Abstract
Summary
Angiotensin-converting enzyme inhibitor use in women was associated with lower femoral neck and lumbar spine bone mineral density as well as trabecular bone score compared to non-users. No differences were identified for men or for those who used ARB medications.
Purpose
Many individuals at high fracture risk use medications such as angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB) that could affect bone; thus, this study aimed to investigate whether there are any differences in bone mineral density (BMD) and trabecular bone score (TBS) between ACEI users, ARB users, and non-users.
Methods
Participants (685 men, 573 women) were from the Geelong Osteoporosis Study. Current medication use was self-reported. BMD at the femoral neck (FNBMD) and lumbar spine (LSBMD) were measured using DXA. TBS was calculated using TBS iNsight software. Linear regression models were used to investigate associations between ACEI or ARB use and bone measures, adjusting for other potential confounders. Due to interaction terms, data were stratified by age.
Results
There were 88 (12.8%) men and 41 (7.2%) women taking an ACEI medication, and 71 (10.4%) men and 76 (13.3%) women taking an ARB medication. Compared to non-users, ACEI use was associated with lower FNBMD (− 7.2%), LSBMD (− 12.2%), and TBS (− 9.0%) for women aged < 65 years. Lower TBS was also observed for women aged ≥ 65 years (− 17.3%). No differences were identified for ARB use.
Conclusions
Women who used an ACEI medication had lower values for FNBMD, LSBMD and TBS compared to non-users. No differences were identified for men or for those who used ARB medications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis and hypertension are common conditions affecting older adults, and both will continue to increase in prevalence due to an ageing population. Many of the risk factors for osteoporosis overlap with hypertension, such as older age, poor diet, smoking status, and physical inactivity [1].
Medications prescribed to reduce blood pressure include angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs). These work through the renin angiotensin system; ACEIs prevent the conversion of angiotensin I to angiotensin II by blocking the activity of the angiotensin-converting enzyme (ACE), while ARBs block the action of the angiotensin II receptor type 1 (AT1R) [2, 3]. Both medication types prevent the downstream effects of angiotensin II, ultimately resulting in a reduction in blood pressure. Guidelines from the Australian National Heart Foundation state that ACEIs, ARBs, thiazide diuretics, and calcium channel blockers are first-line antihypertensive agents due to high efficacy and safety profiles [4]. These guidelines also advise against the use of ACEI and ARB medications in combination therapies for treatment of hypertension, as this increases the risk of adverse effects.
Components of the renin angiotensin system are also present in bone; however, the effect of ACEI or ARB medications on bone is still unclear. Specifically, receptors for angiotensin II are present on osteoclasts and osteoblasts [2]. Angiotensin II is detrimental to bone, as it activates the AT1R on osteoblasts, which upregulates receptor activator of nuclear factor kappa-Β ligand (RANKL), leading to the activation of osteoclasts [5, 6]. Local activation of the renin angiotensin system promotes bone resorption and inhibits bone formation. Therefore, it is plausible that blockade of the renin angiotensin system in bone using ACEIs or ARBs could lead to improved bone health and potentially reduce fracture rates. However, ACEI or ARB medications reduce renal acid elimination, through inhibition of the renin angiotensin system. Bone contains calcium carbonate, which is an important contributor in mechanisms that buffer protons in an acidic environment [7]. Due to this buffering, in combination with osteoclast-mediated bone resorption in acidic conditions, it is possible that long-term use of ACEI and ARB medications may promote bone loss and lead to osteoporosis [7].
Several animal studies have reported that metabolic changes do occur with ACEI or ARB treatment and that these are linked to changes in bone. For example, Asaba et al. [8] reported that overexpressing the human renin and angiotensinogen genes in transgenic mice resulted in osteoporosis, with an increased bone resorption. Treatment of these mice with enalapril (ACEI) reduced the development of osteoporosis; however, treatment with losartan (ARB) resulted in greater bone mass deficits. Another study by Shimizu et al. [9] reported similar results using spontaneously hypertensive rats, which underwent ovariectomy to induce estrogen deficiency, and this resulted in bone loss. Treatment of these rats using imidapril (ACEI) resulted in a reduced bone loss and prevention of osteoporosis. However, contrasting results have also been reported. A study by Kang et al. [10] showed that compared to control mice, ovariectomised mice treated with enalapril (ACEI) had an increased rate of bone loss, while treatment with telmisartan (ARB) reduced the rate of bone loss. Another study [11] reported similar findings for ARB treatment; ovariectomised rats treated with losartan (ARB) showed reduced bone loss compared to controls. Overall, these previous animal studies suggest that activation or inhibition of the renin angiotensin system can potentially affect bone.
