Abstract
Grape pomace is the main by-product of grape processing industries which is a good source of hydrocolloids such as pectin and fibers. The possibility of substitution of pectin with the grape pomace powder (GPP) in a fruit filling was evaluated. In terms of rheological point of view, the more GPP content, the more elastic and viscose modulus, loss tangent and the complex viscosity were observed. The closest treatment to the Control sample (0.5 g high-methoxyl pectin), statistically (p < 0.05) was seen to be T3 (7.5 g GPP). The obtained results from confocal laser scanning microscopy (CLSM) and the fluorescence microscopy assays were in agreement with the rheological tests. Moreover, the outcomes of the extrusion test revealed that not only increasing the amount of GPP has a direct correlation with the force and total work, but also, after the exposure of filling to heat, Control, T3 and T4 (10 g GPP) showed bake stability features and their physical characteristics before baking had no significant difference (p < 0.05) with their features after baking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the beneficial effects of grape on human health and its economic importance, it is a widely grown and eaten fruit around the world [1]. The agro-industrial residue of grape is mostly its pomace. Depending on the conditions of the grapes during the time they are harvested, they may result in 13.5–14.5% pomace of the total volume of grapes [2]. The grape pomace contains hydrocolloids such as pectin and dietary fiber, which due to their gelling and water binding capacities, they have the thickening ability, causing stabilization of aqueous systems which make them of great importance ingredients in food industry, especially in bakery and confectionery. The thickening effect of grape pomace is dependent on the type of hydrocolloid, concentration, food matrix, pH of food system and temperature [3]. Pectin belongs to the group of hydrocolloids which are relatively stable under thermal conditions and has the ability to absorb water and form a loose to firm gel structure, depending on its concentration [4].
Bakery and confectionery products containing natural fruit fillings are a worldwide favorite type of meals and well accepted among consumers of all ages. Fruit fillings are generally consisted of sugar, humectants, stabilizers and fats. Depending on the final application of fruit filling, the ratio of ingredients and their types could be significantly varied. Stabilizers and humectants are two essential ingredients defining the structure and water activity of the fillings. Cropotova et al. [5], and Taoukis and Richardson [6], respectively, conducted researches on the influence of different hydrocolloids and humectants on physicochemical features of fruit fillings. Nowadays one of the significant issues related to the filled bakery products backs to the water content and water activity of the dry bakery products such as hard dough biscuits. This parameter is the driving criterion to migrate of moisture from ordinary fruit fillings due to their high-water activity to the biscuit. In such condition, biscuit moisture increases and fruit filling becomes hard and elastic [6]. Another issue is regarding the thermal instability of the used fruit fillings meaning that being exposed to high temperature could lead to water evaporation from fruit filling, causing it to become hard [4, 5]. The requirement for heat-stable fruit fillings should also possess a number of textural parameters which are essential for syneresis control, physicochemical degradation and overall sensory acceptance. Heat-stability is a function of stabilizer content and state, as well as soluble solids content and type of raw materials [7].
Textural parameters are influenced by a quantity and quality of certain ingredient exists in the whole fruit filling system. Hardness is directly dependent on percentage of hydrocolloids and soluble solids, while consistency and chewiness are mostly related to molecular weight of the complex carbohydrates.
This paper aimed to characterize grape pomace in terms of chemical composition and evaluation of rheological properties and confocal laser scanning microscopy (CLSM) to explain the structure of fruit fillings in which were replaced by grape pomace powder to evaluate the feasibility of producing low-water activity bake-stable fruit filling.
Materials and methods
Materials
High-methoxyl pectin (DM 65.2%) was obtained from Degussa Food Ingredients (France). Fluorescein-5-isothiocyanate (FITC) and Fluorescein amid (FA) were purchased from sigma aldrich. High Fructose Corn Syrup (HFCS 55) was acquired from Zarnam Co. (Hashtgerd, Alborz, Iran). The rest of the ingredients including sugar (Debel Khazaei, Khuzestan province, Iran), sorbitol syrup 70% (Gujarat Ambuja, India) and glycerin (Timur oleochemicals, Selangor, Malaysia) were purchased from their suppliers.
