Abstract
Purpose
Obesity has been associated with reduced vagal function and increased sympathetic activity. Cardiac autonomic dysfunction has emerged as a major risk factor in the development of cardiovascular disease. Cardiac autonomic function (CAF) can be assessed by heart rate variability (HRV), an independent predictor of mortality based on changes in time intervals between adjacent heartbeats (RR). Bariatric surgery is considered the most effective treatment for obesity and its comorbidities, with sleeve gastrectomy (SG) being the most frequent bariatric procedure. There are few studies on HRV changes in women with obesity after SG. The aim of this study was to evaluate the short-term impact of SG on CAF and its relationship with weight loss.
Materials and Methods
An observational cohort study was conducted. Twenty-three female patients were assessed before SG and at 1 and 3 months after surgery. CAF was evaluated by analyzing HRV from 5-min records of RR intervals while the subject was supine. HRV was analyzed in time and frequency domains and with a nonlinear method.
Results
Patients (36.0 ± 11.1 years old, BMI 35.1 ± 3.4 kg/m2) presented higher HRV values, on average, in all domains both at 1 and 3 months after SG (p < 0.05). In addition, all anthropometric parameters improved (p < 0.001) although there was no relationship between HRV improvements and anthropometric changes.
Conclusion
SG seems to be effective at reducing excess weight and improving HRV at the short term, and these changes are detectable as early as the first month after surgery. HRV assessment appears as a promising low-cost tool that deserves further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a disease which affects over 650 million adults worldwide [1] with a higher prevalence in women [2]. It has been reported that obesity implies a greater cardiovascular disease (CVD) risk for women than men [3]. Obesity is associated with numerous comorbidities such as dyslipidemia, diabetes, osteoarthritis, some cancers [4], hypertension, atrial fibrillation, heart failure, stroke, and coronary heart disease [5,6,7,8]. CVD may occur due to structural and functional changes of the myocardium induced by the excess adipose tissue and other mechanisms related to obesity [5, 9].
Obesity is also characterized by autonomic dysfunction with elevated sympathetic and decreased parasympathetic system activity, leading to an autonomic imbalance across the cardiovascular system [10]. Hormonal changes observed in subjects with obesity, such as an increase in insulin and leptin plasmatic levels, have been established as factors that contribute to autonomic dysfunction and the development of obesity-related cardiac diseases [11, 12]. It has been described that a 10% increase in body weight is enough to decrease parasympathetic activity and to increase the activity of the sympathetic system; the latter would be an adaptive mechanism to increase the energy expenditure at rest and to promote the restoration of the previous weight [12].
Altered cardiovascular autonomic regulation resulting from obesity can be detected by assessing heart rate variability (HRV), the change in time intervals between adjacent heartbeats [13]. HRV has been widely described [14,15,16,17,18,19], proving to be an independent predictor of mortality [14, 20,21,22,23,24] associated with cardiac health [25].
HRV reductions in obese women have been previously reported [13], with a higher rate of sudden cardiac death among obese people, compared with those in adults with normal body weight [26,27,28].
Conventional obesity treatment has shown limited success in reducing body weight over time [29]. The number of bariatric procedures has increased over recent years [30] and more than 70% of these have been performed on women [31]. Although evidence exists regarding the efficacy of bariatric surgery on weight reduction and comorbidities [32,33,34,35], there is insufficient information on the changes that sleeve gastrectomy (SG), the most common bariatric procedure in the world [30], can induce on HRV. The aim of this study was to describe the short-term HRV changes following SG and their relationship to weight loss.
Materials and Methods
Study Design, Participants, and Procedures
In this analytical observational cohort study, participants were recruited over a 2-year period (2015 and 2016). The sample size was calculated with an alpha error of 5% and a statistical power of 90%, considering previously reported high-frequency (HF) power data [36], which gave a required cohort size of 17 patients. The present study included 23 adult women with obesity (BMI ≥ 30 kg/m2) who were undergoing SG. Participants with arrhythmias, severe CVD, chronic renal insufficiency, chronic obstructive pulmonary disease, a smoking habit, used beta blockers, or a postmenopausal status or who had undergone previous bariatric surgery were excluded.
