Abstract
Background
Blastocystis hominis (B. hominis) is a protozoan parasite that has a worldwide distribution. Some studies have suggested a link between B. hominis and the development of irritable bowel syndrome (IBS). The objective of this study was to determine the prevalence of B. hominis in patients with IBS compared to healthy individuals.
Material and methods
A total of 65 stool samples from patients with IBS and 65 samples from healthy individuals in northern Iran were examined. The samples were tested using various methods including direct smear, formalin ether sedimentation and culture to detect the presence of B. hominis. Additionally, polymerase chain reaction (PCR) was performed on all culture-positive isolates to confirm the results and identify the genotype.
Results
B. hominis was detected in 15.38% of IBS patients and 9.2% of the healthy group. The culture in RPMI1640 was found to be better than the formalin ether and direct smear methods. Positive samples were confirmed using the molecular method. No significant difference was observed in the order of B. hominis infection between the two groups.
Conclusions
The results of our study indicate that no significant difference was observed in the order of B. hominis infection between IBS patients and healthy groups. Therefore, further study is necessary to determine the potential pathogenic effects of this parasite and its role in causing IBS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blastocystis hominis (B. hominis) is a zoonotic protozoa that influence some animals and humans [1], has a global widespread, mostly present in developing countries [2]. Transmission of this protozoon is fecal–oral, its rate rises with low hygiene and a high level of animal contact and transmission may occur from animal to human or from human to human [1, 3, 4]. B. hominis can present via various gastrointestinal symptoms and signs including nausea, diarrhea, abdominal pain and cramps [5]. The other agents have been proposed to be correlated with B. hominis symptoms, such as the factors related to the host, the parasite load or subtypes of it [6,7,8]. The prevalence of B. hominis has been reported about one billion, among 5% to 20% in developed countries and more than 60% in developing countries [1, 2]. This infection is related to contaminated food and especially water contamination [3, 9], and more prevalent in some seasons too [10, 11]. Although B. hominis was described by Alekseev more than 100 years ago [12, 13], the pathogenicity of this infection is a topic under discussion, since it has been found in both symptomatic and asymptomatic persons [14, 15].
Transmission of B. hominis is fecal oral, mostly infects adults and also older children, particularly persons in the main path of the transmission. Of course, molecular researches indicate that zoonotic transmission can occur [16]. Data around subtypes of B. hominis in Iran are limited to some researches [1, 17,18,19,20,21,22,23] and few studies have surveyed the relation between IBS and B. hominis [19, 24]. Research based on the comparison of rRNA gene of small nuclear subunit shows that B. hominis has a wide molecular diversity. 23 subtypes (STs) have been explained, however in humans just ST1–ST9 and ST12 have been found [25, 26]. Between identified subtypes in humans, the most common are ST1–ST4 with about 90% of infections [27]. The most digestive indicators defined in B. hominis carriers are alike to those attributed to IBS, a syndrome that is specified by discomfort with defecation, abdominal pain, and alterations of form of feces [28].
The cause of IBS is mysterious. Changes in the gut microbiota including bacteria, fungi, parasites and even viruses. Strangely, in some studies post-infection gastroenteritis (chiefly parasites) has been approved as one of IBS risk factors [29, 30]. There are researches that have suggested a probable connection among B. hominis and IBS, though some researches did not notice any relationship [31,32,33]. In Iran, although IBS is very common and the prevalence of 1.1–45% has been stated [24, 34], prevalence and its genetic diversity of the B. hominis between IBS people has been neglected. Our study purpose is to measure the prevalence of B. hominis among healthy individuals and IBS patients by wet mount smear, formalin ether, culture and confirmation and genotyping of isolates by molecular method to assess the possible association among IBS and the B. hominis infection.
Subject and Method
Ethical Statement
This study adhered to ethical principles and national standards for conducting medical research in Iran, as reviewed and confirmed by IR.UMSHA.REC.1398.408. Written informed consent was obtained from participants.
