Abstract
Summary
In a prospective cohort study of 5,995 older American men (MrOS), users of angiotensin-converting enzyme (ACE) inhibitors had a small but significant increase in bone loss at the hip over 4 years after adjustment for confounders. Use of angiotensin II AT1 receptor blockers (ARB) was not significantly associated with bone loss.
Introduction
Experimental evidence suggests that angiotensin II promotes bone loss by its effects on osteoblasts. It is therefore plausible that ACE inhibitor and ARB may reduce rates of bone loss. The objective of this study is to examine the independent effects of ACE inhibitor and ARB on bone loss in older men.
Methods
Out of 5,995 American men (87.2%) aged ≥65 years, 5,229 were followed up for an average of 4.6 years in a prospective six-center cohort study—The Osteoporotic Fractures in Men Study (MrOS). Bone mineral densities (BMD) at total hip, femoral neck, and trochanter were measured by Hologic densitometer (QDR 4500) at baseline and year 4.
Results
Out of 3,494 eligible subjects with complete data, 1,166 and 433 subjects reported use of ACE inhibitors and ARBs, respectively. When compared with nonusers, continuous use of ACE inhibitors was associated with a small (0.004 g/cm2) but significant increase in the average rate of BMD loss at total hip and trochanter over 4 years after adjustment for confounders. Use of ARB was not significantly associated with bone loss.
Conclusion
Use of ACE inhibitors but not ARB may marginally increase bone loss in older men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II AT1 receptor blockers (ARB) are commonly used antihypertensive agents, because of their good side effect profile and renoprotective effects in diabetic patients [1, 2]. ACE inhibitors are thought to lower tissue angiotensin II by inhibiting its production, and ARB specifically blocks the effects of angiotensin II AT1 receptors. There are experimental data to suggest that angiotensin II stimulates osteoclastic activities via its effects on osteoblasts [3], but it may also stimulate proliferation of osteoblasts [4]. We have previously reported that ACE inhibitor use was associated with greater bone mineral density (BMD) in older Chinese men and women [5]. A case–control study also showed that ACE inhibitors were associated with a slightly reduced risk of fractures [6]. One prospective uncontrolled trial in hypertensive people showed that quinapril and enalapril reduced calciuria and serum 1,25-hydroxy vitamin D, but urinary deoxypyridinoline—a marker of bone resorption—was not significantly changed [7].
We therefore hypothesized that ACE inhibitors and ARB decrease the rates of bone loss in older people. In order to test these hypotheses, the data from a large cohort study of community-dwelling older American men (The Osteoporotic Fractures in Men (MrOS)) [8] were examined for the independent effects of ACE inhibitors and ARB on rates of bone loss.
Materials and methods
Participants
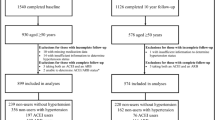
From March 2000 to April 2002, men aged ≥65 years participated in the baseline examination of the prospective cohort study of risk factors for osteoporotic fracture in older men (MrOS) [8]. MrOS is a multicenter study; the design, recruitment methods, and measurements have been described previously [9]. Briefly, 5,995 men were recruited in six areas of the USA: Birmingham, AL; Minneapolis, MN; the Monongahela Valley near Pittsburgh, PA; Palo Alto, CA; Portland, OR; and San Diego, CA. Potential volunteers who could not provide informed consent or self-reported data, could not walk, had hip replacement, or had severe medical conditions that would preclude participation in follow-up were excluded. All subjects were invited to be followed up at years 2 and 4. Only those who were successfully followed up and who did not use osteoporosis medications at any stage were included in the present analyses. Subjects on androgen replacement or androgen deprivation therapy were excluded. The MrOS protocol was approved by the institutional review boards of each participating institution. Written informed consent was obtained from all participants.
Measurements
Demographic information, smoking habits, and personal medical history, including hypertension, diabetes mellitus, cardiac failure, and medication history were obtained by self-administered questionnaire or by face-to-face interviews performed by certificated research assistants. Physical activity was quantified using the Physical Activity Scale for the Elderly (PASE) [10].
