Abstract
Margarine, an emulsion constituted mainly of fat up to 90%, is used for highly variable cooking purposes. The susceptibility of lipids to oxidation needs processing operations/strategies that increase their shelf life and resistance to high temperatures such as the addition of synthetic antioxidants. Due to the toxicity of these later, the demand and search for natural antioxidants are continuously increasing by consumers and industrials. In this study, the antioxidant potential of Moringa oleífera leaves extract (MOLE), obtained from dry leaves with 80% methanol, for stabilization of margarine was evaluated under accelerated storage (30 days at 65 °C). MOLE was added to margarine at 100, 400, 600, and 800 mg/kg and compared with the control (no MOLE or additive) and reference margarine supplemented with a standard synthetic antioxidant (100 mg/kg of tocopheryl acetate). The progression of oxidation was monitored by analyzing the formation of primary and secondary lipid oxidation compounds as well as fatty acid, tocopherol, tocotrienol, and sterol profiles. The results revealed that 600 and 800 mg/kg MOLE were endowed with the highest antioxidant properties during accelerated storage of margarine. MOLE effectively reduced the peroxide and p-anisidine values as well as the induction time by Rancimat, even at 100 mg/kg. Color changes and degradation of fatty acids, especially linoleic acid (C18:2), were also statistically reduced. Interestingly, MOLE also exhibited a strong ability to protect endogenous α-tocopherol against oxidative degradation. It revealed also that MOLE expressed an effective antioxidant effect and increased the thermal stability of margarine under hot storage. Thus, the application of MOLE as a natural antioxidant can be of particular interest in lipid based agro-food industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Margarine, a lipidic emulsion-based product, is commonly used for thermal processes. Lipid oxidation during storage and heating is one of the principal causes of its quality deterioration [1,2,3]. In order to delay, reduce, or prevent the oxidation of margarine, industries add natural and synthetic antioxidants, being DL-alpha tocopherol the most widely used [4]. Synthetic antioxidants are effective at low temperatures [5] but become unstable during heating, which induces their quick degradation producing volatile compounds or generating adduct products with food [6]. Indeed, due to the toxic effects of synthetic additives, the demand for natural antioxidants and the research of alternative resources by consumers and industries is continually increasing [7, 8].
Many investigations are focused on natural antioxidants able to improve the oxidative stability of food lipid systems [9, 10]. Scientists have made appreciable progress in replacing these synthetic antioxidants with different natural antioxidant sources from oilseeds, spices, fruits, vegetables, nuts, and barks and numerous products are under investigation regarding their stability property and antioxidant quality [11, 12]. Guo et al. [13] employed the ethanol extract of rosemary in palm oil during accelerated storage. Fruehwirth et al. [14] used green tea extract as a promising antioxidant in margarine. Similarly, tomato by-product antioxidants were used to increase the storage ability of traditional Tunisian butter [9], and Opuntia ficus-indica peels extract was added as a natural antioxidant to ameliorate the storage potential of margarine [4].
Among the plant that have been studied, Moringa oleífera, is the most widely cultivated species of Moringaceae monogeneric family, which is native to the sub-Himalayan tracts of India, Pakistan, Bangladesh, and Afghanistan. It’s being grown in Africa, Tropical Asia, Latin America, Caribbean, Florida, and Pacific Islands [15]. In 2001, this plant was introduced in Southwestern Algeria by Sahrawi refugees [16].
Due to its substantial benefits to human health and important nutritional valor (proteins, vitamins A and C, potassium, calcium, and iron), Moringa oleífera is qualified as “the miracle tree” [7, 17]. Numerous therapeutic and pharmacologic properties of different parts of this plant have been studied [18, 19]. M. oleífera was used traditionally as hepatoprotective, antihypertensive, antispasmodic, antiseptic, antiepileptic, and diuretic as well as used as stimulant and tonic. This plant expressed several pharmacological properties such as anti-tumor, anti-inflammatory, antidiabetes, and antimicrobial activities and is implicated in the regulation of the thyroid status and cholesterol level [17, 20]. The majority of its medicinal and biological properties have been attributed to the presence of some vitamins, minerals, carotenoids, glucosinolates, isothiocyanates, and phenolics [21, 22]. M. oleífera leaves are endowed particularly with high phenolic content and expressed a high antioxidant capacity [23]. Moreover, the antioxidant potential of Moringa oleífera leaf extracts, obtained by different solvents, has demonstrated the interesting oxidative stability of sunflower oil subjected to accelerated storage [24]. Another accelerated oxidation study performed by Arabshahi-Delouee et al. [25] confirmed the effectiveness of Moringa oleífera leaf extract (MOLE) in enhancing the oxidative stability of soybean oil.
In a perspective to valorize the antioxidant potential of Moringa oleífera leaves, the supplementation of different food matrices with compatible extracts is an interesting approach. The present study comes in the same line as our recent work devoted to the structural, textural properties, and oxidative stability of margarine (palm oil basis) enriched with MOLE under refrigeration storage [26]. Indeed, the present investigation is focused on phytochemicals preservation (vitamin E profile and sterol composition) and the chemical stability of margarine (soybean oil base) supplemented by MOLE under heat-accelerated storage.