There are few previous studies that have investigated ACEI or ARB use and bone mineral density (BMD) involving older adults, with inconsistent results. One study that examined cross-sectional differences in BMD showed, compared to non-users, that men using ACEIs had higher BMD at the femoral neck, total hip, and lumbar spine and women had higher femoral neck BMD [12]. Other studies have examined rates of bone loss over time. For example, one study showed that women taking ACEIs had a greater rate of bone loss at the femoral neck and total hip than non-users; however, there were no differences observed for men [13]. Another USA study reported that long-term use of ACEIs was associated with a lower rate of bone loss over time, as well as a higher BMD at all time points studied at the total hip, femoral neck, and whole body for black men, but not for black women or white men/women [14]. Only one previous study has included ARB users, which reported that ACEI use was associated with an increased rate of bone loss at the total hip and trochanter, whereas ARB use was associated with no change in the rate of bone loss [6].
There are few studies in the literature that have examined associations between ACEI or ARB use and BMD, and none in an Australian population. Additionally, to our knowledge, no studies as yet have included trabecular bone score (TBS). Thus, the aim of this study was to investigate associations between measures of bone and the use of ACEI or ARB medications in a cohort of Australian men and women.
Methods
Participants
Participants for this study were men and women enrolled in the ongoing, longitudinal, population-based, Geelong Osteoporosis Study (GOS). Participants were randomly selected from the electoral roll and have been followed up every few years. Baseline assessments occurred in 1993–1997 for women and 2001–2006 for men. Further details of the study have been published elsewhere [15].
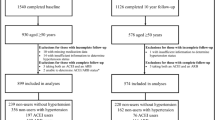
This cross-sectional study used data from the 5-year follow-up for men (2007–2011) and the 15-year follow-up for women (2011–2014). These follow-up phases were selected because they occurred after the introduction of ARBs into Australian clinical practice, which occurred in 1997 [16]. These follow-up phases were also selected as these were the first time points following the introduction of ARBs where data for TBS were available. Participants were included if they provided information regarding medication use and had at least one of the bone measures performed. There were 888 men and 773 women who provided sufficient information for the analyses. Of these, 27 men and 68 women had a BMI outside the validated range for TBS (15–37 kg/m2 [17]) and were excluded from the study. Seven men and three women were excluded because they were using both an ACEI and an ARB medication. Further exclusions were applied for participants with diabetes (70 men, 39 women), those taking bone active medication (such as bisphosphonates or anabolic agents; 13 men, 41 women), participants taking glucocorticoids (8 men, 17 women), and those with a history of cancer (78 men, 32 women). Cancer data were obtained from linkage with the Victorian Cancer Registry. Thus, 685 men and 573 women aged 25–95 years were included in the analyses. A participant flow chart is shown in Fig. 1.
All participants provided written, informed consent. Barwon Health Human Research Ethics Committee approved the study (projects 92/01 and 00/56).
Medication use
Participants self-reported their current medication use, including dose, frequency, and date started. They were asked to bring their medications with them when they attended appointments with the research team. Participants were grouped as (1) using an ACEI, (2) using an ARB, or (3) non-users, taking neither an ACEI nor an ARB. Use of other cardiovascular medications was also considered, which included calcium channel blockers, beta blockers, diuretics, and statins.
Bone measures
Dual-energy X-ray absorptiometry (DXA) was used to measure femoral neck (FNBMD) and lumbar spine BMD (LSBMD) for all participants (GE-Prodigy: Prodigy; GE Lunar, Madison, WI, USA). To ensure correct calibration of the DXA machine over time, an anthropomorphic phantom was scanned daily prior to use. Qualified personnel also conducted annual routine maintenance and servicing. TBS was retrospectively calculated from lumbar spine scans using TBS iNsight software (version 2.2; Medimaps Group, Geneva, Switzerland).