At each visit, participants were asked to bring all the medications they had taken in the past 30 days to the clinical center for data collection. Only prescription medications were included at baseline. Use of calcium and vitamin D supplements was obtained from a modified food frequency questionnaire developed specifically for MrOS by Block Dietary Data Systems. At follow-up visits at years 2 and 4, both prescribed and over-the-counter medications were recorded. All medications recorded by the clinics were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA). The recorded specific medications of interest for each person included ACE inhibitors, ARB, thiazides, loop diuretics, nitrates, statins, beta-blockers, calcium antagonists, glitazones, alpha-blockers, androgen, antiandrogen, oral glucocorticoid, inhaled steroid, vitamin D, and calcium. The duration of drug use was estimated by the number of visits when the drug use was reported: three visits—4 years or continuous, two visits—2 to 4 years, and one visit only—less than 2 years.
Body weight was measured in participants wearing indoor clothing without shoes using a balance beam or electronic scale. The scale at each clinical center was calibrated monthly. Weight change was calculated by subtracting weight at the baseline examination from weight at the year 4 follow-up examination and was expressed as a percentage of the baseline value. Ankle–brachial index (ABI) to estimate the extent of atherosclerosis was measured as follows: supine blood pressure in right arm and both ankles were measured in duplicates, using a standard mercury sphygmomanometer and an 8-MHz Doppler probe. ABI was calculated for each leg by dividing the posterior tibial systolic pressure by brachial systolic pressure. The lower ankle ABI was used in statistical analysis [11].
Morning fasting blood was taken. Serum creatinine was measured by using a Roche COBAS Integra 800 automated analyzer (Roche Diagnostics Corp., Indianapolis, IN), which was calibrated daily in the clinical laboratory. The estimated glomerular filtration rate (eGFR) was calculated from the following Modification of Diet in Renal Disease study equation [12]:
eGFR of less than 60 mL/min/1.73 m2 is indicative of moderate (stage 3) chronic kidney disease. This cutoff value of eGFR was used to define chronic kidney disease in this study.
BMD of the total hip, femoral neck, and trochanter was measured at each visit using by dual-energy X-ray absorptiometry with Hologic QDR 4500 bone densitometers (Hologic, Waltham, MA, USA). Only BMD data at baseline and year 4 were used in this analysis. Standardized procedures for positioning, scanning, and analysis were executed for all scans. All measurements were performed on the right hip unless the participant had right hip replacement or had other metal internal fixation materials. Extensive quality assurance protocols were used throughout, including central training and certification of technicians and regular phantom scans and calibration within and across centers. The interscanner coefficient of variation was 0.9%.
Statistical analysis
Users of ACE inhibitors and ARB were compared with nonusers of either drug in clinical characteristics using ANOVA for continuous variables or chi-square test for categorical variables. Univariate analysis was performed to examine the associations between BMD change at total hip and potential confounders which included race, education level, chronic kidney disease as defined by eGFR, ankle brachial index, history of diabetes mellitus, cardiac failure, hypertension at any visit, and estimated duration of use of thiazide, loop diuretic, nitrate, statin, beta-blocker, calcium antagonist, glitazone, and alpha-blocker. Those variables which showed significant association at P level of <0.05 were included into the final multivariable regression models examining the independent effects of use of ACE inhibitors and ARB on bone loss at total hip, femoral neck, and trochanter. Baseline age, age squared, current smoking, body weight, percentage weight change, and duration of use of calcium and vitamin D supplements, androgen, antiandrogen, oral glucocorticoid, and inhaled steroid were regarded as recognized factors of bone loss and were predetermined to be covariates in the final multivariable regression models. As some patients might have taken ARBs after developing side effects from ACE inhibitors or concomitantly with ACE inhibitors, duration of use of ARB or ACE inhibitor was entered a covariate in the final multivariable regression models.
Propensity score analysis was also performed. Propensity scores indicate the likelihood of a participant’s use of ACE inhibitors or ARB. Significant factors for the use of ACE inhibitors and ARB use were determined by stepwise logistic regression. As proportional odds assumption was not satisfied, generalized logits model was used. Linear regression was then performed to examine the independent association between the use of ACE inhibitors or ARB and rates of bone loss, by adjusting for propensity scores. Covariates associated with bone loss but not used to calculate propensity scores were also entered into the models for adjustment [13].