To provide a scientific basis for the replacement of synthetic antioxidants with MOLE, this study aims to compare the oxidative stability under accelerated storage of margarines enriched with four different MOLE concentrations (100, 400, 600, and 800 mg/kg) with control margarine (without any addition) and reference margarine (supplemented with synthetic vitamin E). The margarine oxidation is monitored by the measurement of primary and secondary lipid oxidation products (peroxide value (PV), p-Anisidine value (PAV), and Rancimat (induction time) and composition profiles (fatty acid, tocopherols, and sterols).
Materials and methods
Moringa oleífera leaves preparation and antioxidants extraction
The Moringa oleífera leaves harvested from Oud Souf department (Algeria) were dried in free air under shadow and temperatures ranging from 20 to 28 °C, then ground to pass a 0.5 mm sieve (Retsch Analytical sieve shaker AS 200), and defatted by Soxhlet extraction with hexane. Extraction was performed by maceration of powder of M. oleífera leaves for two hours in 80% methanol under magnetic stirring [27]. The hydro-alcoholic extract was filtered and centrifuged for 30 min at 11,000 g. The dried extract was obtained after solvent removal using a rotary evaporator (40 °C) followed by lyophilization using a freeze drier system (ALPHA 1–4 LD plus, lyophilizer, Christ, Osterode, Germany) to insuring maximum moisture removal.
Margarines preparation
In the present study, margarine was produced at a laboratory scale in COGB Labelle fat industry (Bejaia Algeria) according to the process applied by the company. Three types of margarines were prepared: negative control margarine (margarine without any antioxidant/extract addition), enriched margarines (added with Moringa Oleífera leaves extract (MOLE) at different concentrations (100, 400, 600, 800 mg/kg MOLE, mg of dry Moriga oleífera leaves extract/kg of margarine), and reference margarine (the commercial margarine was added with 100 mg/kg of vitamin E (DL-alpha tocopheryl acetate ≥ 98% purity) as a synthetic antioxidant), as commercially available in Algeria. The MOLE concentrations used were chosen according to the literature and after some tested concentrations.
Lipid (84%) and aqueous phases (16% ) were prepared separately. The lipid phase consisted of a mixture of vegetable oils mainly from refined soybean oil and three esterified oils (palm, stearin, and soybean), the emulsifiers (0.28%) mono and diglycerides (E471), and 0.2% soy lecithin (E322). The aqueous phase consisted of osmosis water and 0.28% salt (NaCl). The lyophilized MOLE was incorporated in the aqueous phase of the enriched margarines while 100 mg/kg of vitamin E was incorporated into the lipid phase of the reference margarine. After mixing the two phases for approximately 10 min to create the emulsion, the mixtures were cooled with iced water for 30 min for the crystallization of the product. All margarines were prepared on the same day under the same conditions. Margarine emulsions produced were packed in sticks of 250 g. Each margarine was prepared in triplicate and each sample was at least analyzed two times.
Storage procedure
The margarine samples were subjected to accelerated oxidation following Shin et al. [28]. This test, used by most researchers to estimate the stability of margarines to oxidation, is representative of commonly used methods for food thermal processing as cooking. Margarines were packed in glass jar containers, opened to the atmosphere, and subjected to storage under accelerated temperature using 65 °C in a controlled temperature oven (Memmert, Schwabach, Germany) for 30 days. The analyses were performed for samples stored for 0, 5, 10, 15, 20, 25, and 30 days.
Analytical methods
Extraction and estimation of total phenolic content of enriched margarines
The extraction of phenolic compounds was performed using 10 g of margarine that was dissolved in hexane (20 mL) and the solution was subjected to extraction three times successively with 20 mL of mixture methanol/water (60/40). The mixture was shaken and the polar phase was recovered after centrifugation (4000 rpm/10min).
The determination of total phenolic content was estimated by the Folin-Ciocalteu method, as described by Škerget et al. [29] and using gallic acid as a standard reference. For this, an aliquot of margarine extract (500 µL) was mixed with 2.5 mL of Folin–Ciocalteu (10%). After 5 min, 2 mL of sodium carbonate (75 g/L) was added. The mixture was incubated at 50 °C for 5 min and the absorbance was read at 760 nm. Results were expressed as milligrams of gallic acid equivalent per 100 g of margarine (mg GAE/100 g).
Induction period by Rancimat
The induction period of prepared margarines was determined by Rancimat test according to ISO-6886 method [30]. About 3 g of each lipid phase of margarine sample was weighed in individual reaction vessels of the Rancimat apparatus (Metrohm CH 679, Herisau, Switzerland) and vessels were placed in a heating block. After that, the air was supplied by a flow rate of 15 L/h and the temperature was adjusted to 120 ± 1.6 °C. The volatile compounds being collected in water and the time necessary to reach the conductivity inflection was recorded.
Lipid oxidation
Before analysis, samples were heated just above the melting point, then sodium sulfate was added and the mixture was stirred thoroughly, filtered then centrifuged. The peroxide index was determined following AOAC methods [31]. The p-anisidine value, used as an indicator of unsaturated aldehydes during secondary oxidation was determined according to ISO 6885 method [32].
Color
Color coordinates of fresh and stored margarines were determined on the homogenized samples using a Chroma Meter CR-400 (Konica Minolta, Tokyo, Japan). The color of samples was described by L*, a*, and b* color scale calibrated with a white tile, measuring lightness (L*), redness (a*, red/green), and yellowness (b*, yellow/blue) [33].
