou Jiangya fire equipment co. LTD. Foam solutions used in this study are prepared by mixing foam concentrates with fresh water according to the volume ratio of 3:97.
2.2 Apparatus and Methods
2.2.1 Properties of Foam Solutions
Surface tension and interfacial tension of AFFF solutions are measured using a QBZY-1 full automatic surface tension meter in a 20°C water bath. The dynamic viscosity of foam solutions is measured with a DV-1 digital viscometer. Each experiment is repeated three times. The spreading coefficient is calculated by Eq. (1). It is a standard method for spreading coefficient provided by many international standards listed in Ref. [29].
where S is spreading coefficient, \(\gamma_{o}\) is surface tension of liquid fuel, \(\gamma_{w}\) is surface tension of foam solution, \(\gamma_{ow}\) is interfacial tension between liquid fuel and foam solution.
2.2.2 Foam Generation, Drainage and Spread Property
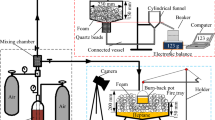
The experimental system established in this study can be used to perform foam generation, foam drainage and foam spread tests. A compressed-air foam generation system consisting of compressed air cylinders, gas and liquid flow meters, mixing chamber, valves and pipes was used to create foam, as illustrated in Fig. 2. The mixing chamber was a cylindrical container made of quartz glass with inner diameter of 80 mm and length of 200 mm, and filled up with spherical quartz beads (Diameter: 3 mm). Foams are generated by mixing foam solution with compressed air at the ration of 15 L/h foam solution and 75 L/h compressed air in the mixing chamber. The similar experimental system was described in detail elsewhere [29]. Foam drainage apparatus was established based on the law of connected vessel. Several similar foam drainage apparatuses were used in previous studies [14, 18, 29]. Foam solution is added into the connected vessel to make sure that the same level of foam solution on the surfaces at both ends of the connected vessel is reached before the test. The instantaneous mass of liquid drained out of foam can be obtained by use of this apparatus. For all the experiments of drainage, foam is collected by foam container for 90 s at the same flowrate. The process of foam spread on a circle fire tray filled up heptane is recorded by camera, and then numerical analysis of foam spread property is conducted by use of the image processing technique of MATLAB. Similar foam spread apparatus was used in previous study [13]. Particularly, in order to make sure the accuracy of experimental apparatus, each experiment of foam drainage and foam spread was repeated at least three times until three complete overlapped curves of drainage or spread were obtained.
2.2.3 Fire Extinguishing and Burn-Back Tests
Fire extinguishing and burn-back tests were conducted based on the Chinese small-scale testing standard GB15308-2006. The testing method provided by GB15308-2006 is similar to many international standard methods listed in Ref. [29]. Briefly, the test method is the following: the 9 L n-heptane in fire tray is ignited, and ignition time is recorded as 0 s. Foam is applied to the fire tray by use of a stainless steel pipe with inner diameter of 15 mm from the center of the fire tray at a rate of 750 g/min after 60 s pre-burning. The time when the flame is extinguished is recorded as fire extinguishment time. The foam application is stopped after 180 s. The 1 L n-heptane in the burn-back pot is ignited at 240 s. The height of the burn-back pot is dynamically adjusted so that the pot lip is kept at the same level with the foam surface. The duration from the time of igniting the burn-back pot to the moment when the entire fire tray is eventually covered again is recorded as 100% burn-back time. All the fire extinguishing and burn-back tests were repeated at least three times in order to make sure that the error of fire extinguishing time and burn-back time is no more than 3%.