All statistical analyses were performed using the statistical package SAS, version 9.1 (SAS Institute, Inc., Cary, North Carolina). An α level of 5% was used as the level of significance.
Results
Five thousand nine hundred ninety-five men were recruited at baseline. Of these, 5,229 (87.2%) were followed up at year 4. The average duration of follow-up was 4.6 years. Out of these, 330 participants were excluded from analysis because of use of osteoporosis-related medications; 1,032 participants were excluded because of missing drug use and supplement data at baseline (N = 381) or follow-up (N = 651); 281 participants were excluded due to missing serum creatinine data; and 92 participants were excluded because of missing BMD data at baseline or follow-up. When compared with eligible subjects with complete data, those who were excluded for the above reasons were significantly older (mean 75.0 versus 72.8 years old, P < 0.0001), but their proportions of ACE inhibitor or ARB users were similar.
The clinical characteristics and the 4-year BMD changes of the remaining participants who used ACE inhibitors or ARB were compared with those of nonusers in Table 1. One hundred thirty-nine ever users of ACE inhibitors took ARB at some stage, 64% of them for less than 2 years. Twenty-one subjects had taken ACE inhibitors and ARB concomitantly, but only one did so continuously. ACE inhibitor and ARB users were heavier, more likely to be diabetic and have cardiovascular and chronic kidney disease, and had lower ankle brachial index than nonusers. Continuous users of ACE inhibitors or ARBs had significantly greater femoral neck BMDs than nonusers after adjustment for age and body weight. Over 4 years, ACE inhibitor use of any duration was associated with higher rates of bone loss at all three hip sites, after adjusting for age and body weight. Only continuous users of ARB use had significantly greater bone loss at femoral neck.
In order to further examine the independent effect of the duration of ACE inhibitor use or ARB use on rates of bone loss, multiple variable regression models were constructed using the significant and predetermined confounders as covariates (listed in footnote of Table 2). The results shown in Table 2 indicated that on both multivariable and propensity score analyses, use of ACE inhibitors was associated with significantly greater bone loss at total hip and trochanter. The average absolute difference between continuous ACE inhibitor users and nonusers in total hip BMD changes over 4 years was approximately 0.004 g/cm2. Use of ARB was not significantly associated with bone loss at any site after adjustment for confounders by both methods of analysis.
Discussion
In this prospective cohort study of older men, use of ACE inhibitor was associated with a small but significant increase in rates of bone loss at total hip, independent of potential confounders. The average absolute difference in BMD loss associated with continuous use of ACE inhibitors over 4 years was approximately 0.004 g/cm2.
Consistent with a cross-sectional study of older Chinese men and women [5], the average femoral neck BMD of continuous users of ACE inhibitors was significantly greater than that of nonusers in this study. Similar finding was also observed in continuous ARB users, but it was somewhat surprising to observe such group differences in short-term ARB users as well. This suggests that the cross-sectional group differences in BMDs might have been confounded by the associated medical conditions and concomitant drug use.
As ACE inhibitors and ARBs are commonly prescribed for hypertension and cardiac failure, especially in diabetic people, it was not unexpected that users of these two classes of drugs had higher BMI and lower ABI, were more likely to have diabetes and chronic kidney disease, and were users of antihypertensive drugs, statins, diuretics, and nitrates. It is essential to adjust for confounding effects of these factors, as a number of cardiovascular medication, peripheral vascular disease, and chronic kidney disease have been reported to be associated with bone loss [14–20]. Noninsulin-dependent diabetes mellitus is associated with greater fracture risk but not with bone loss [21, 22], though use of glitazone has been associated with greater bone loss [23].
In order to adjust for confounding effects by drug indications, we analyzed the data by both multivariable analysis and the propensity score method which is an alternative to the traditional multivariable model, designed to reduce bias when comparing exposure groups in observational studies. In this study, both methods yielded similar results, thus strengthening the validity of the findings.