Fatty acids profile
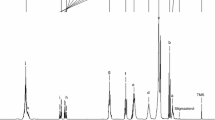
Fatty acids (FA) composition was determined by gas-liquid chromatography with flame ionization detection (GC-FID) (Palo Alto, CA, USA), equipped with a Select FAME column (100 m 50 × 0.25mmi.d.; Agilent J&W). Briefly, the margarine oil glycerides were hydrolyzed with boiling methanolic potassium hydroxide solution (0.5 M). The released FA were converted to methyl esters by the reaction with boron trifluoride-methanol solution (BF3/MeOH, 14%), and extracted with n-heptane. The fatty acids profile was analyzed with a Chrompack CP 9001 chromatography (Chrompack, Middelburg, Netherlands). The temperatures of 250 and 260 °C were set for the injector and detector, respectively. Helium was used as carrier gas at an internal pressure of 190 kPa. The column temperature was 180 °C, for a 3 min hold, and then programmed to increase to 250 °C at a rate of 3 °C/min and then held for 10 min. The split ratio was 1:50, with a total run time of 60 min. The results were expressed as grams per 100 g of margarine oil. A certified fatty acids methyl ester (FAME) mixture (37 fatty acids from C4:0 to C24:0) from Supelco (TraceCERT, Bellefonte, PA, USA) was used as standards [34].
Free vitamin E profile
Free vitamin E contents were analyzed by HPLC with fluorescence detection (Jasco, Japan) following Malheiro et al. [35]. A 20 mg portion of fresh margarine oils or about 80 mg amount of stored margarine oils was blended with 7.5 µL of internal standard solution (tocol) in a 1 mL volume of n-hexane and homogenized by stirring. The mixture was centrifuged for 5 min at 3500 rpm and the supernatant was analyzed by HPLC. The chromatographic separation was achieved on a Supelcosil™ LC-SI column (3 μm) 75 × 3.0 mm (Supelco, Bellefonte, PA), conditioned at 23 °C (ECOM, ECO 2000, Czech Republic). A mixture of n-hexane: dioxane (97:3) was used as eluent, at a flow rate of 1.0 mL/min with a total run time of 12 min. The analysis of results was performed with ChromNAV Control Center — JASCO Chromatography Data Station (Japan). The identification of compounds was achieved by comparison to authentic standards. Quantification was based on the internal standard method using the fluorescence signal response and individual calibration curves for each compound (α, β, γ, and δ tocopherols and tocotrienols).
Sterol composition
Sterols composition was evaluated by GC-FID (TRACE GC, Thermo Finnigan, Milan, Italy) equipped with a DB-5MS column (30 m × 0.25 mm, 0.25 mm; Agilent J&W). The procedure was based on alkaline saponification using α-cholestanol (0.2%) as the internal standard, followed by extraction and conversion to trimethylsilyl esters [36]. A temperature gradient from 250 to 280 °C was used with a helium flow rate of 1 mL/min for a total run time of 45 min. Individual sterol amount was reported in mg per kg of margarine oil, based on the area ratio to the internal standard.
Statistical data treatment
All analyses were carried out in triplicate and data were reported as mean ± standard deviation. Differences between the treatments regarding the stability of margarines under accelerated storage were tested using STATISTICA software (5.5) following the one-way ANOVA and Fisher LSD post hoc test. Differences were considered statistically significant at p < 0.05.
Results and discussion
Total phenolic content
The results of TPC at time zero were presented in Table 1. Margarines represented by the control, supplemented with 100 mg/kg vitamin E and 100 mg/kg MOLE expressed statistically similar and lower TPC. The latter increased significantly with the increase of MOLE concentrations from 100 to 800 mg/kg, demonstrating the enrichment of elaborated margarines with moringa phenolics.
Previous results from our laboratory team on the characterization of phenolic profile by high-resolution mass spectrometry (HPLC-DAD-ESI-MSn) on the same sample of Moringa oleífera leaves harvested from Oued Souf (south Algeria), led to the identification of twelve compounds, mainly phenolics, with chlorogenic acids (3-caffeoylquinic and 4-caffeoylquinic acids) and quercetin glycosides (quercetin-3-O-glucoside, quercetin-3-O-galactoside, and quercetin 3-O-(6′′-malonyl glucoside) that expressed a high quenching capacity towards DPPH radical [37].
Some other works demonstrated the enrichment of elaborated products with phenolic extracted from natural products. Abid et al. [9] observed that the incorporation of tomato processing by-product extract in traditional Tunisian butter enriched it in bioactive compounds endowed with potent antioxidative properties. Similarly, the butter supplemented with cinnamon, characterized by an elevated level of bioactive compounds, revealed a high TPC and expressed better antioxidant activity than the control butter [38].
Induction period
The measurement of Rancimat induction period represents another interesting parameter that indicates on resistance aptitude of lipids to oxidation induced by heating under oxygen flow. From the results, it was noticed that the control margarine at time zero had the lowest induction period (4 h), the addition of vitamin E at 100 mg/kg or MOLE at 100 and 400 mg/kg ameliorate significantly the oxidative properties of corresponding margarines (Table 1). The induction period was gradually prolonged with the increase of moringa extract concentrations, and this was most likely related to TPC.
MOLE addition at concentrations of 400, 600, or 800 mg/kg to margarine induced an increase in oxidation resistance, with better antioxidant properties as compared to margarine with vitamin E or that without antioxidants. Peroxide and acid values of MOLE supplemented margarines were considerably reduced demonstrating the efficiency of Moringa oleífera leaves [12]. The results reported by Nadeem et al. [39] concluded also that Moringa oleífera leaf extract at 600 mg/kg was proposed to improve the oxidative stability of butter oil.