All subjects followed the usual bariatric post-surgical diet indications [37] and were given recommendations for increasing physical activity levels.
Subjects were asked to fast for at least 3 h before each evaluation; they were also asked to wear comfortable clothes and to refrain from drinking caffeinated and alcoholic beverages and performing intense physical exercise over the preceding 24 h. All assessments were performed in the morning to avoid variations in the circadian rhythm.
A complete assessment of anthropometric parameters and HRV was conducted 7–14 days prior to surgery, as well as 1 month and 3 months following the SG.
All procedures were performed in accordance with the standards set out in the 1964 Helsinki declaration and its later amendments, and all patients signed an informed consent.
Anthropometrics
The body mass index (BMI) and the waist circumference (at the level of the iliac crests) were determined using a DETECTO 439 balance scale and a Rosscraft anthropometric tape, respectively [38]. Weight loss was expressed as the percentage of total weight loss (%TWL) and the percentage of excess weight loss (%EWL) [39].
Heart Rate Variability
The duration of RR intervals was recorded using a Polar RS800CX telemetry heart rate monitor (Polar Electro Oy, Kempele, Finland) [40,41,42]. After resting in the supine position for 5 min, the heart rate RR intervals were continuously recorded for 10 min in the same position in a quiet, temperature-controlled room (22–24 °C) while breathing at a controlled rate (14 breaths per minute) using a metronome [43]. The time domain, frequency domain, and nonlinear analysis of HRV were determined from the 5-min resting RR record with the lowest average heart rate.
RR data were analyzed with Kubios HRV Premium software (3.0.2 version) and preprocessed to remove abnormal intervals and artifacts [43, 44]. The time domain HRV variables analyzed were standard deviation of all RR intervals (SDNN), root mean square of successive differences in RR intervals (RMSSD), and percentage of consecutive RR intervals that differ by more than 50 ms (pNN50) [16, 23]. The frequency domain analysis was computed using the fast Fourier transform and the measures included the low-frequency (LF) power, HF power, and LF to HF power ratio (LF/HF) [14]. HF and LF were expressed in absolute and logarithm values (Ln).
Nonlinear parameters included from Poincaré plot were standard deviation 1 (SD1), which represents short-term variability, the major axis which represents standard deviation 2 (SD2), meaning long-term variability (compared with SD1), and the sample entropy, which measures the regularity and complexity of a time series [18, 25].
The ratio of change of the HRV variables was expressed as a percentage and was calculated by subtracting the pre-surgical value from the post-surgical data and dividing it by the pre-surgical values.
Surgical Technique
The surgical procedures were performed by three certified bariatric surgeons. All the patients underwent laparoscopic SG, as described previously [45], leaving an estimated stomach capacity of 120–150 ml.
Statistical Analysis
For data distribution, the Shapiro-Wilk normality test was used. All HRV values were expressed as medians [minimum–maximum] whereas the anthropometric values were expressed as means ± standard deviation (SD). Differences in HRV over the three time points were analyzed using the Friedman test, with the Wilcoxon test being employed for pairwise comparison. To compare the anthropometric measurements over the three assessments, we used ANOVA with Bonferroni post hoc analysis. The Spearman test was applied for correlation analysis. Statistical analysis was performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, U.S.). A p value of < 0.05 was considered statistically significant.
Results
Thirty-five women with an indication for SG were recruited; however, five of them were excluded and two patients did not consent to participate in the study. Of the 28 patients initially included, five were lost during the study (Fig. 1).
The study finally included 23 women (36.0 ± 11.1 years old; excess weight 26.0 ± 9.2 kg; BMI 35.1 ± 3.4 kg/m2), two of which did not participate in the 1-month postoperative evaluation. Regarding comorbidities, 2 of them had controlled arterial hypertension, 7 had controlled hypothyroidism, and 14 had non-alcoholic fatty liver disease.