Other variables
Weight and height were measured to the nearest 0.1 kg and 0.1 cm and body mass index (BMI, kg/m2) was calculated. Blood pressure was measured while the participant was seated, using an automated meter (Takeda Medical UA-751). Prior low trauma fractures (excluding fingers, toes, and skull/face) were identified by self-report and confirmed with radiological reports where possible. Mobility was documented using a 7-point scale including very active, active, sedentary, limited, inactive, chair or bedridden, and bedfast. These data were dichotomised into high mobility that included “very active” and “active,” and low mobility, that pooled the remaining responses. Smoking status was defined as currently smoking or not. Alcohol consumption was collected using a food frequency questionnaire developed by the Victorian Cancer Council [18] and dichotomised into < 30 g or ≥ 30 g of alcohol per day.
Comorbidities were ascertained using the Charlson comorbidity index (CCI) [19] which included myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, mild liver disease, hemiplegia, renal disease, moderate or severe liver disease, and acquired immune deficiency syndrome. Data for the CCI were collected by self-report and a score was calculated for each participant as described in Charlson et al. [19]. Data for congestive heart failure and peripheral vascular disease were not available for women. Since participants with diabetes and cancer were excluded from this study, these conditions were consequently not included in the CCI.
Statistical analyses
Normality of continuous variables was tested using the Shapiro–Wilk test. All were normal except for age and CCI, which were described using median (interquartile range (IQR)), while the other variables were described using mean and standard deviation (SD). Differences between groups were assessed using Kruskal–Wallis tests for age and CCI; other variables were assessed using ANOVA. Categorical data were expressed as n(%) and differences were assessed using Chi squared tests.
Unadjusted differences in bone measures for ACEI, ARB, and non-users were assessed using ANOVA. Linear regression models were used to investigate associations between ACEI and ARB use and measures of bone (FNBMD, LSBMD, and TBS), adjusted for other variables. A categorical variable was generated and included in the models which indicated whether participants (i) used an ACEI medication, (ii) used an ARB medication, or (iii) were non-users of either ACEI or ARB medications. Other variables tested in the models included age, weight, height, systolic blood pressure (continuous), diastolic blood pressure (continuous), prior fracture, mobility, smoking, alcohol consumption, calcium channel blockers, beta blockers, diuretics, statins, comorbidities, ACEI/ARB medication dose, and duration of ACEI/ARB medication use, and these were retained if p < 0.05.
The associations between age and the bone measures are different between men and women, as we have previously reported [20,21,22]. Therefore, for the models in men, a linear age term was used. For women, a quadratic age term was used for FNBMD and cubic for LSBMD/TBS.
There was an ACEI use*age interaction in the model for FNBMD in men (p = 0.032) and for LSBMD in women (p = 0.007). Thus, data were stratified by age (< 65 years versus ≥ 65 years). No other interaction terms were identified. Homoscedasticity of residuals was assessed using the White’s and Breusch-Pagan tests, as well as a visual inspection of residual plots. All models met homoscedasticity assumptions.
Analyses were completed using Minitab (Minitab, version 18, State College, PA, USA) and STATA (Version 15.1. StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
There were 88 (12.8%) men and 41 (7.2%) women taking an ACEI medication and 71 (10.4%) men and 76 (13.3%) women taking an ARB medication. The median (IQR) duration of ACEI medication use was 5 (2–12) years in men and 4 (2–10) years in women. For ARB medication use, the median duration was 4 (2–12) years for men and 6 (3–11) years in women.
Descriptive characteristics
Table 1 shows the descriptive characteristics for men and women stratified by ACEI or ARB medication use.
For both men and women, participants using ACEIs or ARBs were older and shorter, had higher systolic blood pressure and lower mobility, and were more likely to be taking a beta blocker, diuretic, and/or statin. In men, those taking an ACEI or ARB had a higher BMI and were more likely to be taking a calcium channel blocker. Men taking an ACEI medication also had a higher CCI score. In women, those using an ARB medication had a higher weight, BMI, and CCI score. Additionally, women using an ACEI or ARB had a higher diastolic blood pressure and were more likely to have sustained a prior fracture.