3 Results and Discussion
3.1 Aqueous Solution Properties
Aqueous solution properties of fluorine-free foams and AFFF are listed in Table 2. The range in surface tension of aqueous solutions of fluorine-free foams is from 20mN/m to 30mN/m, and the interfacial tension of all the foam solutions is below to 3mN/m. For fluorine-free foams, FF-2# has the minimum value of both surface tension and interfacial tension, while FF-3# shows the maximum value of both surface tension and interfacial tension. Apparently, the aqueous solutions of AFFF exhibits the lower surface tension than that of fluorine-free foams, indicating that the aqueous solution of AFFF has the higher surface activity. This should be attributed to the higher ability of fluorocarbon surfactant in AFFF to lower surface tension of water. But, for interfacial tension, FF-2# and FF-5# exhibit the lower value than AFFF, indicating that Silok-2235 and Silok-2232 have the stronger ability to lower interfacial tension compared with other silicone surfactants.
The spreading coefficients of aqueous solutions of fluorine-free foams on cyclohexane are negative except FF-2#, indicating that theoretically only FF-2# among all the aqueous solutions of fluorine-free foams can form aqueous film on the surface of liquid fuel. However, for the aqueous solutions of all the fluorine-free foams, no obvious aqueous film was observed on the surface of cyclohexane during film-forming test, even though the spreading coefficient of FF-2# is positive. Similar results occurred in some previous studies [23, 30,31,32]. Hetzer et al. thought that a positive spreading coefficient (S > 0) is just only a necessary but not a sufficient requirement for the water film formation [23]. This phenomenon was ascribed to non-equilibrium surface tension effects in the aqueous layer [32]. The viscosity of aqueous solutions of all the fluorine-free foams is near to each other and higher than that of AFFF.
3.2 Properties of Foam Containing Different Silicone Surfactants
3.2.1 Foaming Ability of Foam Solutions
Figure 3 shows the initial foam height of aqueous solutions of fluorine-free foams and AFFF. Generally, fluorine-free foams have an approximate value of initial foam height to AFFF. Foaming ability of fluorine-free foams is affected slightly by different silicone surfactants. Specifically, the initial foam height of FF-1#, FF-3#, FF-4# and FF-5# shows slightly higher than that of AFFF, while FF-2# is lower than that of AFFF, indicating that Silok-2235 tends to reduce foaming ability of fluorine-free foam. It should be pointed out that FF-2# containing Silok-2235 has the lowest surface tension and interfacial tension, but it shows the worst foaming ability. This result is not in accordance with Rosen’s study result [33]. Rosen et al. indicated that lower surface tension promotes foaming of surfactant solutions. This maybe resulted from defoaming effect caused by the interaction between Silok-2235 and other components in fluorine-free foam.
3.2.2 Foam Drainage Property
Figure 4 shows the variation in mass of liquid drained out of foams versus time. Obviously, the drainage mass curves of fluorine-free foams are lower than that of AFFF, indicating that the fluorine-free foams show a much higher stability than AFFF. This can be attributed to the addition of high-efficiency foam stabilizers into fluorine-free foams besides silicone surfactant. In terms of the fluorine-free foams, the drainage mass curves of foams containing different silicone surfactants show a similar change trend generally, while the difference among the curves in details still exists. The drainage mass curve of FF-2# is obviously higher than that of others. It is at 96 s that the drainage mass of FF-2# increases rapidly after a slow increase, but the rapid growth in drainage mass of FF-1#, FF-3#, FF-4# and FF-5# occurs until 300 s. Besides, the drainage mass curve of FF-2# is apparently higher than other fluorine-free foams within equal times, implying that the foam stability of FF-2# is the worst among fluorine-free foams. The drainage mass curve of FF-1# is obviously lower than that of FF-2# but slightly higher than that of FF-3#, demonstrating that the foam stability of FF-1# is slightly stronger than that of FF-3# and obviously weaker than that of FF-2#. The drainage mass curves of FF-4# and FF-5# almost overlap each other completely and slightly lower than that of FF-3#, implying that FF-4# and FF-5# have the strongest stability among fluorine-free foams. The results indicate that the five silicone surfactants show different ability to stabilize fluorine-free foam. Tegopren-6950 and Silok-2232 exhibit the relative better ability to stabilize foam, while Silok-2235 is the worst. Foam stability was affected by silicone surfactants due to the change of surface tension and the interaction between molecular of silicone surfactant and other components with addition of silicone surfactants into foam solutions.