Grape pomace powder preparation
Grapes (Vitis vinifera L.) (65 kg) were collected by the authors directly from Malayer city (2020 harvest season) located in Hamedan province, Iran. The grapes were cleaned and pressed using an industrial depulper, separating the pomace and pulp for the extraction of grape juice. Then, the juicy pomace was put in a deck oven (D-2, Sveba Dahlen, Fristad, Sweden) for 4 h at 60 °C. Finally, the grape pomace particles were milled using a grinder (GCS2700, Hard stone, Iran) while 80% of the particles passed through mesh 200.
Proximate analysis of dried grape pomace powder
Acidity, pH, ash and moisture content
The pH was determined by a pH-meter, (MS Tecnopon, model mPA210), calibrated in buffer solutions of pH 4.0 and 7.0, respectively. Determination of moisture was performed using AOAC method (930.15–1930, 1999) [8]. Ash content was measured by incineration in a furnace at 550 °C until constant weight. All analyses were performed in triplicate.
Fat, protein, total dietary fiber, and total carbohydrate
Fat content was acquired according to the analytical standards of AACC method 30 − 25 which is the soxhlet extraction [9]. Protein was determined using the micro-Kjeldahl method by selenium and copper sulphate as catalysts and boric acid as the receiver solution in the distillation of ammonia. Then, the samples were titrated with 0.1 N hydrochloric acid. As recommended by Association of Official Analytical Chemistry (AOAC, 976.05, 1995), the conversion factor of 6.25 was used to convert nitrogen into protein. Total dietary fiber (TDF) was obtained by adding the soluble and insoluble fractions, according to the enzymatic-gravimetric method of Prosky et al. [8]. Total carbohydrate was determined by the difference method: 100-(weight in grams [moisture + ash + protein + total fat + total dietary fiber in 100 g of food]).
Pectin content
Pectin content determination was done which was involved with the neutralization the overall charge of free uronic acid residues by calcium ions causing gelation and precipitation of pectin. The results were expressed in grams of calcium pectate per 100 g of sample [10].
Fruit filling preparation
The samples were prepared in a food processor cooker (Qbo8, Roboqbo, Italy) equipped with temperature and stirring speed control according to Carcelli et al. [11] method with some modifications. The first step in preparing the samples was well distribution of pectin in the sugar and its addition to mixture of HFCS 55, glycerin, sorbitol syrup 70% which was pre-heated to 60 °C and kept stirring at 70 rpm for 5 min. Then the temperature was increased to 80 °C until sugar and pectin were totally dissolved. Calcium chloride, dissolved in a small amount of water reserved for this step and was added to the mixture containing pectin and stirring continued for a further 5 min. At the last step, the pH was adjusted to 3.8 ± 0.2, using citric acid solution (1:2 ratio of citric acid to water), and stirring and heating continued for a further 5 min. For the preparation of treatments which contained GPP, no pectin was added. The levels of GPP used are given in Table 1.
The samples were transferred to different plastic containers and held in cool condition (8 °C) for 24 h (freshly prepared samples). After the aging period (24 h), half of each filling treatment was baked at 200 °C for 10 min [12] and named as After baking (baked). Before testing, the samples were stabilized for 1 h at ambient temperature.