Men
Unadjusted analyses showed that men using ACEI medications had lower FNBMD than non-users (mean ± SD; non-users: 0.996 ± 0.136, ACEI: 0.955 ± 0.156, ARB: 0.980 ± 0.118 g/cm2, p = 0.033). TBS was lower for both users of ACEI and ARB medications compared to non-users (mean ± SD; non-users: 1.262 ± 0.140, ACEI: 1.173 ± 0.160, ARB: 1.162 ± 0.125, p < 0.001). However, LSBMD was higher for ARB users compared to non-users (mean ± SD; non-users: 1.278 ± 0.178, ACEI: 1.318 ± 0.248, ARB: 1.367 ± 0.201 g/cm2, p = 0.001).
The results for adjusted analyses in men are shown in Table 2. No differences were observed for any of the bone measures between men who used ACEI or ARB medications and non-users.
Women
In unadjusted analyses, women who used ACEI or ARB medications had lower FNBMD than non-users (mean ± SD; non-users: 0.942 ± 0.146, ACEI: 0.838 ± 0.123, ARB: 0.878 ± 0.130 g/cm2, p < 0.001). TBS was also lower for women using ACEI or ARB medications (mean ± SD; non-users: 1.337 ± 0.131, ACEI: 1.201 ± 0.129, ARB: 1.240 ± 0.125, p < 0.001). Additionally, women using an ACEI medication had lower LSBMD than non-users (mean ± SD; non-users: 1.218 ± 0.178, ACEI: 1.137 ± 0.201, ARB: 1.195 ± 0.170 g/cm2, p = 0.015).
Women aged < 65 years who were using an ACEI medication had lower FNBMD (vs non-users: − 7.2%), LSBMD (vs non-users: − 12.2%), and TBS (vs non-users: − 9.0%) than the other two groups (Table 3). Women aged ≥ 65 years who used an ACEI medication had lower TBS (vs non-users: − 17.3%) compared to the other two groups (Table 3). No other differences were observed.
The association (compared to non-users) for lower TBS in women aged < 65 years taking ACEIs was independent of LSBMD (ACEI: p < 0.001). This was also true for women aged ≥ 65 years (ACEI: p = 0.002). The lack of associations observed for ARB use was also independent of LSBMD for both women aged < 65 years (p = 0.135) and ≥ 65 years (p = 0.204).
Discussion
In this study, we report that ACEI use was associated with lower FNBMD, LSBMD, and TBS in younger women (< 65 years), while older women (≥ 65 years) who used ACEIs had lower TBS. No differences were detected for ARB use in either men or women. To our knowledge, this is the first study to report differences in TBS between users of ACEI medications and non-users.
It is difficult to compare our results with previous literature, as many have examined changes in BMD over time, rather than cross-sectionally. There is one similar cross-sectional study that reported higher BMD in association with use of ACEIs [12], which is different from what we report in this study. However, this previous study included participants from a different geographical region (Hong Kong) and no data were available for ACEI dose or duration. ARB users were also not included in this previous study. Our results do agree with other studies examining changes in BMD over time, in that ACEI use is associated with deleterious effects on BMD [6, 13] and that ARB use does not show this same effect [6]. Overall, further studies are needed in this area, especially including ARB use.
We report lower BMD and TBS in some groups with ACEI use; however, it is not clear whether this would also correspond to an increased fracture risk. Other literature has investigated this question, with inconsistent results. For example, Choi et al. [23] showed that ARB use was associated with no difference in fracture rates compared to non-users, whereas those using ACEIs had higher rates of fracture. Another study reported that new users of ARBs had a lower risk of osteoporotic fracture than new users of calcium channel blockers (used as a control group), while no associations were observed for new users of ACEIs [24]. A study which compared ACEIs and ARBs showed that ARBs were associated with lower incidence of non-vertebral fracture in men over a study period of 6.8 years [25]. This is not consistent with two other studies which showed that neither ACEI nor ARB use was associated with fracture risk [26, 27]. Two other studies have reported that ACEI use was associated with a lower risk of fracture compared to non-users [28, 29].