Sampling and Information Collection
This case–control study was designed to evaluate the frequency and subtype of B. hominis in persons with IBS (case) and healthy people (control) form Mazandaran province located in the north of Iran, which is humid area with moderate climate [35]. Demographic and clinical data were collected from participants. The collected feces from 65 patients with IBS symptoms (19 males and 46 females), and 65 healthy participants (29 males and 36 females) were surveyed during October 2010 to October 2020. Exclusion criteria for both groups were persons up 70 years, presence of other intestinal parasites except B. hominis, viral or bacterial diarrhea, immunosuppressed diseases and having received any effective drug against the Blastocystis before study inclusion (secnidazole, iodoquinol, cotrimoxazole, paromomycin, albendazole, metronidazole, ivermectin, and nitazoxanide). Also, by using the Rome IV criteria, IBS patients were selected based on physical examination and achieved data about the gastrointestinal tract symptoms in the last 3 months [36].
Group of control was selected from healthy persons without any history of gastrointestinal diseases and symptoms that for check-up referred to health centers. The participants’ information was recorded in the questionnaires. Questionnaire of study including: (1) digestive problems (abdominal pain, nausea, diarrhea, constipation, heartburn, vomiting, feeling of fullness and bloating, abdominal distension, belching). (2) One of the following symptoms for at least 3 days in one month (up to the last 3 months); (A) relieving abdominal pain with defecation, (B) the onset of abdominal pain with changes in the frequency of defecation, (C) the onset of abdominal pain with a change in the appearance of stool. (3) Feeling of incomplete emptying after defecation. (4) Intensification of abdominal pain after eating. (5) Intensification of abdominal pain after emotional stress. (6) In women: worsening of abdominal pain in premenstrual and menstrual periods. (7) Intensification of digestive symptoms after consuming high-fat foods. (8) Nocturnal diarrhea. (9) Smoking. (10) Severe stress and emotional pressure in the last few weeks. (11) Use of antidepressants. (12) History of taking certain drugs. From all participants, one fresh fecal sample were obtained and were transported to the parasitology department in Mazandaran University of medical science for microscopy surveying and culturing.
Microscopy
Stool samples before culture using direct wet mounts (saline solution 0.9% and Lugol’s iodine stain) and also formalin ether sedimentation technique were tested by light microscopy at 100× and 400× magnification.
Culture
For culture, RPMI1640 medium (associated with12 mg/ml ampicillin and 4 mg/ml streptomycin) and also fetal bovine serum (FBS) 10% was used. Samples after culturing were incubated at 37 °C for 72 h. If no B. hominis was seen in 3 days of microscopic examination, the cultured samples were considered negative. Positive isolates were passage into fresh medium for another 3 days, then 500 µL of them were transferred to 2 mL microtubes and stored at −20 °C until PCR test [37, 38].
Molecular Procedures
Molecular test done at the Parasitology Reference and Research Laboratory (Hamadan university of medical sciences, Hamadan, Iran). The positive samples preserved in 70% ethanol were provided for DNA extraction. 100 μl of positive cultured samples using the DNA stool Kit (SinaClon, Iran) according to producer protocol were extracted. The extracted DNA were stored at −20 °C until PCR processing. The parasite was confirmed by using the following primers F1 5’- GGA GGT AGT GAC AAT AAA TC -3’ and BHCRseq3 5’- TAA GAC TAC GAG GGT ATC TA -3’ [39] that amplified a 550-bp variable region of the SSU rRNA gene of the Blastocystis. PCR was done in a total volume of 25 μl containing 0.5 μM of each of the primers. initial denaturation at 95 °C for 7 min, 35 cycles of, denaturation at 94 °C for one min, annealing at 56 °C for 45 s, extension at 72 °C for 45 s and last extension at 72 °C for 5 min. Amplicons were analyzed by agarose 1% gel electrophoresis in 1× TBE. The amplified fragments of isolates were visualized on a UV transilluminator.
Sequencing
Isolates were purified and sequenced with an ABI 3500X sequencer. The Chromas software (version 2.6.5.0) was used to align the sequences, and also compared via available sequences in the GenBank database.