Drainage rate is the derivative of the drainage mass of foam versus time. The drainage rates of the firefighting foams are analyzed systematically, as shown in Fig. 5. Significant difference exists among the drainage rate curves of fluorine-free foam and AFFF. The drainage rate of AFFF decreases rapidly following a sharp increase, and then gradually decreases to zero after reaching CPD (critical point of drainage). The explanation about CPD in details can be found in previous references [34]. For fluorine-free foams, the drainage rate curve of FF-2# shows significant difference from other foams. At approximate 90 s, the drainage rate of FF-2# increases dramatically and reaches the maximum value 0.41 g/s. After a short decrease, a relative stable value of drainage rate is subsequently reached and lasted approximate 200 s when the value of drainage rate of FF-2# drops to 0.3 g/s. Then, drainage rate keeps decreasing gradually. CPD occurs on the drainage rate curve of FF-2# at 655 s when drainage rate decreases to 0.23 g/s. The drainage rate of FF-2# obviously changes no longer but is not zero after 1800 s. The drainage rate curves of FF-1#, FF-3#, FF-4# and FF-5# almost overlap each other completely. The drainage rate curves of them increases rapidly after keeping a relative stable for more than 200 s from 0 s, and then reach relative stable again after the maximum value occurred. The time lasted for the second relative stable stage of drainage rate curves of FF-1#, FF-3#, FF-4# and FF-5# are 550 s, 660 s, 705 s and 720 s, respectively. It should be pointed out that no CPD occurs on the drainage rate curves of the four foams, which should be ascribed to their smaller value of the maximum drainage rate. Note that, the drainage rate curves of FF-1#, FF-3#, FF-4# and FF-5# are higher than that of AFFF and FF-2# at later stage of foam drainage, which is mainly attributed to the higher liquid fraction in the fluorine-free foams of FF-1#, FF-3#, FF-4# and FF-5# at later stage [33].
We can conclude from the analysis above that silicone surfactants with different molecule structure resulted in the difference in stability of fluorine-free foams. In terms of the five silicone surfactants used in this study, Tegopren-6950 and Silok-2232 shows better ability to stabilize foam compared with other silicone surfactants. Silok-2235 shows the worst ability to stabilize foam though it has the strongest ability to lower the surface tension of water and interfacial tension between water and liquid fuel.
3.2.3 Foam Spread Property
Figure 6 shows the variation in spread area of the firefighting foams on the fire tray versus time. The time required for firefighting foams to cover the whole fire tray is denoted as foam spread time (FST). AFFF exhibits the shortest FST 34 s, which is obviously lower than that of fluorine-free foams. The spread area curve of AFFF exhibits three stages, including rapid increase, linear increase and slow increase, while the spread area curves of fluorine-free foams just show two stages, linear increase and slow increase. The main reason why AFFF spreads faster is that the aqueous solution of AFFF has higher surface activity and lower viscosity. For fluorine-free foams, the FSTs of FF-1#, FF-2#, FF-3#, FF-4# and FF-5# are 63 s, 48 s, 67 s, 63 s and 58 s, respectively. Apparently, FF-2# spreads on heptane surface faster than other foams. The spread area curves of FF-1#, FF-3#, FF-4# and FF-5# almost overlap each other. The addition of Silok-2235 leads to the fastest spread of FF-2# on heptane surface, which is attributed to the stronger ability of Silok-2235 to lower surface tension of water.
The variation in the instantaneous spread rate of foams on heptane surface versus time is plotted in Fig. 7. Similar change trends were observed on the spread rate curves of the fluorine-free foams. For all the fluorine-free foams, the spread rates keep a relative stable after a transient increase, and then gradually decrease to zero. FF-2# shows the maximum value of foam spread rate compared with other fluorine-free foams. But, more fluctuations with bigger amplitude occurred on the foam spread rate curve of FF-2#. Notably, the change trends of AFFF in foam spread rate curve is obviously different from fluorine-free foams. The maximum value of the spread rate of AFFF reaches 0.026 m2/s, which is much higher than that of fluorine-free foams.