Rheological assessment
Rheological measurements were carried out using a rheometer (VT550, Thermo Haake, Karlsruhe, Germany) with a parallel-plate geometry probe (50 mm diameter and 1 mm gap) at 20 °C on the treatments which were prepared 24 h before the experiments. In addition, the frequency sweep tests ranging from 0.01 to 10 Hz were performed, and storage modulus (G´) and loss modulus (G″) were obtained. Frequency sweep tests were conducted at constant strain within the linear viscoelastic range. The obtained data from frequency sweep were fitted to the Power Law model (Eqs. 1–4) [13]:
The coefficients\({G{^\prime }}_{\left(\omega 1\right)}\) and \({G{^\prime }{^\prime }}_{\left(\omega 1\right)}\)represent the elastic and viscous moduli, respectively, at different frequencies, \({{\eta}}^{\text{*}}\)defines the complex viscosity at the temperatures between 30 and 60 °C and \(\text{T}\text{a}\text{n} \left({\delta }\right)\) describes the damping factor (loss tangent). Exponents a and b quantify the dependence degree of these moduli and the loss tangent with the oscillation frequency.
Moisture content and water activity of fruit fillings
The moisture content and water activity of fruit fillings were measured both for before (freshly prepared) and after baking (baked). The water content and water activity (aw) were determined using AACC method (AACC, 44-15.02) [14] and a Novasina analyzer (Model Labmaster, Switzerland) at 25 °C respectively [3, 14, 15].
Extrusion test
The extrusion properties were measured using a texture analyzer (TA-XT Plus, Godalming, Surrey, England) equipped with a 50 Kg load cell and a 50 mm diameter back extrusion cell (A/BE Back Extrusion Rig) with a 10 mm gap between the sample container and the disc plunger. The trigger point was 10 g the compression speed was set at 10 mm/s. The test duration was adjusted on 15 s [16]. The force profiles were recorded and the maximum extrusion force (Fmax in N) as an index of firmness, the area under the curve (AUC) as an index of consistency and the adhesiveness force between the sample and the probe were obtained [12]. This test was done for fruit fillings, both before and after baking.
Confocal laser scanning microscopy (CLSM) and fluorescence microscopy
Ruthenium red (RR) was used to visualize the pectin and fibers in red color and FITC was to discriminate the hydroxyl groups in green color. A solution containing 0.17 mg/mL of RR and 0.5 mg/mL fluorescein isothiocyanate (FITC) was prepared using dimethylformamide as solvent. The fluorophores were kept and protected from light at -20 °C to avoid any possible degradation, and the required amount of solution was freshly prepared. Eight fruit filling samples were taken from each treatment and the resulting image was an average of 8 scanned focal planes. Samples (slice of 10 μm of thickness) were cut off in the cryostat microtome chamber (HM 500 OM, Microm International, USA) set at -25 °C. Before the slide cover was placed on the top, fruit filling slices were covered with 15 µL of fluorophore solution. Mounted slides were stored for 24 h at room temperature (25 °C) before observation to ensure a complete diffusion of the fluorophores. For excitation purpose, a Nikon CLSM (Model A1) with a green laser at 488 nm was used. The fluorescence emissions of the dyes were detected between 580 and 670 nm and spectral de-convolution was performed [17, 18]. To analyze the resulted images, the color histogram tool of the Image J software (The National Institutes of Health, USA) was used.
To prepare the fluorescence microscope images, the fruit filling treatments were visualized using a FITC filter cube equipped fluorescence microscope (Nikon mod. Ti-U). In case of having experiments done, a solution of 1% Rhodamine B (Sigma–Aldrich, Germany) was added at the concentration of 1 mL/5 g of the fruit fillings to let their microstructures are visualized under blue light excitation (kem = 520 nm, kex = 496 nm and the exposure time = 1 s). Prior to starting fluorescence microanalysis, 1 h reaction time between the analyzed fillings treatments and the colorant was considered. Then, the images were processed in Image J according to the following protocol: Firstly, all images were turned from RGB to 8-bit format. After that, they were analyzed using Plot Surface and the distance were set between 0 and 350 pixels.