Result
The following epidemiological data of this study were gathered from of structured questionnaire: 48 men and 82 women were presented. B. hominis were detected in (16/130) 12.3% of the total participants, with (10/16) 15.38% of IBS patients and (6/16) 9.2% of healthy group. Demographic information is given in Table 1. There was no difference in detection of B. hominis by direct smear method via saline wet mount compared to Lugol’s iodine. The formalin ether method was better than direct smear method. As expected, the culture in RPMI1640 was better than the formalin ether and two direct smear methods. In over 99% of cases, the vacuolar form of parasite was observed (Fig. 1). Furthermore, in both symptomatic and asymptomatic individuals, the number of the parasite was more than 5 in each oil-immersion microscopic field.
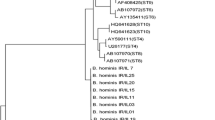
The positive cases were initially detected using parasitology methods, then confirmed through PCR. 50% (8/16) of isolates were diagnosis just by culture and were not recognized by the formalin ether method and direct smear. Positive samples were confirmed by PCR method (Fig. 2). The 87.5% of isolates seen at the age group of up 40 (14/16) and the lower prevalence under 40 (2/16). 10 positive samples in IBS patients and 6 positive samples were detected in healthy group. In two groups no significant difference was observed in order of B. hominis infection. In sequencing, 13 isolates were genotype 3, one IBS sample was genotype 7 (IBS195), and also two isolates were unknown (IBS76 and IBS78). Our isolates were uploaded to GenBank with accession numbers: OQ945168–OQ945181 (Table 2). Abdominal pain and flatulence and abdominal fullness were the most symptoms in IBS patients (Table 3). In addition, in isolates that were detected by formalin ether and direct smear methods, more than 2–3 parasites were not observed in a microscopic field. So, they did not need treatment. One IBS person and one person of healthy group, were infected with Giardia lamblia and were excluded from our study. Metronidazole was prescribed for giardiasis who they were cured.
Discussion
Our study did not find significant difference in the prevalence of B. hominis among the IBS and control groups (P = 0.5 <), regardless of the diagnostic method used. This is consistent with rates reported in other regions of Iran and around the world [40], also is consistent with some previous studies that have not found a correlation between the presence of B. hominis in fecal samples and IBS diagnosis [41]. Also, this result contradicts one study conducted in the western region of Iran [24] and several researches from France, Turkey, Mexico, and India that reported a higher prevalence of B. hominis among individuals with IBS compared to control groups [42,43,44,45]. However, our results are consistent with studies from Thailand and Denmark that also reported similar rates of B. hominis infection in individuals with and without IBS [33, 46]. Gastrointestinal symptoms associated with the presence of B. hominis in stool are non-specific, and many carriers of the parasite do not exhibit any symptoms. Additionally, there could be other probable causes of the symptoms, such as other viral, bacterial, or parasitic agents, which makes it challenging to attribute symptoms to the presence of B. hominis. In the case of IBS, conflicting results have been reported in studies. For instance, a study by Shafiei et al. found a higher percentage of B. hominis between patients with IBS (15%) compared to asymptomatic controls (6%) [40].
Reciprocally, Krogsgaard et al. were reported a higher prevalence of parasite was found between control group (22%) compared with the proportion detected in IBS group (15%) [33]. Our findings align with those of other studies, such as the research conducted by VargasSánchez et al., who evaluated 50 asymptomatic individuals and 50 IBS patients who tested positive for finding of B. hominis. They found that both groups had similar parasitological loads, which were measured using PCR [47]. Functional gastrointestinal disorder known as IBS is widely prevalent around the world [48]. For example, research conducted in Europe and the Middle East has revealed that B. hominis infection is present in about 30–40% of individuals diagnosed with IBS [48, 49]. The presence of B. hominis has been observed in both symptomatic and asymptomatic persons, leading to differing opinions regarding its role as a pathogen. Nevertheless, a growing number of studies suggest that B. hominis does have the potential to cause disease [3, 50, 51].