Some studies have combined ACEI and ARB use. Carbone et al. [30] reported that the duration of ACEI and/or ARB use was important, with short (≤ 3 years) and longer term (> 3 years) use being associated with a higher and lower risk of fracture, respectively. Three other studies reported that use of ACEIs and/or ARBs was associated with lower rates of fracture compared to the non-user groups [31,32,33]. In these studies, it is not clear if the lower fracture rates are driven by ACEI use, ARB use, or both, as other studies have reported that the two different classes of medication have differing associations with bone and fracture.
One previous study by Ruths et al. [34] showed an interaction with age, where individuals using ACEIs who were aged < 80 years had an increased risk of fracture than those aged ≥ 80 years over a 5-year follow-up. Overall, ARB use appears to be associated with a lower, or not, different risk of fracture compared to non-users, while the data for ACEI use are inconsistent.
In this study, we have reported associations in bone measures that differed by age and sex. For example, younger women (< 65 years) who used ACEI medication had lower FNBMD, LSBMD, and TBS than the other two groups. Increasing age is an important factor for reduced BMD values [20, 21], and older individuals are also more likely to be taking ACEI or ARB medications. We also observed differing results by age for LSBMD and TBS results in women. For younger women (< 65 years), both LSBMD and TBS were lower for those using ACEI medications; however, for older women (≥ 65 years), TBS was lower but LSBMD was not different to the non-users group. However, it is possible in this study that we observed a greater number of associations with younger women because for older women, other factors such as increasing age, comorbidity, and longer time since menopause become more important influences on BMD and TBS than the use of ACEI or ARB medication. This is supported by the Ruths et al. study [34], described above, which reported an increase in fracture risk for individuals aged < 80 years who used ACEI medications.
The associations observed in this study show different results for ACEI and ARB use, despite both medications acting on the renin angiotensin system. One reason for the differences observed could include a genetic component. There is a polymorphism in the angiotensin-converting enzyme gene, which has two alleles, I and D, referring to the insertion (I) or deletion (D) of a particular sequence in the gene [35]. Individuals with the D allele produce more angiotensin-converting enzyme and thus experience greater effects with the inhibition of this enzyme. One study has examined the effect of this polymorphism on BMD, showing that women with at least one copy of the I allele (II or ID) had a higher BMD at the start of the study than women who had the DD polymorphism and had a decrease in BMD over time after treatment with ACEIs [35]. However, those with the DD polymorphism, despite having a lower BMD at the start of the study, showed an increase in BMD over time, opposite to the other group.
Another possibility relates to the existence of two receptors for angiotensin II, known as angiotensin II receptors 1 and 2 (AT1R and AT2R). While ACEIs inhibit the production of angiotensin II, ARBs block the AT1R specifically [36]. Thus, in the case of ACEIs, all functions mediated through AT1R and AT2R are blocked, while for ARBs, functions mediated through AT2R will still occur. This receptor is not well characterised, and it is unclear if AT2R functions would impact bone [36]. Additionally, angiotensin II can be produced through multiple pathways and as a result, long-term use of ACEIs can lead to a return of angiotensin II levels to baseline levels over time in some patients [3, 6, 25]. However, this effect does not occur for ARB use, because ARBs block the action of the receptor, rather than the angiotensin-converting enzyme. In our study, the median duration of use for ACEIs was (median (IQR)) 5 (2–12) years in men and 4 (2–10) years in women, which corresponds to a longer duration of use, which may partly explain why ACEIs were associated with lower bone measures, as angiotensin II levels may have returned towards baseline values in some participants.
Another important consideration is that ACE inhibition may lead to increased inflammation [2, 3, 37] which can be detrimental to bone as inflammatory cytokines affect both osteoclasts and osteoblasts, leading to increased bone resorption and reduced formation [38, 39]. ARBs do not have this effect and may have anti-inflammatory properties mediated through the AT2R [3]. Angiotensin II is also an important contributor to acid–base balance within the body. Angiotensin II increases glomerular filtration rate and secretion of aldosterone; which is the main stimulator of renal acid elimination [40]. Chronic use of ACEI or ARB medications could result in an increased accumulation of acids within the body, leading to an increase in respiratory excretion of CO2, as well as buffering of metabolic acids by carbonate and phosphate in bones. Additionally, the mechanism of acid-sensing by preosteoclasts and osteoclasts is tightly coupled to the promotion of differentiation, survival, and bone reabsorption activity of osteoclasts [41]. Therefore, it is possible that an increase in metabolic acids resulting from blockade of angiotensin II may lead to increased bone resorption, leading to lower BMD and TBS.