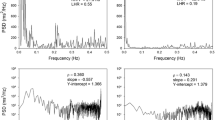
There was a significant improvement in all the anthropometric measurements at both the first and the third month after surgery (Table 1). Regarding the HRV analysis, an improvement in all time domain variables was observed among the three assessments, SDNN (p = 0.003; Cohen’s d = 0.68), RMSSD (p = 0.006; Cohen’s d = 0.87), and pNN50 (Fig. 2) (Cohen’s d = 0.80), with all the improvements being statistically significant from the first month post-surgery (Table 2). In the frequency domain analysis, there was an improvement in HF power from the preoperative to postoperative assessments (both in absolute and Ln values, p = 0.015; a Cohen’s d for absolute HF power = 0.75), with a higher spectral power from the first month following SG (Table 2). On the other hand, the LF power showed a tendency to improve among the three assessment points (p = 0.076), with a significant change only between the preoperative assessment and the third month in absolute values (p = 0.030; Cohen’s d = 0.22) and Ln values (p = 0.007; Cohen’s d = 0.58) (Table 2). There were no significant changes in the LF/HF ratio (p = 0.201).
The nonlinear analysis showed an improvement in SD1 and SD2 (Fig. 3), and no changes in sample entropy (p = 0.217), with higher variability in the Poincaré plot from the first month in SD1 and SD2 (Table 2) (Cohen’s d for SD1 = 0.87).
There was no relationship between the weight changes and HRV improvements observed in our patients.
Discussion
This study showed that SG is effective at inducing significant weight loss and improving HRV indices, beginning as soon as the first month after surgery.
Only three previous studies have reported the effect of SG on HRV [36, 46, 47]. The study by Casellini et al. (on 56 patients with SG) showed an improvement in the HRV time domain 6 months after bariatric surgery. Unfortunately, the authors did not include either a frequency domain analysis or a nonlinear HRV analysis [46].
The work of Kokkinos et al. (on 23 patients with SG) showed similar results to our study related to improved HF and LF power both 3 and 6 months after SG, with no changes in the LF/HF ratio. The authors did not include time domain or nonlinear analysis results. There was also no information regarding the gender distribution of the sample [36].
Finally, in the work by Wu et al. on a sample of 18 patients with SG (50% women), the authors found statistically significant HRV improvements 6 months after SG, both in terms of the time and frequency domain analyses, although these changes were not apparent 3 months after surgery. Moreover, they found no changes in the nonlinear analysis. The findings of Wu et al. clearly differ from our results and theirs is the only work that showed changes in the LF/HF ratio after SG [47].
The increase in the RMSSD and pNN50 following SG that we observed in our study, which is directly related to vagus nerve activity [19], indicates enhanced parasympathetic activity [48]. The spectral power in HF, which increased in our patients from the first month following SG, is a well-known marker of parasympathetic tone [19]. In contrast, the LF, which only increased 3 months after SG in our study, reflects both sympathetic and vagal influences [19]. Likewise, the improvement we observed in the Poincaré plot indexes, SD1 and SD2, has been described as a reliable indicator of better parasympathetic system functioning [17].
Moreover, the SDNN, which also increased from the first month post SG in our study, is negatively influenced by the sympathetic component of the autonomic nervous system [15, 19]. This might be due to the fact that, after bariatric surgery, there is a severe caloric restriction, mainly in the first months. It has been reported that these dietary changes produce a global reduction in sympathetic activity [49, 50] with implications regarding resting energy expenditure as an adaptive response to a caloric restriction [50, 51].
The improvement in parasympathetic tone following SG that we observed in our patients may be beneficial to their cardiovascular system, as previously reported [52,53,54], although it has not yet been established how much the vagal activity markers need to increase to provide protection for the heart.