In the same line, the addition of green tea extract in margarine preparation was enhanced significantly the induction period of oxidation, suggesting that polyphenols of green tea decrease lipid mobility at the surface of the water droplets and might chelate the transition metals at the interface thus decreasing lipid oxidation [14].
Margarines were supplemented with 100–800 mg/kg of MOLE or 100 mg/kg of vitamin E; TPC, total phenolic content expressed as mg gallic acid equivalent/100 g margarine; IP, induction period; Results in the same column with different letters are statistically different (p < 0.05, a > b > c > d); mean ± SD; N = 3.
Accelerated oxidation test
Effect of MOLE on peroxide value (PV)
PV is commonly used as a measure of the degree of primary oxidation. The influence of MOLE addition during accelerated storage of margarines was detailed in Fig. 1 A, where a continuous increase in PV during the storage period was observed for all samples. This increase in PV was attributed to the continual formation of peroxides (hydroperoxides, dihydroperoxides, and cyclic peroxides). Initially, the progression in PV was relatively slow, but a rapid increase was observed after the 5th day of storage. At the end of the 30 days of storage at 65 °C, the control sample (margarine without any antioxidant/extract) exhibited a significantly higher (p < 0.05) peroxide value (136 meq/kg), while the lowest value was registered in the 800 mg/kg MOLE margarine (103 meq/kg). The results also showed that the addition of MOLE in the margarines provided statistically lower PV than the reference margarine with vitamin E, with exception of margarine added with 100 mg/kg MOLE, and this effect was concentration dependent.
The interesting protective effect of MOLE in the first stages of oxidation might be attributed to the ability of the extract in stabilizing peroxides, thus inhibiting the propagation stage of lipid oxidation. The present results were in good agreement with those reported by Arabshahi-Delouee et al. [25]. This effect can be directly related to the presence of MOLE bioactive compounds, particularly phenolic compounds known to offer a great deal of resistance toward oxidation in the accelerated oxidation reaction [40].
Effect of MOLE on p-anisidine value
Anisidine analysis is commonly used for evaluating secondary lipid oxidation, measuring the accumulation of aldehyde carbonyl bonds generated during the secondary lipid oxidation [13]. P-Anisidine value (PAV) of margarines incorporated with MOLE at 65 °C during the 30 days of sampling were detailed in Fig. 1B. The addition of MOLE into margarine reduces the PAV significantly (p < 0.05) in comparison with the control and reference margarines. There were no statistically significant differences between PAV for 400, 600, and 800 mg/kg MOLE margarines during the first 20 days (p > 0.05) nor between 600 and 800 mg/kg MOLE during the entire storage period. At the 10th day, PAV of margarine with 100 mg/kg MOLE had also no significant difference compared to 400, 600, and 800 mg/kg MOLE but had a significantly lower PAV compared to both control and reference margarines (p < 0.05). However, for longer storage times the 100 mg/kg MOLE margarine had no significant difference compared to reference margarine with vitamin E and control margarines.
According to peroxide values and PAV as well as Rancimat results of margarines over the 30 days of storage at elevated temperature, it can be concluded that 800 and 600 mg/kg MOLE margarines expressed the highest resistance to oxidation. Similar results were observed between the samples prepared with 100 mg/kg MOLE and vitamin E.
In addition, MOLE contained a large number of diverse compounds that can probably act in synergy [41] at a high temperature compared to the synthetic antioxidant (vitamin E) that most likely becomes unstable under the used storage temperature [13].
Oxidative stability of margarines during the 30 days of storage at 65 °C. (A) Peroxide index (B) p-anisidine value
Margarines were supplemented with 100–800 mg/kg of MOLE or 100 mg/kg of vitamin E; for each time, results with different letters are statistically different at p < 0.05 with a < b < c < d (mean ± SD, N = 3)
Color
The effect of the supplementation by MOLE on the color of margarine during storage at elevated temperature was investigated and CieLab coordinates (L*, a*, b*) were given in Table 2. L* values of all samples were significantly (p < 0.05) decreased during the first period of storage and this is due to the reduction of the lightness of margarine. In fact, hydroperoxides were formed during hot storage, from fatty acids, particularly the unsaturated ones, where their broken led to the formation of ketones and aldehydes. These last react with amines of phospholipid through the Maillard reaction resulting in components that increase the darkening thus reducing lightness. Due to the instability of Maillard compounds, the degradation of these formed molecules occurred later resulting in the increase of lightness [42]. The decomposition products consisted of non-volatile polar compounds (in particular aldehydes, alkylbenzenes, and other aromatic hydrocarbons), triacylglycerol dimers and polymers, and cyclic compounds produced and accumulated during hot storage [43] lead to the decrease in a* value. The formation and degradation of several compounds during the heating process caused fluctuations of yellowness (+ b*) of margarines. The heat treatment of the fat leads to very complex reactions of formation and degradation of compounds which affect the color. It was noticed that the color change of samples with MOLE at 400, 600, and 800 mg/kg was relatively smaller than that of the control demonstrating the contribution of moringa extract to maintaining margarine quality.