The small but significant increases in bone loss at total hip and trochanter with the use of ACE inhibitors were unexpected. But with this finding, we could reject our initial hypothesis that use of ACE inhibitors or ARBs reduces rates of bone loss in older men. An association between ACE inhibitor use and increased bone loss has been similarly found in a cohort study of middle-aged Japanese people [24]. Whether ACE inhibitors may marginally increase rates of bone loss warrants further study as many patients take these drugs for many years.
ACE is the major enzyme for the production of angiotensin II in humans, and angiotensin II indirectly stimulates osteoclastic activities by promoting RANKL production in osteoblasts [25]. It is therefore surprising that we should find that ACE inhibitor use to be associated with greater bone loss. Indeed in hypertensive rats, ACE inhibitor attenuated bone loss after ovariectomy [26]. One possible explanation is that in the animal studies, the models were designed to have abnormally high levels of angiotensin II. On the other hand, the RAS may not necessarily be activated in normal older people who are prone to have high salt intakes [27]. It may actually be suppressed in those with diabetes mellitus and hypertension because of sodium retention [28]. Another possible explanation is that chronic use of ACE inhibitors is not effective in lowering local angiotensin II production, because of reactive increase in plasma rennin and angiotensin I, and other non-ACE-related mechanisms in which angiotensin I can be converted into angiotensin II [29].
These observations could explain why ACE inhibitor did not attenuate bone loss in men as expected from animal studies but could not explain the observed increase in rates of bone loss. One possible explanation is that ACE inhibitors may lower serum sex hormone concentrations. One prospective study of lisinopril in hypertensive patients showed a significant decrease in free testosterone over 6 months in men and an increase in sex hormone-binding globulin in women [30]. In addition, a cross-sectional study of older Chinese men found that ACE inhibitor use was associated with lower serum dehydroepiandrosterone [31].
In contrast with ACE inhibitors, ARB was not significantly associated with bone loss. The clinical profiles of users of ACE inhibitors and ARBs were very similar. In the USA, ARB was usually prescribed when ACE inhibitor was not tolerated, thus explaining the smaller number of ARB users. Based on the educational levels, there was no evidence to suggest that ARB users were economically better off than ACE inhibitor users. ARBs are designed to block the actions of angiotensin II on the AT1 receptors but have none of the peptidase actions of ACE inhibitors. The effects of angiotensin II on the vasculature have been known to mediate through AT1 receptors. But animal and cell studies suggest that the osteoclastic stimulating effects of angiotensin II are primarily mediated through AT2 rather than AT1 receptors on osteoblasts [32–34]. AT1 receptors on the other hand are mechanoreceptors for the osteoblastic response to mechanical stress [35]. We have observed that losartan, an ARB, had no significant effect on BMD in orchidectomized rats [36]. This study also suggested that use of ARBs does not have any significant overall effect on bone loss in older men.
Although prospective, the study is only observational. The rate of follow-up was high, but a significant number of subjects were excluded because of incomplete data. The excluded subjects were significantly older, but their use of ACE inhibitors or ARB was similar to the subjects included in the analysis. Unless there is a significant interaction between age and the potential effects of ACE inhibitors or ARBs on bone loss, the older age of the excluded subjects should not have biased our results. Other limitations include the reliance on self-report for medical conditions and the imprecise information on drug exposure. In addition, the number of ARB users was limited. A small effect of ARB on bone loss can therefore not be ruled out. On the other hand, the strengths of this study include the large sample size of community-dwelling older adults from six different geographic regions and the ability to control (statistically) for concomitant medications and coexisting diseases.
In summary, use of ACE inhibitors but not ARB was associated with significantly greater bone loss at the total hip over 4 years in older men. But the difference was small and was unlikely to have a significant impact on fracture risk in the short term. Whether this marginal difference may be more significant and consistent in postmenopausal women who have greater bone loss warrants further study. With the recent introduction of renin inhibitors [37] and ARB with coexisting inhibitory effect on ACE [34], there is a need to evaluate their long-term effects on bone. Better understanding on how the components of the renin–angiotensin system influence bone turnover might improve the choice of antihypertensive therapies which promote bone health.