Statistical analysis
To run the analysis of variance (one-way ANOVA) and the significance mean levels (Duncan) (p < 0.05), all the data obtained were processed through the SAS software version 9.2 (SAS Institute, Cary, NC). Principal component analysis (PCA) was done using Minitab 18 software (Minitab, LLC, Penn State University) for the clustering of results.
Results and discussion
Proximate features of GPP
The measured parameters were pH, ash, moisture content, fat, protein, total dietary fiber, total carbohydrate and pectin content and were found to be 3.45 ± 0.01, 4.10 ± 0.06, 6.16 ± 0.07, 15.32 ± 0.86, 5.1 ± 0.1, 32.83 ± 1.74, 14.1 ± 0.96 and 5.73 ± 0.42%, respectively which were in accordance with the findings by Tolve et al. [19] and Baldan et al. [20].
Viscoelastic properties
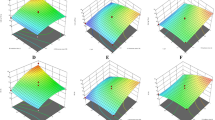
Mechanical spectra of different treatments prepared using LM pectin and also complete substituition of it with GPP in different amounts are presented in Fig. 1. As it is apparent from Fig. 1A, all treatments resulted in a gel structure, with G´ greater than G″. However, the rheology of all treatments were significantly different (p < 0.05) from each other except the control and T3 which had no significant difference (p > 0.05). Considering the pectin content,GPP, T1, T2, T3 and T4, respectively, possibly contained 0.143, 0.286, 0.429 and 0.573 g, respectively. Also, GPP contains over 32.83% fiber including both soluble and insoluble fibers. Fibers are largely contribute in water absorption to increase the viscosity and also decreasing the water activity [21]. Hence, it could be the reason of there was no significant difference (p > 0.05) among the control with 0.5 g LM pectin and the T3 treatment with 0.429 g pectin. From Fig. 1A, it is clear that the T1 had the lowest viscoelatic properties with the encountered shear force which indicates the weakest gel structure. On the other hand, T4 showed even higher viscoelastic properties than the control. But it is worth mentioning that the increasing of G´ and G″ as a matter of using GPP, was not linear; Meaning usig 2.5 g extra GPP in each treatment from T1 to T2, T2 to T3 and T3 to T4, caused G´ to be increased 9%, 8.4% and 8%, respectively, which is hypothesized to be due to the lack of free water to be absorbed [22]. Regarding the G″ this manner was 11.3%, 18% and 6.8%. It could be seen that a jump in viscosity was seen in T2 to T3 that is assumed to be as a result of high water binding of fibers in GPP which their main role is to bind the water and increase the viscosity and less gelation [23].
Complex viscosity defines the total resistance to flow as a matter of applied stress [24]. As could be seen in Fig. 1B which shows the complex viscosity of the treatments, the maximum and the minimum stress needed to flow the fruit fillings were seen in T4 and T1, respectively. Although, T3 and the control sample had no significant difference (p > 0.05) at the beginning of mixing, a steep slope was seen in the complex viscosity of the control, while, T3 showed lower rate in increasing of the strain. The reason could be refered to the disintegration of gel structure caused by pectin as a matter of shearing, while, the viscosity of T3 caused by both pectin and fiber that does not form a structure like pectin [25]. Therefore, lower rate of disintegration viscose feature of all treatments was higher than their elastic feature which is in agreementvisc of the total structure caused lower rate of strain changes. The interesting point was that 1133 s (19 min) after applying shear, T3, T4 and control treatments had the same complex viscosity; meaning, despite the difference in the total pectin and fiber content of these treatments, 1133 s mixing led to enough structure disintegration (Fig. 1B). From Fig. 1C, it could be found that the viscose feature of all treatments was higher than their elastic feature which is in agreement with the results showed in Fig. 1A. The highest and the lowest amount of loss tangent were seen to be T3 and T1, respectively; However, no significant difference (p > 0.05) was seen among T3, T4 and Control. It is hypothesized that the remained free water for binding to the pectin or fibers was very low regarding the T3, T4 and the Control. Hence, the changes were not significant for these three treatments.