Fatty acid profile
Determination of fatty acid balance ratios is an effective way to measure the degree of oil degradation. The modifications of fatty acid composition can be used as a good parameter to estimate the magnitude of margarine oxidation. Initial and final fatty acid profiles of the margarines stored at accelerated conditions were determined (Table 3). The major fatty acids in all margarines were linoleic acid (C18:2), palmitic acid (C16:0), and oleic acid (C18:1). Stearic acid (C18:0) and linolenic acid (C18:3) were also detected but with low amounts. The fatty acid composition reflects the fatty acid content of the oils used for margarine preparation.
The margarines freshly produced have practically the same fatty acids profile. Therefore, the inclusion of MOLE at all levels did not affect appreciably the fatty acid composition of different margarines produced. When comparing the amounts of fatty acids before and after storage, a clear reduction of unsaturated fatty acids was observed. The decrease was particularly noticeable for fatty acids that are more prone to oxidation such as linoleic acid, clearly behind the formation of primary and secondary products [44]. However, the decrease in C18:2 was sensitively lower in margarines enriched with MOLE at 400, 600, and 800 mg/kg. Additionally, the ratio of linoleic acid/palmitic acid (C18:2/C16:0), used as an indicator of the level of oxidative deterioration [45], decreased from 1.21 at day zero, to around 0.76–0.90 on all margarines after 30 days being higher in the margarine with 600 and 800 mg/kg.
Unsaturated fats expressed a high susceptibility to thermal oxidation but the addition of bioactive compounds such as those of M. oleífera can prevent or reduce the oxidation reactions of fatty acids. MOLE at all used concentrations reduce sensitively the degradation of polyunsaturated fatty acids especially linoleic acid (C18:2) compared to the control without addition. The smaller efficiency of vitamin E in comparison with MOLE extract that requires more than 400 mg/kg for equivalent effect can be due to the nature of antioxidants, particularly their polarity/solubility. MOLE compounds were probably more concentrated in the aqueous phase interface, while α-tocopherol should be totally incorporated in the oil phase which is related to the chemical affinity and the polarity of molecules thus expressing different protection mechanisms according to the “Polar Paradox” [46]. Once more, this data indicates that the addition of MOLE to margarine seems to be beneficial during the accelerated storage, and corroborates with the data obtained for PV, PAV, and Rancimat test.
.
Vitamin E composition
Vitamin E, particularly tocopherols, is an important component contributing to the nutritional value, both by its vitaminic actions and antioxidant properties. Its presence is also important from a technological point of view due to its antioxidant activity, being generally oxidized to quinidine and dimers while protecting the fat [47]. Table 4 presented the changes that occurred in the tocopherol and tocotrienol contents for the first 10 days of storage. The choice of analyzing vitamin E after only the first period of storage was due to its sensitivity and will be highly probable to disappear after 30 days.
Based on the HPLC analysis, margarines contained four major tocopherols and four tocotrienols (α, β, γ, and δ analogs) with α- and γ-tocopherols being the most relevant being natural components of refined soybean oil. The storage at 65 °C caused a huge decrease for all compounds but was more noticeable for α-tocopherol. The loss of α-tocotrienol was lower in margarines containing 600 and 800 mg/kg MOLE compared to margarines with 100 and 400 mg/kg MOLE, reference, and control ones. The storage under heating affected differently tocopherol and tocotrienol contents. Indeed, α analogs decreased the most followed by β and then γ forms while δ analogs were the best preserved. Overall, the loss of vitamin E in margarines containing 600 and 800 mg/kg of MOLE (34% of loss) was lower than in margarines with 400 and 100 mg/kg MOLE (42% of loss) followed by control and reference margarines (50% of loss). The MOLE addition, particularly at high concentrations, could potentially delay the depletion of endogenous vitamin E. Different degradation patterns were also observed by Chen et al. [48] using storage at 45 °C in base algae oil and water-in-algae oil emulsions had different patterns, α-tocopherol disappeared first, followed by γ-tocopherol, while δ- tocopherol was the most stable one. Strong correlations were also found between α- and γ-tocopherols degradation and the oxidation extent of many oils, such as purified rapeseed oil, but not for δ-tocopherol [49].
Indeed, Wang et al. [50] when studying the dependence of autoxidation of perilla oil and tocopherol degradation, have found that phenolic compounds were implicated in oxidative stability by protecting tocopherols from degradation, particularly in the first steps of oil autoxidation. Similarly, grape phenolics were endowed with a good ability to protect α-tocopherol levels of fish muscle during frozen storage that preserves the endogenous antioxidant system [51]. Maintaining vitamin E with antioxidants such as polyphenols is a good measure to prevent lipid oxidation.
Sterol
In margarine, four phytosterols (campesterol, stigmasterol, β-sitosterol, 5-avenasterol) and two phytostanols (Δ-7-stigmastenol and Δ-7-avenasterol) were identified (Table 5). Overall, no statistical differences were found in total amounts of sterols at the beginning of the experiment between control margarine and margarine with 800 mg/kg MOLE. The main components were β-Sitosterol with 1437 mg/kg for the control margarine and 1461 g/kg for 800 mg/kg MOLE margarine. At the end of storage, a loss of total sterols was observed and was statistically lower in margarine containing 800 mg/kg MOLE (1874 mg/kg) than control margarine (1806 mg/kg). Panpipat et al. [52] found similarly that the sterols amount was also reduced in margarine stored at elevated temperature (55 °C). However, Soupas et al. [53] have not found losses in phytosterols content when storage was done using a microcrystalline suspension of different fats/oils at 4 °C for 12 months, demonstrating thus the deleterious effect of heating on phytosterols.