The faster spread of AFFF should be attributed to its lower viscosity, smaller surface tension and greater spreading coefficient. Nonetheless, for fluorine-free foams containing different silicone surfactants, FF-1# and FF-4# has lower viscosity than FF-2#, but they spread more slowly than FF-2#. These results indicated that the viscosity is not the main reason for the different spread rate of fluorine-free foams. The spreading coefficient and surface tension are the key parameters resulting in different spreading rate of fluorine-free foams.
3.3 Fire Extinguishing and Burn-Back Performance of Foams
3.3.1 Process of Fire Extinguishing and Burn-Back
Figure 8 shows the process of fire extinguishing and burn-back of FF-1#. Other firefighting foams exhibit the same process of fire extinguishing and burn-back. Heptane is ignited at 0 s. After 60 s, foam is supplied into fire tray, and flame is gradually controlled with foam spread on heptane surface. Flame height decreases rapidly once foam covers the whole fire tray, and finally flame is extinguished with lasting supply of foam, as illustrated in Fig. 8a.
Figure 8b shows the process of burn-back of fluorine-free foam FF-1#. Obvious expansion of fluorine-free foam caused by heated air in foam occurred at the early stage of burn-back process, denoting as foam expansion stage. Then, the upper surface of foam layer declined gradually as burn-back experiment went on, denoting as foam attenuation stage. No fuel vapor or “ghost flame” was observed during foam decay. And no pores were observed even though the thin foam blanket was left on the surface of heptane. This is different from the burn-back process of AFFF described in Ref. [29]. At 700 s, foam blanket can still inhibit fuel vapor effectively. As time went on, boiling combustion of the heptane in burn-back pot occurred, resulting in a rapid decrease in foam blanket. Small flame caused by the ignition of fuel vapor escaping from beneath the foam layer occurred in fire tray, and cannot be effectively inhibited by foam blanket. On the contrary, the small flame evolved into bigger flame, and finally led to the entire burn-back of heptane in fire tray, denoting as flame burn-back stage. The phenomenon is different from the burn-back process of AFFF described in Ref. [29]. At the process of burn-back test of AFFF, small flame was extinguished rapidly by foam once it occurred in foam layer, and a thin foam layer can still inhibit small flame effectively. Burn-back test of AFFF can last a relative long time even though small flame occurred in foam layer. The main reason is that fluorine-free foams have worse spread property and lower self-sealing capacity than AFFF.
3.3.2 Fire Extinguishing Time and Burn-Back Time
Fire extinguishing and burn-back experiments are conducted based on the analysis results of foam drainage and spread. It should be pointed out that the evaluation of fire extinguishing and burn-back performance of FF-3# and FF-5# are not conducted because the curves of both foam drainage and foam spread of them almost overlapped with FF-4# completely. The fire extinguishing and burn-back performance of the four firefighting foams, FF-1#, FF-2#, FF-4# and AFFF, were tested. The results are listed in Table 3.
All the foams can extinguish heptane fire rapidly and resist on the thermal radiation of flame for more than 600 s, indicating that the fire extinguishing and burn-back performance of the fluorine-free foams prepared in this study meet the requirements of GB15308-2006. Fire extinguishing time of FF-1# and FF-2# is lower than that of AFFF, while FF-4# shows longer fire extinguishing time than AFFF. For burn-back performance, FF-1# and FF-4# are better than AFFF, but FF-2# is worse than AFFF.