This study has several strengths, including randomly selected participants from a population-based setting. In addition, we were able to adjust for a number of confounders, such as weight, blood pressure, other hypertensive medications, and comorbidities. Hypertension has been reported to affect bone [42,43,44], and thus it is important that we were able to adjust for blood pressure. We recognise as limitations that there may be residual confounding and we were not able to determine the compliance of medication use. Additionally, although participants were asked to bring their medications with them, not all did and consequently recall bias may have affected self-reporting of medication duration or dose. Some of the other data were self-reported, and this may also have introduced some bias. The study was cross-sectional and thus we cannot make any conclusions regarding causation, only associations. Further studies are needed to confirm these findings. Longitudinal studies are also needed to investigate whether the differences in bone measures observed in this study affect incident fracture risk. We did not have data on genetic polymorphisms that may have affected response to ACEI use. The sample size for this study was small following stratification by age and sex. We also did not have sufficient numbers of participants using different agents within these drug categories (e.g., perindopril and enalapril for ACEIs), and each of these could have different effects on bone [5]. Further studies with larger sample sizes are needed to confirm the findings of this study.
Conclusions
The use of ACEI medication was associated with lower bone parameters in women, particularly those aged less than 65 years. Further work is needed to determine the mechanisms responsible for the differences observed.
Data availability
Data are available upon reasonable request.
References
Ghosh M, Majumdar SR (2014) Antihypertensive medications, bone mineral density, and fractures: a review of old cardiac drugs that provides new insights into osteoporosis. Endocrine 46:397–405. https://doi.org/10.1007/s12020-014-0167-4
Bislev LS, Sikjær T, Rolighed L, Rejnmark L (2015) Relationship between aldosterone and parathyroid hormone, and the effect of angiotensin and aldosterone inhibition on bone health. Clin Rev Bone Miner Metab 13:194–205. https://doi.org/10.1007/s12018-015-9182-0
Unger T, Stoppelhaar M (2007) Rationale for double renin-angiotensin-aldosterone system blockade. Am J Cardiol 100:S25–S31. https://doi.org/10.1016/j.amjcard.2007.05.011
National Heart Foundation of Australia (2016) Guideline for the diagnosis and management of hypertension in adults - 2016. Melbourne: National Heart Foundation of Australia
Tamargo J, Caballero R, Delpón E (2015) The renin–angiotensin system and bone. Clin Rev Bone Miner Metab 13:125–148. https://doi.org/10.1007/s12018-015-9189-6
Kwok T, Leung J, Zhang YF et al (2012) Does the use of ACE inhibitors or angiotensin receptor blockers affect bone loss in older men? Osteoporos Int 23:2159–2167. https://doi.org/10.1007/s00198-011-1831-7
Krieger NS, Frick KK, Bushinsky DA (2004) Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens 13:423–436
Asaba Y, Ito M, Fumoto T et al (2009) Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J Bone Miner Res 24:241–250. https://doi.org/10.1359/jbmr.081006
Shimizu H, Nakagami H, Osako MK et al (2009) Prevention of osteoporosis by angiotensin-converting enzyme inhibitor in spontaneous hypertensive rats. Hypertens Res 32:786–790. https://doi.org/10.1038/hr.2009.99
Kang KY, Kang Y, Kim M et al (2013) The effects of antihypertensive drugs on bone mineral density in ovariectomized mice. J Korean Med Sci 28:1139–1144. https://doi.org/10.3346/jkms.2013.28.8.1139