Conclusion
In this study, Moringa oleífera leaves extract (MOLE) was used to improve the stability of margarine under accelerated thermal storage (65 °C for 30 days). The supplemented margarines with the different concentration of moringa leaf extract demonstrated a significant increase in phenolic compounds and manifested an amelioration of induction period of oxidation proportional to increase of MOLE concentration. The concentrations of 400, 600, and 800 mg/kg MOLE decreased the incidence of lipids oxidation evaluated by peroxide and p-anisidine test. The hot storage of margarines revealed also that MOLE at particularly the concentrations of 600 and 800 mg/kg protect unsaturated fatty acids and tocopherols from oxidation. Thus, Moringa oleífera leaves extract at concentrations of 600 and 800 mg/kg demonstrated interesting protective properties against the peroxidation and degradation of margarine lipids. Thus, the moringa extract can be recommended as an alternative source of natural antioxidants for lipid base products, in particular for margarines.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Y. Lin, D. Knol, I. Valk, V. van Andel, S. Friedrichs, D. Lütjohann, K. Hrncirik, E.A. Trautwein, Thermal stability of plant sterols and formation of their oxidation products in vegetable oils and margarines upon controlled heating. Chem. Phys. Lipids 207, 99 (2017). https://doi.org/10.1016/j.chemphyslip.2017.01.007
M. Rudzińska, R. Przybylski, E. Wąsowicz, Degradation of phytosterols during storage of enriched margarines. Food Chem. 142, 294 (2014). https://doi.org/10.1016/j.foodchem.2013.07.041
B. Scholz, N. Menzel, V. Lander, K.-H. Engel, Heating two types of enriched margarine: complementary analysis of phytosteryl/phytostanyl fatty acid esters and phytosterol/phytostanol oxidation products. J. Agric. Food Chem. 64, 2699 (2016). https://doi.org/10.1021/acs.jafc.6b00617
N. Chougui, N. Djerroud, F. Naraoui, S. Hadjal, K. Aliane, B. Zeroual, R. Larbat, Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 173, 382 (2015). https://doi.org/10.1016/j.foodchem.2014.10.025
N. Nenadis, I. Zafiropoulou, M. Tsimidou, Commonly used food antioxidants: a comparative study in dispersed systems. Food Chem. 82, 403 (2003). https://doi.org/10.1016/S0308-8146(02)00579-4
C.R. Kiran, I. Sasidharan, D.S. Kumar, A. Sundaresan, Influence of natural and synthetic antioxidants on the degradation of soybean oil at frying temperature. Int. J. Food Sci. Technol. 52, 5370 (2015) https://dx.doi.org/10.1007%2Fs13197-015-1774-7
M.S. Jahan, D.D. Zawawi, A.R. Abdulkadir, Effect of chlorophyll content and maturity on total phenolic, total flavonoid contents and antioxidant activity of Moringa oleifera leaf (miracle tree). J. Chem. Pharm. Res. 7, 1147 (2015)
C. Kaur, H.C. Kapoor, Antioxidants in fruits and vegetables–the millennium’s health. Int. J. Food Sci. Technol. 36, 703 (2001). https://doi.org/10.1111/j.1365-2621.2001.00513.x
Y. Abid, S. Azabou, M. Jridi, I. Khemakhem, M. Bouaziz, H. Attia, Storage stability of traditional tunisian butter enriched with antioxidant extract from tomato processing by-products. Food Chem. 233, 476 (2017). https://doi.org/10.1016/j.foodchem.2017.04.125
G. Kaanin-Boudraa, F. Brahmi, M. Wrona, C. Nerín, S. Hadjal, K. Madani, L. Boulekbache‐Makhlouf, Citrus× paradisi essential oil as a promising agent for margarine storage stability: composition and antioxidant capacity. J. Food Process. Preserv 45, e15374 (2021). https://doi.org/10.1111/jfpp.15374
S. Admassu, M. Kebede, Application of antioxidants in food processing industry: options to improve the extraction yields and market value of natural products. J. Food Technol. Nutr. Sci. 5, 38 (2019). https://doi.org/10.17140/AFTNSOJ-5-155
A. Zeb, Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 44, e13394 (2020). https://doi.org/10.1111/jfbc.13394
Q. Guo, S. Gao, Y. Sun, Y. Gao, X. Wang, Z. Zhang, Antioxidant efficacy of rosemary ethanol extract in palm oil during frying and accelerated storage. Ind. Crops Prod. 94, 82 (2016). https://doi.org/10.1016/j.indcrop.2016.08.032
S. Fruehwirth, S. Egger, D. Kurzbach, J. Windisch, F. Jirsa, T. Flecker, M. Ressler, A.T. Reiner, N. Firat, M. Pignitter, Ingredient-dependent extent of lipid oxidation in margarine. Antioxidants 10, 105 (2021) https://www.mdpi.com/2076-3921/10/1/105#
J.W. Fahey, Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 1, 1 (2005). https://doi.org/10.1201/9781420039078.ch12
A. Leone, G. Fiorillo, F. Criscuoli, S. Ravasenghi, L. Santagostini, G. Fico, A. Spadafranca, A. Battezzati, A. Schiraldi, F. Pozzi, Nutritional characterization and phenolic profiling of Moringa oleifera leaves grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 16, 18923 (2015). https://doi.org/10.3390/ijms160818923