However, other studies, including those by Boorom et al. [52] and Yakoob et al. [53], have suggested an association between B. hominis and IBS, with higher frequency of B. hominis detected in IBS patients compared to control groups. Some researchers have proposed that the abnormal conditions of the intestine may create an environment that favors the growth of B. hominis. It is plausible that blastocystosis indicates an underlying intestinal disorder rather than serving as a direct cause of IBS. When B. hominis is identified in the fecal samples of IBS person, it does not necessarily imply that the symptoms are related to this parasite. Therefore, other potential infections should be thoroughly checked [41, 54]. The B. hominis subtype is another factor that has been associated with IBS. In one study of Indonesia conducted among senior high school students, a correlation was found between B. hominis ST1 and IBS [55]. We in this study detected the ST3 subtype and one isolate of the ST7 subtype, which were also identified in other studies.
Research suggests that the pathogenic potential of B. hominis may be linked to its molecular variations and different subtypes [3, 53]. Our study found that ST3 had a prevalence in both the IBS and healthy individuals. In other similar studies, ST3 is the most commonly found subtype in both two groups, followed by ST1 [54, 56, 57]. In the study conducted by Dogruman-Al et al., subtypes 2 and 3 were related to chronic infection in symptomatic and asymptomatic individuals with chronic diarrhea, IBS, inflammatory bowel disease (IBD), and asymptomatic persons in Turkey [56]. Also, earlier studies have suggested a potential association among subtype 2 of B. hominis and asymptomatic infections [56]; but in our study, subtype 2 was not detected in either the IBS or control groups. In some previous research has indicated that zoonotic subtypes (subtypes of 4, 6, 7), are not frequently identified in patients with chronic B. hominis infections [52]. In our study, we only observed one case of a zoonotic subtype (IBS195), which was strain 8 of ST7 too. Findings of our study suggest that there is no significant difference in the distribution of B. hominis subtypes between the IBS and control groups.
It is possible that symptoms may be associated with factors such as host genetics, immunity, or the intensity of infection, rather than the specific subtype of B. hominis. Comparable event has been observed in amoebiasis, where the same strain of the parasite can cause either asymptomatic or severe symptomatic infections in different individuals [58]. Similar to the infection with Entamoeba histolytica, the manifestation of symptoms in B. hominis infection may be linked to the presence of effective cytokines. In the IBS population, there is an over-representation of high levels of TNF-α cytokine and low levels of immunosuppressive IL-10, which might play a role in expression of symptoms [58, 59]. Furthermore, a parasite-associated protease and a 29-kDa protein have been detected as potential markers of this protozoa pathogenicity [60].
B. hominis uses various pathological factors such as intestinal cells apoptosis, disturbance of epithelial barrier function, and modulation of the host’s immune response [61]. According to some research, the primary cause of gastrointestinal symptoms in individuals with IBS can believed to be the production of serine proteases [62]. This enzyme is specific to protozoa and is not present in bacterial or viral infections. In IBS patients, the high levels of proteases can damage intestinal permeability, resulting in diarrhea [63]. Additionally, B. hominis produces proteases to break down secretory IgA, which is a defense mechanism. Symptomatic cases of B. hominis are often associated with the release of high levels of IgA, while asymptomatic cases are not affected in the same way [64].
One of the remarkable features of our study was that the control group was selected from among healthy people without digestive symptoms in all seasons of the year, which can be generalized to the entire society of northern Iran. The prevalence and diversity of B. hominis subtypes vary across different regions and countries, with subtype 3 being the most common globally such as this study [38, 63]. However, studies have shown variations in the predominant subtypes among IBS patients, with ST1 being dominant in Pakistan and Egypt (29, 30), and STs 3 and 4 being common in the UK and France. These differences may be attributed to various aspects such as geographical location, temperature, cultural practices, transmission routes and exposure to reservoir hosts [45]. While subtype 4 is limited to Europe, it is rarely reported in other regions like Asia, the Middle East, and South America [27, 38]. Studies from Turkey, Italy, and Sweden have reported varying prevalence of ST4 among patients with abdominal pain, IBS, and IBD [65]. In a study conducted in Italy, ST4 was identified in 21.7% of patients with IBD and IBS (34), and Forsell et al. in Sweden found ST4 in 20.6% of patients in the Stockholm (33).