Confocal laser scanning microscopy (CLSM) and fluorescence microscopy
CLSM could distinguish the components of a complex matrix through color discrimination. As it is apparent from Fig. 2, the highest amount of pectin and fibers could be found in image E (T4) which was followed by A (Control), D (T3), C (T2) and B (T1), respectively. However, the amount of red areas in images A and D seems to be very close to each other;which means 7.5% of GPP could have almost the same active ingredients as 0.5% LM pectin. Also, it could be found that the amount of free hydroxyl groups (green areas) in image B is the highest that refers to the lowest capability of its active ingredients to bind the free hydroxyl groups, while T4 showed the lowest free hydroxyl groups which shows the highest amount of water binding ingredients. Based on the image analysis of the images of Fig. 2 from A to E, the ratio of red areas (pectin and fibers) to the green areas (the rest of fruit filing matrix) has been calculated to be 0.991, 0.579, 0.839, 0.954 and 1.150, respectively.
Regarding the fluorescence imgaes, it was possible to indirectly identify the water associated with sugar molecules (bright zones displayed in the micrographs) and separately, water associated with hydrocolloids (darker zones). Figure 3A-E presents fluorescent micrograph of the fruit filling treatments where hydrocolloid gel network is shown as continuous dark regions which could be seen that the greater amount of GPP in the treatments resulted in more darker zones, indicating a more uniform gel structure [7].
Moisture content and water activity
Regarding the Fig. 4 which shows the moisture content and the aw of the prepared treatments before (A) and after (B) baking. From Fig. 4A, it is clear that the highest and the lowest moisture contents were seen to be the Control and T4 which were significantly (p < 0.05) different from each other, while Control and T1, T1 and T2, T2 and T3 and finally, T3 and T4, had no significant difference with each other (p > 0.05), separatly. Regarding the water activity, T1 had the highest aw which was followed by T2, T3, Control and T4, respectively. However, T3 and Control had no significant difference (p > 0.05). Hence, it could be realized that, although, the moisture content of T3 and control were significantly different (p < 0.05) with each other; their water activity had no significant difference (p > 0.05). The results of moisture contents and water activities after baking were different than before baking. After exposure of the fruit filling to heat (Fig. 4B), as a matter of lower capability of retaining of moisture by T1, it had the lowest moisture content (p < 0.05) [26], while its aw had no significant difference (p > 0.05) with T3 and Control. However, Control, T3 and T4 had no significant difference (p > 0.05) with each other.
It is worth mentioning that as the GPP content increased, the structure of fruit filling was firmer due to having less unbonded water which result in higher viscosity (Fig. 1B).
Extrusion test
As it could be seen from the Table 2, the highest and the lowest maximum forces before baking (freshly prepared) were related to the T4 and T1, while Control and T3 showed no significant difference (p > 0.05). However, the results of baked fillings were different; meaning that the highest and the lowest maximum forces were seen to be T4 and T2. The rest of the treatments including T1, T3 and Control had no significant difference (p > 0.05). The point is that the maximum force of before baking and after baking of Control, T3 and T4 had no significant difference (p > 0.05) with each other which means that the amount of unbonded water was very low which let them to keep their moisture during baking and keep their structure which was in agreement with results found by Cropotova et al. [5], and Razak et al. [3]. On the contrary, after baking, the maximum force of T1 and T2 increased drastically which is hypothesized to be due to the lack of water binding agents in their matrix. Agudelo et al. [12] reported that the increasing of the needed force to deform the fruit filling after baking in comparison to its freshly prepared form, indicates to the loss of quality in terms of softness due to removal of moisture content. Area under the curve (AUC) shows the amount of the work done by the probe to penetrate and compress the fruit filling. The results were in complete agrrement with the results of maximum force, both for the freshly prepared and baked fruit fillings. Hence, the highest and the lowest amount of AUC in freshly prepared fruit fillings and in baked fruit fillings were T4 and T1 and T4 and T2, respectively. The adhesiveness value was seen to be increased as the GPP increased. Therefore, regarding the freshly prepared fruit fillings T4 had significantly (p < 0.05) higher adhesiveness than the other treatments, while Control and T3 had no significant difference (p > 0.05) with each other. Despite the maximum force and AUC of baked fruit fillings, the adhesiveness value of T1 was significantly different (p < 0.05) with the Control and T3 (Table 2). The reason could be due to increasing the hardness of the filling as a matter of baking procedure and the loss of water content [12].