S. Dixit, A. Tripathi, P. Kumar, Medicinal properties of Moringa oleifera: a review. Int. J. Educ. Res. Rev. 3, 173 (2016)
A. Bhattacharya, P. Tiwari, P.K. Sahu, S.J.J.o.p. Kumar, b. sciences, a review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 10, 181 (2018)
S.O. Salawu, E.O. Ibukun, I.A. Esan, Nutraceutical values of hot water infusions of moringa leaf (Moringa oleifera) and licorice root (Glycyrrhiza glabra) and their effects on liver biomarkers in Wistar rats. J. Food Meas. Charact. 13, 602 (2019). https://doi.org/10.1007/s11694-018-9973-3
Z.F. Ma, J. Ahmad, H. Zhang, I. Khan, S. Muhammad, Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. S Afr. J. Bot. 129, 40 (2020). https://doi.org/10.1016/j.sajb.2018.12.002
L. Gopalakrishnan, K. Doriya, D.S. Kumar, Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 5, 49 (2016). https://doi.org/10.1016/j.fshw.2016.04.001
F. Al Juhaimi, K. Ghafoor, I.A. Mohamed Ahmed, E.E. Babiker, M.M. Özcan, Comparative study of mineral and oxidative status of Sonchus oleraceus, Moringa oleifera and Moringa peregrina leaves. J. Food Meas. Charact. 11, 1745 (2017) https://doi.org/10.1007/s11694-017-9555-9
P. Nobosse, E.N. Fombang, C.M.F. Mbofung, The effect of steam blanching and drying method on nutrients, phytochemicals and antioxidant activity of Moringa (Moringa oleifera L.) leaves. Am. J. Food Technol. 5, 53 (2017). https://doi.org/10.12691/ajfst-5-2-4
A. Siddiq, F. Anwar, M. Manzoor, A. Fatima, Antioxidant activity of different solvent extracts of Moringa oleifera leaves under accelerated storage of sunflower oil. Asian J. Plant. Sci. 4, 630 (2005). https://doi.org/10.3923/ajps.2005.630.635
S. Arabshahi-Delouee, M. Aalami, A. Urooj, Drumstick (Moringa oleifera L.) leaves: a potential source of natural lipid antioxidants. J. Food Process. Eng. 34, 947 (2011). https://doi.org/10.1111/j.1745-4530.2009.00554.x
S. Ouahrani, D.A. Tzompa-Sosa, K. Dewettinck, F. Zaidi, Oxidative stability, structural, and textural properties of margarine enriched with Moringa oleifera leaves extract. J. Am. Oil Chem. ' Soc. 99, 485 (2022). https://doi.org/10.1002/aocs.12586
B.D. Oomah, F. Caspar, L.J. Malcolmson, A.-S. Bellido, Phenolics and antioxidant activity of lentil and pea hulls. Int. Food Res. J. 44, 436 (2011). https://doi.org/10.1016/j.foodres.2010.09.027
D.-M. Shin, J.H. Yune, T.-K. Kim, Y.J. Kim, H.C. Kwon, C.H. Jeong, Y.-S. Choi, S.G. Han, Physicochemical properties and oxidative stability of duck fat-added margarine for reducing the use of fully hydrogenated soybean oil. Food Chem. 363, 130260 (2021). https://doi.org/10.1016/j.foodchem.2021.130260
M. Škerget, P. Kotnik, M. Hadolin, A.R. Hraš, M. Simonič, Ž Knez, Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 89, 191 (2005). https://doi.org/10.1016/j.foodchem.2004.02.025
ISO-6886, Animal and Vegetable fats and oils-determination of Oxidation Stability (Accelerated Oxidation test) (International Organization for Standardization, Geneva, Switzerland, 1997), p. 1
AOAC, Association of Official Analytical Chemists - Official methods of analysis of the Association of Official Analytic Chemists (AOAC), 16th ed; Gaithersburg, USA. (1998)
ISO-6885, Animal and Vegetable fats and oils - Determination of Anisidine Value (International Organization for Standardization, Geneva, Switzerland, 2008), p. 1
M. Aniołowska, A. Kita, The effect of frying on glycidyl esters content in palm oil. Food Chem. 203, 95 (2016). https://doi.org/10.1016/j.foodchem.2016.02.028
ISO-12966-2, Animal and Vegetable fats and oils-gas Chromatography of Fatty acid Methyl esters-Part 2: Preparation of Methyl Esters of Fatty Acids (International Organization for Standardization, Geneva, Switzerland, 2011), p. 1
R. Malheiro, S. Casal, H. Lamas, A. Bento, J.A. Pereira, Can tea extracts protect extra virgin olive oil from oxidation during microwave heating? Nt. Food Res. J. 48, 148 (2012). https://doi.org/10.1016/j.foodres.2012.03.005
EEC-2568/91. Commission Regulation - The characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Official Journal L 248 (5 September 1991), European Union, 1 (1991)
F. Braham, D. Carvalho, C. Almeida, F. Zaidi, J. Magalhães, L. Guido, M. Gonçalves, Online HPLC-DPPH screening method for evaluation of radical scavenging phenols extracted from Moringa oleifera leaves. S Afr. J. Bot. 129, 146 (2020). https://doi.org/10.1016/j.sajb.2019.04.001
S. Vidanagamage, P. Pathiraje, O. Perera, Effects of Cinnamon (Cinnamomum verum) extract on functional properties of butter. Procedia Food Sci. 6, 136 (2016). https://doi.org/10.1016/j.profoo.2016.02.033
M. Nadeem, M. Abdullah, A. Khalique, I. Hussain, A. Mahmud, S. Inayat, The effect of Moringa oleifera leaf extract as antioxidant on stabilization of butter oil with modified fatty acid profile. J. Agric. Sci. Technol. 15, 919 (2013)