Notably, the times fluorine-free foams lasted at foam expansion stage of burn-back tests are nearly equal to that of AFFF. But the times fluorine-free foams lasted at foam attenuation stage of burn-back test are much longer than that of AFFF. The times fluorine-free foams lasted at flame burn-back stage of burn-back tests are much shorter than that of AFFF. This should be attributed to greater stability and lower self-sealing capacity. At foam attenuation stage, fluorine-free foams have high stability and few liquids drained out of foams, the spill of fuel vapor can be stopped by the “wet” foam for a longer time. At flame burn-back stage, small flame can be extinguished rapidly by AFFF due to its good self-sealing capacity. But fluorine-free foams cannot extinguish small flame because of their worse self-sealing capacity, leading to the much shorter time of flame burn-back stage than AFFF and the rapid complete burn-back of fire tray.
3.3.3 Mechanism of Fire Extinguishing and Burn-Back of Fluorine-Free Foam
The fire extinguishing mechanism of traditional AFFF is insulation of liquid fuel from oxygen by foam layer and aqueous film, as described in Fig. 9a. During AFFF extinguishing fire, a large amount of liquid drained out of foams due to the relative bad foam stability, and the liquid drained can form an aqueous film on the surface of liquid fuel relying on the very low surface tension of the liquid containing fluorocarbon surfactant. But the excessive liquid drained will sink to the bottom of fire tray after seal-healing aqueous film formed due to the limitation of spreading amount. The ability of foam layer to isolate fuel vapor decreased with the rapid loss of liquid drained from foams.
For the fluorine-free foams, the fire extinguishing mechanism is different from AFFF, which can be explained by Fig. 9b. Few liquids drained out of foam during fire extinguishment of fluorine-free foams due to their high stability provided by hydrocarbon surfactants and mixtures of foam stabilizers and rapid foam spread provided by silicone surfactant. Most of liquids still exist in foams and fuel vapor cannot penetrate foam blanket containing lots of liquid. The foam with high liquid content spread and covered the whole fire tray rapidly, resulting in the rapid extinguishment of flame. Thus, fluorine-free foams can extinguish flame of heptane fire effectively though no aqueous film formed on heptane surface. During burn-back, the declination in the upper surface of foam layer is attributed to thermal radiation of flame from burn-back pot. A large amount of heat from burn-back pot is absorbed by the liquid in the foam blanket. Also, fuel vapor is inhibited high-efficiently by foam containing lots of liquid. Besides, the liquid in foam blanket can continuously cool the surface of fuel of fire tray. The combined effects of these actions resulted in the good burn-back performance of fluorine-free foam.
Essentially, the fluorocarbon surfactant in the traditional AFFF is the key component and provides the very low surface tension, and the addition of hydrocarbon surfactant in AFFF is to provide excellent foaming ability. The very low surface tension of AFFF containing fluorocarbon surfactants results in excellent film-forming property, spread property, foaming ability, foam stability and the corresponding high-effectiveness in fire extinguishing and burn-back performance. In terms of the fluorine-free foam, the silicone surfactant provides a relative low surface tension and good foam spread property, and the hydrocarbon surfactants and mixtures of foam stabilizers provide highly stable foam. The relative low surface tension, good foam spread property, and the high foam stability lead to the high-effectiveness in fire extinguishing and burn-back performance commonly.
4 Conclusions
Fluorine-free firefighting foams are prepared based on the mixture of hydrocarbon and silicone surfactants. The key properties of fluorine-free foams are studied systematically, including surface activity, interfacial activity, viscosity, spreading coefficient, drainage, spreading property, fire extinguishing and burn-back performance. The main conclusions are as following:
The surface activity of aqueous solution of fluorine-free foams is lower than that of AFFF. And no aqueous film was formed on the surface of cyclohexane by aqueous solution of fluorine-free foams, even though spreading coefficient is positive. Fluorine-free foams have the higher stability but the worse spread property on the surface of liquid fuel compared with traditional AFFF.