To get a complete picture of the impact of the different amounts of GPP on the characteristics of the fruit filling, a principal component analysis (PCA) was constructed (Fig. 5). The PCA explained 78.8% and 13.9% of the variability for PC1 and PC2, respectively. Overall, the rheological parameters were the highest for the Control, T3 and T4, while the green area and the aw before baking were the highest for T1 and the aw after baking and water content before baking were the most for T2. The remaining of the measured parameters tended to the Control, T3 and T4 treatments.
PCA graph of rheological and physical characteristics of various fruit fillings using various percentages of the grape pomace powder (GPP) including Control (0 g GPP, 0.5 g pectin), T1 (2.5 g GPP, 0 g pectin), T2 (5 g GPP, 0 g pectin), T3 (7.5 g GPP, 0 g pectin) and T4 (10 g GPP, 0 g pectin). F.P is the abbreviation of the freshly prepared
Conclusion
Data presented in this study highlights the effect of replacing the pectin with GPP on the characteristics of the fruit fillings. In terms of viscoelastic properties, it was seen that increasing the used amount of GPP caused both G´ and G″ to be increased. However, their increasing were not at the same pace. As the GPP increaed, the available water to be bounded to the hydrocolloids was decreased and that could be a possible explanation for decreasing the ratio of viscoelasticity increasing from T1 to T4. The findings of CLSM also revealed that the amount of hydrocolloids in T3 is close to Control and the T1 and T4 had the least and the most content of hydrocolloids. Also, the outcomes of fluorescence microscopy showed that gel structure of the treatment contains 7.5% of GPP is the closest to the Control. Moreover, it was found that the prepared treatments were meant to be bake stable, the Control, T3 and T4 had this capability did not showed significant (p > 0.05) increase in the hardness and AUC from before baking to the baked condition. Also, although the aw of T1 after baking had no significant difference (p > 0.05) with the Control and T3, as its hardness increased significantly (p < 0.05) after baking, it could not be considered as a bake stable fruit filling. All in all, it was seen that the replacement of pectin with 7.5% of GPP had almost the same results in terms of characteristics of final fruit filling.
Data availability
The dataset during and analyzed during the current study are available from the first author on reasonable request.
References
E.C. Sousa, A.M.A. Uchôa-Thomaz, J.O.B. Carioca, S.M. de Morais, A. de Lima, C.G. Martins, C.D. Alexandrino, P.A.T. Ferreira, A.L.M. Rodrigues, S.P. Rodrigues, J. do N., Silva, L.L. Rodrigues, Food Sci. Technol. 34, 135 (2014).
S. Ahmadi, B.A. Siahsar, Ciencia E Investigación Agrar. 38, 291 (2011).
R.A. Razak, R. Karim, R. Sulaiman, N. Hussain, Int. Food Res. J. 25, 1109 (2018).
S. Inbaraj, K. Sridhar, M. Sharma, N.S. Said, I. Fola, Olawuyi, W.Y. Lee, Gels. 9, 732 (2023).
J. Cropotova, S. Popel, L. Parşacova, J. Process. Energy Agric. 17, 20 (2013).
P.S. Taoukis, M. Richardson, Water Activity Foods: Fundamentals and Applications, vol 385 (Blackwell, 2020).