Few studies have reported beneficial effects on cardiac function after SG. One study showed an improvement in systolic function and global longitudinal strain on the left ventricle that correlated with weight loss [55]. Also, after SG, a reduction in the interventricular septum, the thickness of the posterior wall, and the mass of the left ventricle has been demonstrated [56].
A recent meta-analysis showed that SG has a greater effect on the parasympathetic tone than the gastric bypass procedure [57]. The SG surgical technique preserves the vagal trunk of the stomach’s lesser curvature [57], and it has been suggested that the effects of bariatric surgery on the brain-gut axis could be influenced by the surgically induced anatomical alterations [12].
In our patients, we observed improved HRV in the time and frequency domains, as well as in the nonlinear analysis, with no additional intervention. In contrast, it has been previously reported that patients who have undergone a gastric bypass may only show improved HRV if there is a physical training program, with no change in those patients who only received the surgery [58]. This differs from the studies carried out by Bobbioni-Harsch et al., who found improvements in the time domain [59], and by Kokkinos et al., who reported improvements in the frequency domain. In both cases, there was no physical exercise training following surgery [36].
Our results suggest a recovery in cardiac autonomic function and a reversal of vagal impairment following weight loss [19]; this was demonstrated by the increase in SDNN, RMSSD, pNN50, HF, LF, SD1, and SD2, with a predominantly large or medium effect size. However, a difference in HRV between men and women has been described [60] so our results cannot be applied to the male bariatric population. It is also important to consider that age and initial BMI of our participants are lower than mean values previously reported worldwide [31] and might have influenced these positive results.
As HRV is a predictor of cardiovascular disease and early mortality [24] and considering the physiological changes that SG induces on autonomic function, repeated measurements of HRV may provide the data necessary for evaluating cardiac risk and other post-surgical complications [61]. However, we suggest additional research involving larger cohorts (ideally with higher BMI and including older patients and male population), with at least a 12-month follow-up, to confirm our findings and assess the utility of including HRV assessment in the routine practice of bariatric patients.
We should acknowledge that the main limitation of this study is its inability to determine whether weight loss and HRV improvements will be permanent due to the short-term nature of the follow-up. In addition, had we included a control group made up of obese patients who had not undergone surgery, or diet-induced weight loss patients, it would have enabled us to compare the results. Regarding the study’s main strength, we would like to point out that, so far, this is the most comprehensive HRV analysis conducted on a sample of women who have undergone exclusively SG.
References
World Health Organization. Obesity and overweight. [Internet]. [cited 2020 Feb 12]. Available from: www.who.int/mediacentre/factsheets/fs311/en/
Inoue Y, Qin B, Poti J, et al. Epidemiology of obesity in adults: latest trends. Curr Obes Rep. 2018;7(4):276–88. https://doi.org/10.1007/s13679-018-0317-8.
Garcia M, Mulvagh SL, Merz CN, et al. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118(8):1273–93. https://doi.org/10.1161/CIRCRESAHA.116.307547.
Williams EP, Mesidor M, Winters K, et al. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. 2015;4(3):363–70. https://doi.org/10.1007/s13679-015-0169-4.
Mandviwala T, Khalid U, Deswal A. Obesity and cardiovascular disease: a risk factor or a risk marker? Curr Atheroscler Rep. 2016;18(5):21. https://doi.org/10.1007/s11883-016-0575-4.
Pareek M, Bhatt DL, Schiavon CA, et al. Metabolic surgery for hypertension in patients with obesity. Circ Res. 2019;124(7):1009–24. https://doi.org/10.1161/CIRCRESAHA.118.313320.
GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. https://doi.org/10.1056/NEJMoa1614362.
Lavie CJ, De Schutter A, Parto P, et al. Obesity and prevalence of cardiovascular diseases and prognosis—the obesity paradox updated. Prog Cardiovasc Dis. 2016;58(5):537–47. https://doi.org/10.1016/j.pcad.2016.01.008.
Alpert M, Omran J, Mehra A, et al. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56(4):391–400. https://doi.org/10.1016/j.pcad.2013.09.003.