Donmez BO, Ozdemir S, Sarikanat M et al (2012) Effect of angiotensin II type 1 receptor blocker on osteoporotic rat femurs. Pharmacol Reports 64:878–888. https://doi.org/10.1016/S1734-1140(12)70882-4
Lynn H, Kwok T, Wong SYS et al (2006) Angiotensin converting enzyme inhibitor use is associated with higher bone mineral density in elderly Chinese. Bone 38:584–588. https://doi.org/10.1016/j.bone.2005.09.011
Zhang Y, Qin L, Leung P, Kwok TCY (2012) The effect of angiotensin-converting enzyme inhibitor use on bone loss in elderly Chinese. J Bone Miner Metab 30:666–673. https://doi.org/10.1007/s00774-012-0363-3
Rianon N, Ambrose CG, Pervin H et al (2017) Long-term use of angiotensin-converting enzyme inhibitors protects against bone loss in African-American elderly men. Arch Osteoporos 12:94. https://doi.org/10.1007/s11657-017-0387-3
Pasco JA, Nicholson GC, Kotowicz MA (2012) Cohort Profile: Geelong Osteoporosis Study. Int J Epidemiol 41:1565–1575
Adverse Drug Reactions Advisory Committee (1999) Australian Adverse Drug Reactions Bulletin. 18:1–4
Martineau P, Silva BC, Leslie WD (2017) Utility of trabecular bone score in the evaluation of osteoporosis. Curr Opin Endocrinol Diabetes Obes 24:402–410. https://doi.org/10.1097/med.0000000000000365
Giles GG, Ireland PD (1996) Dietary Questionnaire for Epidemiological Studies (Version 2), Melbourne. Cancer Counc Victoria
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Henry MJ, Pasco JA, Korn S et al (2010) Bone mineral density reference ranges for Australian men: Geelong Osteoporosis Study. Osteoporos Int 21:909–917
Henry MJ, Pasco JA, Pocock NA et al (2004) Reference ranges for bone densitometers adopted Australia-wide: Geelong osteoporosis study. Australas Radiol 48:473–475
Anderson KB, Holloway-Kew KL, Hans D et al (2019) Reference ranges for trabecular bone score in Australian men and women: a cross-sectional study. JBMR Plus 3:e10133. https://doi.org/10.1002/jbm4.10133
Choi HJ, Park C, Lee Y-K et al (2015) Risk of fractures in subjects with antihypertensive medications: a nationwide claim study. Int J Cardiol 184:62–67. https://doi.org/10.1016/j.ijcard.2015.01.072
Solomon DH, Mogun H, Garneau K, Fischer MA (2011) Risk of fractures in older adults using antihypertensive medications. J Bone Miner Res 26:1561–1567. https://doi.org/10.1002/jbmr.356
Kwok T, Leung J, Barrett-Connor E, Group for the OF in M (MrOS) R (2017) ARB users exhibit a lower fracture incidence than ACE inhibitor users among older hypertensive men. Age Ageing 46:57–64. https://doi.org/10.1093/ageing/afw150
Bokrantz T, Schiöler L, Boström KB et al (2020) Antihypertensive Drug Classes and the Risk of Hip Fracture: Results From the Swedish Primary Care Cardiovascular Database. J Hypertens 38:167–175
Butt DA, Mamdani M, Gomes T et al (2014) Risk of osteoporotic fractures with angiotensin II receptor blockers versus angiotensin-converting enzyme inhibitors in hypertensive community-dwelling elderly. J Bone Miner Res 29:2483–2488. https://doi.org/10.1002/jbmr.2271
Rejnmark L, Vestergaard P, Mosekilde L (2006) Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case–control study. J Hypertens 24
Kao Y-T, Huang C-Y, Fang Y-A, Liu J-C (2020) The association between renin angiotensin aldosterone system blockers and future osteoporotic fractures in a hypertensive population - a population-based cohort study in Taiwan. Int J Cardiol 305:147–153. https://doi.org/10.1016/j.ijcard.2019.12.069
Carbone LD, Vasan S, Prentice RL et al (2019) The renin-angiotensin aldosterone system and osteoporosis: findings from the Women’s Health Initiative. Osteoporos Int 30:2039–2056. https://doi.org/10.1007/s00198-019-05041-3
Chen C-I, Yeh J-S, Tsao N-W et al (2017) Association between renin-angiotensin-aldosterone system blockade and future osteoporotic fracture risk in hypertensive population: a population-based cohort study in Taiwan. Medicine (Baltimore) 96:e8331–e8331. https://doi.org/10.1097/MD.0000000000008331