Nevertheless, in our study, we did not find any cases of ST4. This could be because ST4 infections are rare in subtropical countries like Iran, as reported in previous studies [38]. In the present study, the 87.5% of isolates seen at the age group of up 40 (14/16) and the lower frequency at the age groups under 40 (2/16). The risk of getting infected with B. hominis parasite may increase with age, especially if someone has a higher exposure to the B. hominis [66]. In our study, it was found that the prevalence of B. hominis was higher in males than females (10 and 6, respectively, 5/10 in IBS group and 5/6 in healthy group), with ST3 was the most common type of the parasite in male. This is consistent with previous research by Forsell et al. that also found ST3 to be more prevalent in males [67]. Also, another study on patients with IBS found that B. hominis was more common in control group males [42]. However, the higher amount of control group males makes it harder to interpret the results, but it is possible that more contact with the B. hominis in males could be the reason of it [67]. Over the years, numerous studies have been conducted to investigate the potential impact of B. hominis on human health. While many studies have associated it with bowel diseases [33, 68], some newer researches indicate that this parasite may play significant role in promoting healthy gastrointestinal and even could be considered as indicator of good digestive health [36].
Conclusions
We did not find any remarkable differences of blastocystosis among IBS patients and healthy individuals, regardless of the diagnostic method used. Therefore, more research is necessary to determine the potential pathogenic effects of this protozoa and its role in causing IBS.
Furthermore, the most commonly detected subtype of B. hominis was ST3, and its prevalence did not differ significantly between the IBS and healthy groups. Identifying the specific subtype of B. hominis in patients could help better know the potential pathogenic effects of this parasite in complicated chronic gastric diseases like IBS.
Limitations
Important limitation of this study was the beginning of the COVID-19 pandemic, which slowed down the sampling process and even stopped this research at some point. But it made us have to do sampling in all seasons of the year, which in our opinion was one of the positive points of our study.
Data availability
The data are available with the correspondence author and can be reached on request.
References
Salehi M et al (2021) Prevalence and subtype analysis of Blastocystis hominis Isolated from patients in the northeast of Iran. J Parasitol Res 2021:8821885. https://doi.org/10.1155/2021/8821885
Fletcher SM et al (2012) Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev 25(3):420–449. https://doi.org/10.1128/CMR.05038-11
Tan KSW (2008) New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev 21(4):639–665. https://doi.org/10.1128/CMR.00022-08
Norouzi P et al (2020) Investigating the prevalence of intestinal parasites with an emphasis on Strongyloides stercoralis infection in hospitalized patients: a regional report from Iran. Ann Parasitol 66(3):365–371. https://doi.org/10.17420/ap6603.275
Bilinski J et al (2021) Eosinophilic gastroenteritis and graft-versus-host disease induced by transmission of Norovirus with fecal microbiota transplant. Transpl Infect Dis 23(1):e13386. https://doi.org/10.1111/tid.13386
Roberts T et al (2013) Subtype distribution of Blastocystis isolates identified in a Sydney population and pathogenic potential of Blastocystis. Eur J Clin Microbiol Infect Dis 32(3):335–343. https://doi.org/10.1007/s10096-012-1746-z