Wijngaarden MA, Pijl H, van Dijk KW, et al. Obesity is associated with an altered autonomic nervous system response to nutrient restriction. Clin Endocrinol. 2013;79(5):648–51. https://doi.org/10.1111/cen.12100.
Straznicky NE, Lambert GW, Lambert EA. Neuroadrenergic dysfunction in obesity: an overview of the effects of weight loss. Curr Opin Lipidol. 2010;21(1):21–30. https://doi.org/10.1097/MOL.0b013e3283329c62.
Guarino D, Nannipieri M, Iervasi G, et al. The role of the autonomic nervous system in the pathophysiology of obesity. Front Psychol. 2017;8:665. https://doi.org/10.3389/fphys.2017.00665.
Triggiani AI, Valenzano A, Ciliberti MA, et al. Heart rate variability is reduced in underweight and overweight healthy adult women. Clin Physiol Funct Imaging. 2017;37(2):162–7. https://doi.org/10.1111/cpf.12281.
Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:1040. https://doi.org/10.3389/fpsyg.2014.01040.
Xhyheri B, Manfrini O, Mazzolini M, et al. Heart rate variability today. Prog Cardiovasc Dis. 2012;55(3):321–31. https://doi.org/10.1016/j.pcad.2012.09.001.
Vigo DE, Siri LN, Cardinali DP. Heart rate variability: a tool to explore autonomic nervous system activity in health and disease. In: Gargiulo P, Mesones Arroyo H, editors. Psychiatry and neuroscience update. Cham: Springer; 2019. p. 113–26.
Rahman S, Habel M, Contrada RJ. Poincaré plot indices as measures of sympathetic cardiac regulation: responses to psychological stress and associations with pre-ejection period. Int J Psychophysiol. 2018;133:79–90. https://doi.org/10.1016/j.ijpsycho.2018.08.005.
Kubičková A, Kozumplík J, Nováková Z, et al. Heart rate variability analysed by Poincaré plot in patients with metabolic syndrome. J Electrocardiol. 2016;49(1):23–8. https://doi.org/10.1016/j.jelectrocard.2015.11.004.
Task Force of the European Society of Cardiology and the North American Society of Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation and clinical use. Eur Heart J. 1996;17(3):354–81.
Ziegler D, Zentai CP, Perz S, et al. Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care. 2008;31(3):556–61. https://doi.org/10.2337/dc07-1615.
Koko KR, McCauley BD, Gaughan JP, et al. Spectral analysis of heart rate variability predicts mortality and instability from vascular injury. J Surg Res. 2018;224:64–71. https://doi.org/10.1016/j.jss.2017.11.029.
Sessa F, Anna V, Messina G, et al. Heart rate variability as predictive factor for sudden cardiac death. Aging. 2018;10(2):166–77. https://doi.org/10.18632/aging.101386.
Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. https://doi.org/10.3389/fpubh.2017.00258.
Wulsin LR, Horn PS, Perry JL, et al. Autonomic imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality. J Clin Endocrinol Metab. 2015;100(6):2443–8. https://doi.org/10.1210/jc.2015-1748.
Rajendra Acharya U, Paul Joseph K, Kannathal N, et al. Heart rate variability: a review. Med Biol Eng Comput. 2006;44(12):1031–51. https://doi.org/10.1007/s11517-006-0119-0.
Sabbag A, Sidi Y, Kivity S, et al. Obesity and exercise-induced ectopic ventricular arrhythmias in apparently healthy middle aged adults. Eur J Prev Cardiol. 2016;23(5):511–7. https://doi.org/10.1177/2047487315591442.
Adabag S, Huxley RR, Lopez FL, et al. Obesity related risk of sudden cardiac death in the atherosclerosis risk in communities study. Heart. 2015;101(3):215–21. https://doi.org/10.1136/heartjnl-2014-306238.
Chiuve SE, Sun Q, Sandhu RK, et al. Adiposity throughout adulthood and risk of sudden cardiac death in women. JACC Clin Electrophysiol. 2015;1(6):520–8. https://doi.org/10.1016/j.jacep.2015.07.011.