J. Cropotova, U. Tylewicz, N. Dellarosa, L. Laghi, S. Romani, and M. Dalla Rosa, Food Chem 195, 71 (2016).
L. Prosky, N.-G. Asp, I. Furda, J.W. Devries, T.F. Schweizer, B.F. Harland, J. AOAC Int. 67, 1044 (1984).
A. Koç, M. Erbaş, J. Food Sci. 87, 2072 (2022)
J. Müller-Maatsch, M. Bencivenni, A. Caligiani, T. Tedeschi, G. Bruggeman, M. Bosch, J. Petrusan, B. Van Droogenbroeck, K. Elst, S. Sforza, Food Chem. 201, 37 (2016).
A. Carcelli, A. Albertini, E. Vittadini, E. Carini, Int. J. Gastronomy Food Sci. 28, 100545 (2022).
A. Agudelo, P. Varela, T. Sanz, S. Fiszman, Food Hydrocoll. 40, 203 (2014).
F. Ronda, B. Oliete, M. Gómez, P.A. Caballero, V. Pando, J. Food Eng. 102, 272 (2011).
AACC, American Association of Cereal Chemists, St. Paul. 11th Editi, (2010).
M. H. F. Felisberto, A. L. Wahanik, C. R. Gomes-Ruffi, M. T. P. S. Clerici, Y. K. Chang, and C. J. Steel, LWT - FOOD SCI TECHNOL. 63, 1049 (2015).
A. Arocas, T. Sanz, S.M. Fiszman, Food Hydrocoll. 23, 2478 (2009).
G. Savary, S. Handschin, B. Conde-Petit, N. Cayot, J.L. Doublier, Food Hydrocoll. 22, 520 (2008).
Q. Ruan, X. Yang, L. Zeng, J. Qi, Food Hydrocoll. 95, 53 (2019).
R. Tolve, B. Simonato, G. Rainero, F. Bianchi, C. Rizzi, M. Cervini, G. Giuberti, Foods. 10, 75 (2021).
Y. Baldán, M. Riveros, M.P. Fabani, R. Rodriguez, Biomass Convers. Biorefinery. 13, 9997 (2023).
M.M. Selani, S.G.C. Brazaca, C.T. Dos Santos Dias, W.S. Ratnayake, R.A. Flores, A. Bianchini, Food Chem. 163, 23 (2014).
Y.S. Ma, Y. Pan, Q.T. Xie, X.M. Li, B. Zhang, H.Q. Chen, Food Chem. 274, 319 (2019).
A.B.B. Bender, C.S. Speroni, P.R. Salvador, B.B. Loureiro, N.M. Lovatto, F.R. Goulart, M.T. Lovatto, M.Z. Miranda, L.P. Silva, N.G. Penna, J. Culin. Sci. Technol. 15, 143 (2017).
S.K. Giri, M.K. Tripathi, N. Kotwaliwale, J. Food Sci. Technol. 55, 1667 (2018).
J.F. Douglas, Gels. 4, 19 (2018).
A. Quintanilla, H. Zhang, J. Powers, S.S. Sablani, Food Bioprocess Technol. 14, 804 (2021).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Farzad Saberi: Conceptualization, Methodology, Writing-Original draft, Writing - Review & Editing. Mostafa Karami: Methodology, Writing - Review & Editing, Supervision. Azam Shiri: Validation, Formal analysis and Methodology. Mousa Rasouli: Resources, Writing - Review & Editing, Rouhollah Karimi: Resources, Visualization, Investigation. Marek Kieliszek: Visualization, Investigation, Formal analysis, Writing-review, editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Neither ethics approval nor consent is needed.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saberi, F., Karami, M., Shiri, A. et al. Using grape pomace powder as a pectin replacer to prepare low water activity bake-stable fruit filling. Food Measure 18, 4314–4322 (2024). https://doi.org/10.1007/s11694-024-02495-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02495-w