Kaya S et al (2007) Pathogenicity of Blastocystis hominis, a clinical reevaluation. Turkiye Parazitol Derg 31(3):184–187. This reference does not have DOI. PMID: 17918055
Salvador F et al (2021) Blastocystis sp. Carriage and Irritable Bowel Syndrome: Is the Association Already Established? Biology 10(4):340. https://doi.org/10.3390/biology10040340
Kuo HY et al (2008) Clinical significance of Blastocystis hominis: experience from a medical center in northern Taiwan. J Microbiol Immunol Infect 41(3):222–226. This reference does not have DOI. PMID: 18629417
Su FH et al (2009) Blastocystis hominis infection in long-term care facilities in Taiwan: prevalence and associated clinical factors. Parasitol Res 105(4):1007–1013. https://doi.org/10.1007/s00436-009-1509-7
Hussein EM et al (2008) Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol Res 102(5):853–860. https://doi.org/10.1007/s00436-007-0833-z
Stensvold CR, Clark CG (2016) Current status of Blastocystis: A personal view. Parasitol Int 65(6 Pt B):763–771. https://doi.org/10.1016/j.parint.2016.05.015
Andersen LO, Stensvold CR (2016) Blastocystis in health and disease: are we moving from a clinical to a public health perspective? J Clin Microbiol 54(3):524–528. https://doi.org/10.1128/jcm.02520-15
Roberts T et al (2014) Update on the pathogenic potential and treatment options for Blastocystis sp. Gut pathogens 6:17–17. https://doi.org/10.1186/1757-4749-6-17
Salvador F et al (2016) Epidemiological and clinical profile of adult patients with Blastocystis sp. infection in Barcelona, Spain. Parasit Vectors 9(1):548. https://doi.org/10.1186/s13071-016-1827-4
Parkar U et al (2007) Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134(Pt 3):359–367. https://doi.org/10.1017/s0031182006001582
Karimi P et al (2023) Molecular identification of Cryptosporidium, Giardia, and Blastocystis from stray and household cats and cat owners in Tehran. Iran Scientific Reports 13(1):1554. https://doi.org/10.1038/s41598-023-28768-w
Taghipour A et al (2023) Frequency, subtypes distribution, and risk factors of Blastocystis spp. in COVID-19 patients in Tehran, capital of Iran: a case-control study. New Microbes and New Infections 51:101063. https://doi.org/10.1016/j.nmni.2022.101063
Azizian M et al (2016) Contribution of Blastocystis hominis subtypes and associated inflammatory factors in development of irritable bowel syndrome. Parasitol Res 115(5):2003–2009. https://doi.org/10.1007/s00436-016-4942-4
Haghighi L et al (2020) Prevalence and subtype identification of Blastocystis isolated from human in Shiraz city, southern Iran. Clinical Epidemiology and Global Health 8(3):840–844. https://doi.org/10.1016/j.cegh.2020.02.010
Khademvatan S et al (2017) Blastocystis and irritable bowel syndrome: Frequency and subtypes from Iranian patients. Parasitol Int 66(2):142–145. https://doi.org/10.1016/j.parint.2017.01.005
Beiromvand M et al (2017) Comparative prevalence of blastocystis in patients with the irritable bowel syndrome and healthy individuals: a case control study. Jundishapur J Microbiol 10(6). https://doi.org/10.5812/jjm.13572
Soleimani Jevinani S et al (2023) Molecular epidemiology and subtyping of Blastocystis sp. and its subtypes in celiac patients; a case control study. Microbial Pathogenesis 179:106086. https://doi.org/10.1016/j.micpath.2023.106086
Mohemmi N et al (2015) The relationship between blastocystis hominis infection and Irritable Bowel Syndrome (IBS) and comparing direct wet mount, stool culture, Formalin-Ether and trichrome staining procedures for identifying organisms. Bimonthly J Hormozgan Univ Med Sci 19(2):77–84. This reference does not have DOI.