Montesi L, El Ghoch M, Brodosi L, et al. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37–46. https://doi.org/10.2147/DMSO.S89836.
Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–94. https://doi.org/10.1007/s11695-018-3450-2.
Welbourn R, Pournaras DJ, Dixon J, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the second IFSO Global Registry Report 2013–2015. Obes Surg. 2018;28(2):313–22. https://doi.org/10.1007/s11695-017-2845-9.
Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;319(3):291–301. https://doi.org/10.1001/jama.2017.21055.
Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14(3):160–9. https://doi.org/10.1038/nrgastro.2016.170.
Yu J, Zhou X, Li L, et al. The long-term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non-randomized evidence. Obes Surg. 2015;25(1):143–58. https://doi.org/10.1007/s11695-014-1460-2.
Bower G, Toma T, Harling L, et al. Bariatric surgery and non-alcoholic fatty liver disease: a systematic review of liver biochemistry and histology. Obes Surg. 2015;25(12):2280–9. https://doi.org/10.1007/s11695-015-1691-x.
Kokkinos A, Alexiadou K, Liaskos C, et al. Improvement in cardiovascular indices after Roux-en-Y gastric bypass or sleeve gastrectomy for morbid obesity. Obes Surg. 2013;23(1):31–8. https://doi.org/10.1007/s11695-012-0743-8.
Snyder-Marlow G, Taylor D, Lenhard MJ. Nutrition care for patients undergoing laparoscopic sleeve gastrectomy for weight loss. J Am Diet Assoc. 2010;110(4):600–7. https://doi.org/10.1016/j.jada.2009.12.022.
Redberg RF, Benjamin EJ, Bittner V, et al. AHA/ACCF 2009 performance measures for primary prevention of cardiovascular disease in adults. Circulation. 2009;120(13):1296–336. https://doi.org/10.1161/CIRCULATIONAHA.109.192617.
Brethauer SA, Kim J, El Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25(4):587–606. https://doi.org/10.1016/j.soard.2015.02.003.
Barbosa MP, da Silva NT, de Azevedo FM, et al. Comparison of Polar® RS800G3™ heart rate monitor with Polar® S810i™ and electrocardiogram to obtain the series of RR intervals and analysis of heart rate variability at rest. Clin Physiol Funct Imaging. 2016;36(2):112–7. https://doi.org/10.1111/cpf.12203.
Hernando D, Garatachea N, Almeida R, et al. Validation of heart rate monitor Polar RS800 for heart rate variability analysis during exercise. J Strength Cond Res. 2018;32(3):716–25. https://doi.org/10.1519/jsc.0000000000001662.
Williams DP, Jarczok MN, Ellis RJ, et al. Two-week test-retest reliability of the Polar® RS800CX™ to record heart rate variability. Clin Physiol Funct Imaging. 2017;37(6):776–81. https://doi.org/10.1111/cpf.12321.
Gisselman AS, D’Amico M, Smoliga JM. Optimizing inter-session reliability of heart rate variability – the effects of artefact correction and breathing type. J Strength Cond Res. 2017:1. https://doi.org/10.1519/JSC.0000000000002258.
Tarvainen MP, Niskanen JP, Lipponen JA, et al. Kubios HRV—heart rate variability analysis software. Comput Methods Prog Biomed. 2014;113(1):210–20. https://doi.org/10.1016/j.cmpb.2013.07.024.
Kissler HJ, Settmacher U. Bariatric surgery to treat obesity. Semin Nephrol. 2013;33(1):75–89. https://doi.org/10.1016/j.semnephrol.2012.12.004.
Casellini CM, Parson HK, Hodges K, et al. Bariatric surgery restores cardiac and sudomotor autonomic C-fiber dysfunction towards normal in obese subjects with type 2 diabetes. PLoS One. 2016;11(5):e0154211. https://doi.org/10.1371/journal.pone.0154211.