M. Nogala-Kalucka, J. Korczak, M. Dratwia, E. Lampart-Szczapa, A. Siger, M. Buchowski, Changes in antioxidant activity and free radical scavenging potential of rosemary extract and tocopherols in isolated rapeseed oil triacylglycerols during accelerated tests. Food Chem. 93, 227 (2005). https://doi.org/10.1016/j.foodchem.2004.09.021
C.S. Romano, K. Abadi, V. Repetto, A.A. Vojnov, S. Moreno, Synergistic antioxidant and antibacterial activity of rosemary plus butylated derivatives. Food Chem. 115, 456 (2009). https://doi.org/10.1016/j.foodchem.2008.12.029
M. Maskan, Change in colour and rheological behaviour of sunflower seed oil during frying and after adsorbent treatment of used oil. Eur. Food Res. Technol. 218, 20 (2003). https://doi.org/10.1007/s00217-003-0807-z
C. Guillaume, F. De Alzaa, L. Ravetti, Evaluation of chemical and physical changes in different commercial oils during heating. Act. Sci. Nutr. Health 2, 2 (2018)
Y. Che Man, C. Tan, Effects of natural and synthetic antioxidants on changes in refined, bleached, and deodorized palm olein during deep-fat frying of potato chips. J. Am. Oil Chem. Soc. 76, 331 (1999). https://doi.org/10.1007/s11746-999-0240-y
E. De Marco, M. Savarese, C. Parisini, I. Battimo, S. Falco, R. Sacchi, Frying performance of a sunflower/palm oil blend in comparison with pure palm oil. Eur. J. Lipid Sci. Technol. 109, 237 (2007). https://doi.org/10.1002/ejlt.200600192
M. Laguerre, C. Bayrasy, A. Panya, J. Weiss, D.J. McClements, J. Lecomte, E.A. Decker, P. Villeneuve, What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 55, 183 (2015). https://doi.org/10.1080/10408398.2011.650335
S. Casal, R. Malheiro, A. Sendas, B.P. Oliveira, J.A. Pereira, Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 48, 2972 (2010). https://doi.org/10.1016/j.fct.2010.07.036
B. Chen, J. Rao, Y. Ding, D.J. McClements, E.A. Decker, Lipid oxidation in base algae oil and water-in-algae oil emulsion: impact of natural antioxidants and emulsifiers. Int. Food Res. J. 85, 162 (2016). https://doi.org/10.1016/j.foodres.2016.04.038
B. Isnardy, K.-H. Wagner, I. Elmadfa, Effects of α-, γ-, and δ-tocopherols on the autoxidation of purified rapeseed oil triacylglycerols in a system containing low oxygen. J. Agric. Food Chem. 51, 7775 (2003). https://doi.org/10.1021/jf0348525
S. Wang, H. Hwang, S. Yoon, E. Choe, Temperature dependence of autoxidation of perilla oil and tocopherol degradation. J. Food Sci. 75, C498 (2010). https://doi.org/10.1111/j.1750-3841.2010.01681.x
M. Pazos, M.J. González, J.M. Gallardo, J.L. Torres, I. Medina, Preservation of the endogenous antioxidant system of fish muscle by grape polyphenols during frozen storage. Eur. Food Res. Technol. 220, 514 (2005). https://doi.org/10.1007/s00217-004-1113-0
W. Panpipat, M. Chaijan, Z. Guo, Oxidative stability of margarine enriched with different structures of β-sitosteryl esters during storage. Food Biosci. 22, 78 (2018). https://doi.org/10.1016/j.fbio.2018.01.009
L. Soupas, L. Huikko, A.-M. Lampi, V. Piironen, Oxidative stability of phytosterols in some food applications. Eur. Food Res. Technol. 222, 266 (2006). https://doi.org/10.1007/s00217-005-0031-0
Acknowledgements
We are grateful to University Abdurrahman Mira of Bejaia and the General Directorate of Scientific Research and Technological Development (Algeria) for providing an internship to Sara Ouahrani. The authors acknowledge COGB Labelle Company for providing ingredients and for the margarine preparation. This work received support from Portuguese Funds (FCT) through project UIDB/50006/2020 and by AgriFood XXI I&D&I project (NORTE-01–0145-FEDER-000041) co-financed by European Regional Development Fund (ERDF), through the NORTE 2020 (Programa Operacional Regional do Norte 2014/2020).
Author information
Authors and Affiliations
Contributions
Sara Ouahrani Writing˗review & editing original draft, Formal analysis, Investigation. Susana Casal, Conceptualization, Mostapha Bachir-bey Writing˗review & editing original draft. Farid Zaidi Supervision, Validation.
Corresponding author
Ethics declarations
Competing interests and Funding
This work was funded by the General Directorate of Scientific Research and Technological Development (Algeria), Portuguese Funds (FCT, UIDB/50006/2020), AgriFood XXI I&D&I project (NORTE-01–0145-FEDER-000041), and European Regional Development Fund.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ouahrani, S., Casal, S., Bachir-bey, M. et al. Impact of Moringa oleífera leaves extract in the stabilization of margarine under accelerated storage. Food Measure 17, 1455–1466 (2023). https://doi.org/10.1007/s11694-022-01714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01714-6