Stensvold CR, Clark CG (2020) Pre-empting Pandora’s box: Blastocystis subtypes revisited. Trends Parasitol 36(3):229–232. https://doi.org/10.1016/j.pt.2019.12.009
Maloney JG et al (2019) Zoonotic and genetically diverse subtypes of Blastocystis in US pre-weaned dairy heifer calves. Parasitol Res 118(2):575–582. https://doi.org/10.1007/s00436-018-6149-3
Clark CG et al (2013) Recent developments in Blastocystis research. Adv Parasitol 82:1–32. https://doi.org/10.1016/b978-0-12-407706-5.00001-0
Lacy BE et al (2016) Bowel disorders. Gastroenterology 150(6):1393-1407.e5. https://doi.org/10.1053/j.gastro.2016.02.031
Botschuijver S et al (2017) Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology 153(4):1026–1039. https://doi.org/10.1053/j.gastro.2017.06.004
Klem F et al (2017) Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 152(5):1042-1054.e1. https://doi.org/10.1053/j.gastro.2016.12.039
Jimenez-Gonzalez DE et al (2012) Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol Res 110(3):1269–1275. https://doi.org/10.1007/s00436-011-2626-7
Yakoob J et al (2004) Irritable bowel syndrome: in search of an etiology: role of Blastocystis hominis. Am J Trop Med Hyg 70(4):383–385. https://doi.org/10.4269/ajtmh.2004.70.383
Krogsgaard LR et al (2015) The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: a population-based case-control study. Clin Gastroenterol Hepatol 13(3):507-513.e2. https://doi.org/10.1016/j.cgh.2014.07.065
Jahangiri P et al. (2012) Irritable Bowel Syndrome in Iran: SEPAHAN Systematic Review No. 1. Int J Prev Med 3(Suppl 1):S1–S9. This reference does not have DOI. PMID: 22826748
Najafi N et al (2016) Disseminated strongyloidiasis in an Iranian immunocompromised patient: a case report. Iran J Parasitol 11(2):279–283. This reference does not have DOI. PMID: 28096866
Mearin F, Rey E, Balboa A (2016) Functional and motor gastrointestinal disorders. Gastroenterol Hepatol 39(Suppl 1):3–13. https://doi.org/10.1016/s0210-5705(16)30169-8
Zman V, Khan KZ (1994) A comparison of direct microscopy with culture for the diagnosis of Blastocystis hominis. Southeast Asian J Trop Med Public Health 25(4):792–793. This reference does not have DOI. PMID: 7667737
Alfellani MA et al (2013) Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop 126(1):11–18. https://doi.org/10.1016/j.actatropica.2012.12.011
Roberts T et al (2011) Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of blastocystis sp. in clinical stool samples. Am J Trop Med Hyg 84(2): 308–312. https://doi.org/10.4269/ajtmh.2011.10-0447
Shafiei Z et al (2020) Parasitic infections in irritable bowel syndrome patients: evidence to propose a possible link, based on a case–control study in the south of Iran. BMC Res Notes 13(1):264. https://doi.org/10.1186/s13104-020-05118-x
Wawrzyniak I et al (2013) Blastocystis, an unrecognized parasite: an overview of pathogenesis and diagnosis. Ther Adv Infect Dis 1(5):167–178. https://doi.org/10.1177/2049936113504754
Nourrisson C et al (2014) Blastocystis is associated with decrease of fecal microbiota protective bacteria: comparative analysis between patients with irritable bowel syndrome and control subjects. PLoS ONE 9(11):e111868. https://doi.org/10.1371/journal.pone.0111868
Cekin AH et al (2012) Blastocystosis in patients with gastrointestinal symptoms: a case-control study. BMC Gastroenterol 12:122. https://doi.org/10.1186/1471-230x-12-122
Ramirez-Miranda ME et al (2010) Parasites in Mexican patients with irritable bowel syndrome: a case-control study. Parasit Vectors 3:96. https://doi.org/10.1186/1756-3305-3-96
Das R et al (2016) Molecular Characterization and Subtyping of Blastocystis Species in Irritable Bowel Syndrome Patients from North India. PLoS ONE 11(1):e0147055. https://doi.org/10.1371/journal.pone.0147055
Surangsrirat S et al (2010) Assessment of the association between Blastocystis infection and irritable bowel syndrome. J Med Assoc Thai 93(Suppl 6):S119–S124. This reference does not have DOI. PMID: 21280524
Vargas-Sanchez GB et al (2015) Blastocystis isolates from patients with irritable bowel syndrome and from asymptomatic carriers exhibit similar parasitological loads, but significantly different generation times and genetic variability across multiple subtypes. PLoS ONE 10(4):e0124006. https://doi.org/10.1371/journal.pone.0124006
Cremonini F, Talley NJ (2005) Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am 34(2):189–204. https://doi.org/10.1016/j.gtc.2005.02.008