In terms of small-scale test, the fire extinguishing and burn-back performances of fluorine-free foams meet the requirements of the standard GB15308-2006. But not all fluorine-free foams containing silicone surfactant performed as well as AFFF. The foam containing OFX-5211 shows the better fire extinguishing and burn-back performance than AFFF. The foam containing Silok-2235 shows better fire extinguishing performance but worse burn-back performance than AFFF. The foam containing Tegopren-6950 shows better burn-back but worse fire extinguishing performance than AFFF.
The mixture of hydrocarbon and silicone surfactants is promising to be used to develop the new generation environmental-friendly firefighting foams instead of fluorocarbon surfactants.
References
Mudan KS (1984) Thermal radiation hazards from hydrocarbon pool fires. Prog Energy Combust Sci 10:59–80
Siddapureddy S, Wehrstedt KD, Prabhu SV (2016) Heat transfer to bodies engulfed in di-tert-butyl peroxide pool fires-Numerical simulations. J Loss Prev Process Ind 44:204–211
Guiberti TF, Cutcher H, Roberts WL, Masri AR (2017) Influence of pilot flame parameters on the stability of turbulent jet flames. Energy Fuels 31:2128–2137.
Saisirirat P, Foucher F, Chanchaona S, Mounaïmrousselle C (2010) Spectroscopic measurements of low-temperature heat release for homogeneous combustion compression ignition (HCCI) n-Heptane/Alcohol mixture combustion. Energy Fuels. 24:5404–5409
Kong D, Liu P, Zhang J, Fan M, Tao C (2017) Small scale experiment study on the characteristics of boilover. J Loss Prev Process Ind 48:101–110
Hu L, Wang Q, Delichatsios M, Lu S, Tang F (2014) Flame radiation fraction behaviors of sooty buoyant turbulent jet diffusion flames in reduced-and normal atmospheric pressures and a global correlation with Reynolds number. Fuel 116:781–786
Bouhafid A, Vantelon JP, Souil JM, Bosseboeuf G, Rongere FX (1989) Characterisation of thermal radiation from freely burning oil pool fires. Fire Saf J 15:367–390
Ditch BD, de Ris JL, Blanchat TK, Chaos M, Bill RGJr, Dorofeev SB (2013) Pool fires—an empirical correlation. Combust Flame 160:2964– 2974
Kong D, Zhang Z, Ping P, Chen G, He X, Yang H (2018) Experimental study on burning behavior of crude oil pool fire in annular ice cavities. Fuel 234:464–472
Hu L, Liu S, Wu L (2013) Flame radiation feedback to fuel surface in medium ethanol and heptane pool fires with cross air flow. Combust Flame 160:295–306
Schaefer TH, Dlugogorski BZ, Kennedy EM (2008) Sealability properties of fluorine free firefighting foams (FfreeF), Fire Technol 44:297–309
Lattimer BY, Hanauska CP, Scheffey JL, Williams FW (2003) The use of small-scale test data to characterize some aspects of firefighting foam for suppression modeling, Fire Saf J 38:117–146
Sheng Y, Jiang N, Lu S, Li C (2018) Fluorinated and fluorine-free firefighting foams spread on heptane surface. Colloid Surface A 552:1–8
Sheng Y, Lu S, Xu M, Wu X, Li C (2016) Effect of Xanthan gum on the performance of aqueous film-forming foam. J Dispers Sci Technol 37:1664–1670
Zhang Q, Wang L, Bi Y, Xu D, Zhi H, Qiu P (2015) Experimental investigation of foam spread and extinguishment of the large-scale methanol pool fire. J Hazard Mater 287:87–92
Laundess AJ, Rayson MS, Kennedy EM, Dlugogorski BZ (2011) Small-scale test protocol for firefighting foams DEF (AUST) 5706: effect of bubble size distribution and ER. Fire Technol 47:149–162
Magrabi SA, Dlugogorski BZ, Jameson GJ (2001) Free drainage in aqueous foams: model and experimental study, AICHE J 47:314–327