Shea C, Witham MD (2020) Association between the use of angiotensin-blocking medications with hip fracture and death in older people. J Frailty Aging 9:107–110. https://doi.org/10.14283/jfa.2019.38
Kunutsor SK, Blom AW, Whitehouse MR et al (2017) Renin-angiotensin system inhibitors and risk of fractures: a prospective cohort study and meta-analysis of published observational cohort studies. Eur J Epidemiol 32:947–959. https://doi.org/10.1007/s10654-017-0285-4
Ruths S, Bakken MS, Ranhoff AH et al (2015) Risk of hip fracture among older people using antihypertensive drugs: a nationwide cohort study. BMC Geriatr 15:153. https://doi.org/10.1186/s12877-015-0154-5
Pérez-Castrillón JL, Silva J, Justo I et al (2003) Effect of quinapril, quinapril-hydrochlorothiazide, and enalapril on the bone mass of hypertensive subjects: relationship with angiotensin converting enzyme polymorphisms. Am J Hypertens 16:453–459. https://doi.org/10.1016/S0895-7061(03)00845-8
Steckelings UM, Kaschina E, Unger T (2005) The AT2 receptor—a matter of love and hate. Peptides 26:1401–1409. https://doi.org/10.1016/j.peptides.2005.03.010
Pasco JA, Kotowicz MA, Henry MJ, Nicholson GC, Spilsbury HJ, Box JDSH (2006) High sensitivity C-reactive protein and fracture risk in elderly women. JAMA 296:1353–1355
Quinn JMW, Gillespie MT (2005) Modulation of osteoclast formation. Biochem Biophys Res Commun 328:739–745. https://doi.org/10.1016/j.bbrc.2004.11.076
Tanabe N, Ito-Kato E, Suzuki N et al (2004) IL-1α affects mineralized nodule formation by rat osteoblasts. Life Sci 75:2317–2327. https://doi.org/10.1016/j.lfs.2004.04.026
Liao W-H, Suendermann C, Steuer AE et al (2018) Aldosterone deficiency in mice burdens respiration and accentuates diet-induced hyperinsulinemia and obesity. JCI Insight 3:e99015. https://doi.org/10.1172/jci.insight.99015
Yuan F-L, Xu M-H, Li X et al (2016) The roles of acidosis in osteoclast biology. Front Physiol 7:222
Butt DA, Alharty R, Leu R, Cheung AM (2015) Hypertension, antihypertensive drugs and the risk of fractures. Clin Rev Bone Miner Metab 13:160–172. https://doi.org/10.1007/s12018-015-9191-z
Hwang JK, Leu R, Butt DA (2015) Hypertension, antihypertensive drugs, and bone mineral density. Clin Rev Bone Miner Metab 13:149–159. https://doi.org/10.1007/s12018-015-9193-x
Ilić K, Obradović N, Vujasinović-Stupar N (2013) The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif Tissue Int 92:217–227. https://doi.org/10.1007/s00223-012-9671-9
Acknowledgements
The authors thank Professor Graham Giles of the Cancer Epidemiology Centre of The Cancer Council Victoria for permission to use the Dietary Questionnaire for Epidemiological Studies (Version 2), Melbourne: The Cancer Council Victoria 1996.
The authors also thank the Victorian Cancer Registry for data linkage.
Funding
The Geelong Osteoporosis Study was supported by grants from the National Health and Medical Research Council (NHMRC; projects 251638, 299831, 628582).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Barwon Health Human Research Ethics Committee approved the study (projects 92/01 and 00/56).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
For this type of study, formal consent is not required.
Conflict of interest
KLH-K is supported by an Alfred Deakin Postdoctoral Research Fellowship and KBA by an Australian Government Research Training Program Scholarship. AGB, JG, MAK, W-HL, MH, and JAP declare no competing interests.
Additional information
Publisher's Nnote
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Holloway-Kew, K.L., Betson, A.G., Anderson, K.B. et al. Association between bone measures and use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Arch Osteoporos 16, 137 (2021). https://doi.org/10.1007/s11657-021-01004-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-021-01004-6