Park DW et al (2010) The differences in prevalence and sociodemographic characteristics of irritable bowel syndrome according to Rome II and Rome III. J Neurogastroenterol Motil 16(2):186–193. https://doi.org/10.5056/jnm.2010.16.2.186
Poirier P et al (2012) New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLoS Pathog 8(3):e1002545. https://doi.org/10.1371/journal.ppat.1002545
Idris NS et al (2010) Intestinal parasitic infection of immunocompromised children with diarrhoea: clinical profile and therapeutic response. J Infect Dev Countries 4(05):309–317. https://doi.org/10.3855/jidc.275
Boorom KF et al (2008) Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit Vectors 1(1):40. https://doi.org/10.1186/1756-3305-1-40
Yakoob J et al (2010) Irritable bowel syndrome: is it associated with genotypes of Blastocystis hominis. Parasitol Res 106(5):1033–1038. https://doi.org/10.1007/s00436-010-1761-x
Jantermtor S et al (2013) Subtype identification of Blastocystis spp. isolated from patients in a major hospital in northeastern Thailand. Parasitol Res 112(4):1781–1786. https://doi.org/10.1007/s00436-012-3218-x
Kesuma Y et al (2019) Blastocystis ST-1 is associated with Irritable Bowel Syndrome-diarrhoea (IBS-D) in Indonesian adolescences. Parasite Epidemiol Control 6:e00112. https://doi.org/10.1016/j.parepi.2019.e00112
Dogruman-Al F et al (2008) A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol Res 103(3):685–689. https://doi.org/10.1007/s00436-008-1031-3
Stensvold CR, Alfellani M, Clark CG (2012) Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infect Genet Evol 12(2):263–273. https://doi.org/10.1016/j.meegid.2011.11.002
Stanley SL (2003) Amoebiasis. The Lancet 361(9362):1025–1034. https://doi.org/10.1016/S0140-6736(03)12830-9
van der Veek PPJ et al (2005) Role of Tumor Necrosis Factor-α and Interleukin-10 Gene Polymorphisms in Irritable Bowel Syndrome. Official J Am Coll Gastroenterol | ACG 100(11):2510–2516. https://doi.org/10.1111/j.1572-0241.2005.00257.x
Abou Gamra MM et al (2011) The potential use of 29 kDa protein as a marker of pathogenicity and diagnosis of symptomatic infections with Blastocystis hominis. Parasitol Res 108(5):1139–1146. https://doi.org/10.1007/s00436-010-2156-8
Puthia MK et al (2006) Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect Immun 74(7):4114–4123. https://doi.org/10.1128/iai.00328-06
Cenac N et al (2007) Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 117(3):636–647. https://doi.org/10.1172/jci29255
Gecse K et al (2008) Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57(5):591–599. https://doi.org/10.1136/gut.2007.140210
Puthia MK et al (2005) Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol Res 97(5):386–389. https://doi.org/10.1007/s00436-005-1461-0
Ozyurt M et al (2008) Molecular epidemiology of Blastocystis infections in Turkey. Parasitol Int 57(3):300–306. https://doi.org/10.1016/j.parint.2008.01.004
Engsbro AL et al (2014) Prevalence, incidence, and risk factors of intestinal parasites in Danish primary care patients with irritable bowel syndrome. Scand J Infect Dis 46(3):204–209. https://doi.org/10.3109/00365548.2013.861609
Forsell J et al (2012) Subtype analysis of Blastocystis isolates in Swedish patients. Eur J Clin Microbiol Infect Dis 31(7):1689–1696. https://doi.org/10.1007/s10096-011-1416-6
Petersen AM et al (2013) Active ulcerative colitis associated with low prevalence of Blastocystis and Dientamoeba fragilis infection. Scand J Gastroenterol 48(5):638–639. https://doi.org/10.3109/00365521.2013.780094
Acknowledgements
We would like to appreciate the assistance offered by the colleagues at Razi Hospital, Mazandaran University of Medical Science, Qaemshahr, Iran.
Funding
Vice Chancellor for Research and Technology, Hamadan University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors state that there is no conflict of interest.
Approval Statement
The current study was supported financially by Hamadan University of Medical Science, Project No. IR.UMSHA.REC.1400.857.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maghsood, A.H., Kayedimajd, S., Motavallihaghi, S. et al. Irritable Bowel Syndrome Associated with Blastocystis hominis or Without Relationship to It? A Case–Control Study and Minireview. Acta Parasit. 69, 639–647 (2024). https://doi.org/10.1007/s11686-023-00787-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00787-7