Magrabi SA, Dlugogorski BZ, Jameson GJ (2002) A comparative study of drainage characteristics in AFFF and FFFP compressed-air firefighting foams. Fire Saf J 37:21–51
Kishi T, Mitsuru A (2008) Study on the generation of perfluorooctane sulfonate from the aqueous film-forming foam. J Hazard Mater 159:81–86
Schaefer CE, Andaya C, Urtiaga A, McKenzie ER, Higgins CP (2015) Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid(PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J Hazard Mater 295:170–175
Rodriguez-Freire L, Abad-Fernández N, Sierra-Alvarez R, Hoppe-Jones C, Peng H, Giesy JP, Keswani M (2016) Sonochemical degradation of perfluorinated chemicals in aqueous film-forming foams. J Hazard Mater 317:275–283
Sheng Y, Wu X, Lu S, Li C (2016) Experimental study on foam properties of mixed systems of silicone and hydrocarbon surfactants. J Surfactants Deterg 19(4): 823–831
Hetzer R, Kümmerlen F, Wirz K, Blunk D (2014) Fire testing a new fluorine-free AFFF based on a novel class of environmentally sound high performance siloxane surfactants. Fire Saf Sci 11:1261–1270o
Wang P (2015) Application of green surfactants developing environment friendly foam extinguishing agent. Fire Technol 51(3):503–511
Vinogradov AV, Kuprin DS, Abduragimov IM, Kuprin GN, Serebriyakov E, Vinogradov VV (2016) Silica foams for fire prevention and firefighting. ACS Appl Mater Inter 8(1):29–301
Kennedy M, Conroy M, Dougherty J, Otto N, Williams B, Ananth R, Fleming J (2015) Bubble coarsening dynamics in fluorinated and non-fluorinated firefighting foams. Colloid Surface A 470:268–279
Hinnant KM, Conroy MW, Ananth R (2017) Influence of fuel on foam degradation for fluorinated and fluorine-free foams. Colloids Surf A 522:1–17
Dlugogorski BZ, Phiyanalinmat S, Kennedy EM (2005) Dynamic surface and interfacial tension of AFFF and Fluorine-Free Class B foam Solutions. In: Fire safety science: Procedings of the eighth international symposium, pp 719–730
Sheng Y, Jiang N, Sun X, Lu S, Li C (2018) Experimental study on effect of foam stabilizers on aqueous film-forming foam. Fire Technol 54(1):211–228
Sheinson RS, Williams BA, Green C, Fleming JW, Anleitner R, Ayers S, Barylski D (2002) The future of aqueous film forming foam (AFFF): performance parameters and requirements. Naval Research Laboratory
Moran HE, Burnett JC, Leonard JT (1971) Suppression of Fuel Evaporation by Aqueous Films of Fluorochemical Surfactant Solutions (No.NRL-7247). Naval Research Lab Washington DC
Svitova T, Hoffmann H, Hill RM (1996) Trisiloxane surfactants: surface/interfacial tension dynamics and spreading on hydrophobic surfaces. Langmuir 12(7):1712–1721
Rosen MJ, Kunjappu JT (2012) Surfactants and interfacial phenomena. Wiley, New Jersey, pp 308–331
Sheng Y, Lu S, Jiang N, Wu X, Li C (2018) Drainage of aqueous film-forming foam stabilized by different foam stabilizers. J Dispers Sci Technol 39(9):1266–1273
Acknowledgements
The present work was supported by The National Natural Science Foundation of China (No. 51904230), China Postdoctoral Science Foundation (No. 2019M653700), Key R&D plan of Shaanxi Province (2017ZDXM-SF-092), Opening Fund of State Key Laboratory of Fire Science (No. HZ2019-KF03), and Doctor Initial Funding of Xi’an University of Science and Technology (No. 6310118032).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheng, Y., Jiang, N., Lu, S. et al. Study of Environmental-Friendly Firefighting Foam Based on the Mixture of Hydrocarbon and Silicone Surfactants. Fire Technol 56, 1059–1075 (2020). https://doi.org/10.1007/s10694-019-00920-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10694-019-00920-x