Wu JM, Yu HJ, Lai HS, et al. Improvement of heart rate variability after decreased insulin resistance after sleeve gastrectomy for morbidly obesity patients. Surg Obes Relat Dis. 2015;11(3):557–63. https://doi.org/10.1016/j.soard.2014.09.011.
Poirier P, Hernandez TL, Weil KM, et al. Impact of diet-induced weight loss on the cardiac autonomic nervous system in severe obesity. Obes Res. 2003;11(9):1040–7. https://doi.org/10.1038/oby.2003.143.
Straznicky NE, Lambert EA, Lambert GW, et al. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90(11):5998–6005. https://doi.org/10.1210/jc.2005-0961.
Rosenbaum M, Hirsch J, Murphy E, et al. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71(6):1421–32. https://doi.org/10.1093/ajcn/71.6.1421.
Bettini S, Bordigato E, Fabris R, et al. Modifications of resting energy expenditure after sleeve gastrectomy. Obes Surg. 2018;28(8):2481–6. https://doi.org/10.1007/s11695-018-3190-3.
Olshansky B, Sabbah HN, Hauptman PJ, et al. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118(8):863–71. https://doi.org/10.1161/CIRCULATIONAHA.107.760405.
Guiraud T, Labrunee M, Gaucher-Cazalis K, et al. High-intensity interval exercise improves vagal tone and decreases arrhythmias in chronic heart failure. Med Sci Sports Exerc. 2013;45(10):1861–7. https://doi.org/10.1249/MSS.0b013e3182967559.
Dimova R, Tankova T, Chakarova N, et al. Cardiovascular autonomic tone relation to metabolic parameters and hsCRP in normoglycemia and prediabetes. Diabetes Res Clin Pract. 2015;109(2):262–70. https://doi.org/10.1016/j.diabres.2015.05.024.
Leung M, Xie M, Durmush E, et al. Weight loss with sleeve gastrectomy in obese type 2 diabetes mellitus: impact on cardiac function. Obes Surg. 2016;26(2):321–6. https://doi.org/10.1007/s11695-015-1748-x.
Cavarretta E, Casella G, Calì B, et al. Cardiac remodeling in obese patients after laparoscopic sleeve gastrectomy. World J Surg. 2013;37(3):565–72. https://doi.org/10.1007/s00268-012-1874-8.
Geronikolou SA, Albanopoulos K, Chrousos G, et al. Evaluating the homeostasis assessment model insulin resistance and the cardiac autonomic system in bariatric surgery patients: a meta-analysis. Adv Exp Med Biol. 2017;988:249–59. https://doi.org/10.1007/978-3-319-56246-9_20.
Castello V, Simões RP, Bassi D, et al. Impact of aerobic exercise training on heart rate variability and functional capacity in obese women after gastric bypass surgery. Obes Surg. 2011;21(11):1739–49. https://doi.org/10.1007/s11695-010-0319-4.
Bobbioni-Harsch E, Sztajzel J, Barthassat V, et al. Independent evolution of heart autonomic function and insulin sensitivity during weight loss. Obesity. 2009;17(2):247–53. https://doi.org/10.1038/oby.2008.532.
Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310. https://doi.org/10.1016/j.neubiorev.2016.03.007.
Lodder AF, Kamath MV, Armstrong D, et al. Bariatric surgery and its effects on heart rate variability. In: Kamath MV, Watanabe MA, Upton ARM, editors. Heart Rate variability (HRV) signal analysis. Clinical Applications. Boca Raton: CRC Press; 2013. p. 279–302.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (registered number 149-2014) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no competing interests.
Informed/Written Consent
Informed consent was approved by the institutional research ethics committee and was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibacache, P., Cárcamo, P., Miranda, C. et al. Improvements in Heart Rate Variability in Women with Obesity: Short-term Effects of Sleeve Gastrectomy. OBES SURG 30, 4038–4045 (2020). https://doi.org/10.1007/s11695-020-04721-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04721-y