References
So WY, Ma RC, Ozaki R et al (2006) Angiotensin-converting enzyme (ACE) inhibition in type 2, diabetic patients—interaction with ACE insertion/deletion polymorphism. Kidney Int 69(8):1438–1443
Brenner BM, Cooper ME, de Zeeuw D et al (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345(12):861–869
Hatton R, Stimpel M, Chambers TJ (1997) Angiotensin II is generated from angiotensin I by bone cells and stimulates osteoclastic bone resorption in vitro. J Endocrinol 152(1):5–10
Hiruma Y, Inoue A, Hirose S et al (1997) Angiotensin II stimulates the proliferation of osteoblast-rich populations of cells from rat calvariae. Biochem Biophys Res Commun 230(1):176–178
Lynn H, Kwok T, Wong SY et al (2006) Angiotensin converting enzyme inhibitor use is associated with higher bone mineral density in elderly Chinese. Bone 38:584–588
Rejnmark L, Vestergaard P, Mosekilde L (2006) Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J Hypertens 24(3):581–589
Pérez-Castrillón JL, Silva J, Justo I et al (2003) Effect of quinapril, quinapril-hydrochlorothiazide, and enalapril on the bone mass of hypertensive subjects: relationship with angiotensin converting enzyme polymorphisms. Am J Hypertens 16(6):453–459
Orwoll E, Black JB, Barrett-Connor E et al (2005) Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26(5):569–585
Blank JB, Cawthon PM, Carrion-Petersen ML et al (2005) Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26(5):557–568
Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46:153–162
Woo J, Lynn H, Wong SY, Hong A, Tang YN, Lau WY, Lau E, Orwoll E, Kwok TC (2006) Correlates for a low ankle–brachial index in elderly Chinese. Atherosclerosis 186(2):360–366
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39(2 Suppl 1):S1–S266
D'Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17:2265–2281
Bolland MJ, Ames RW, Horne AM et al (2007) The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int 18(4):479–486
Uzzan B, Cohen R, Nicolas P et al (2007) Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone 40(6):1581–1587
Rejnmark L, Vestergaard P, Heickendorff L et al (2006) Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: results from a randomized controlled study with bumetanide. J Bone Miner Res 21(1):163–170
Jamal SA, Cummings SR, Hawker GA (2004) Isosorbide mononitrate increases bone formation and decreases bone resorption in postmenopausal women: a randomized trial. J Bone Miner Res 19(9):1512–1517
Pasco JA, Henry MJ, Sanders KM et al (2004) Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res 19(1):19–24
Wong SYS, Kwok T, Lynn H, Griffith JF, Leung J, Tang YYN, Leung PC (2005) Bone mineral density and the risk of peripheral vascular disease in men and women: results from the Mr. and Ms Os, Hong Kong. Osteoporos Int 16:1933–1938
Ishani A, Paudel M, Taylor BC, Barrett-Connor E, Jamal S, Canales M, Steffes M, Fink HA, Orwoll E, Cummings SR, Ensrud KE, Osteoporotic Fractures in Men (MrOS) Study Group (2008) Renal function and rate of hip bone loss in older men: the Osteoporotic Fractures in Men Study. Osteoporos Int 19(11):1549–1556
Inzerillo AM, Epstein S (2004) Osteoporosis and diabetes mellitus. Rev Endocr Metab Disord 5(3):261–268
Gerdhem P, Isaksson A, Akesson K et al (2005) Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int 16(12):1506–1512
Murphy CE, Rodgers PT (2007) Effects of thiazolidinediones on bone loss and fracture. Ann Pharmacother 41(12):2014–2018
Masunari N, Fujiwara S, Nakata Y et al (2008) Effect of angiotensin converting enzyme inhibitor and benzodiazepine intake on bone loss in older Japanese. Hiroshima J Med Sci 57(1):17–25
Shimizu H, Nakagami H, Osako MK et al (2008) Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J 22(7):2465–2475
Shimizu H, Nakagami H, Osako MK, Nakagami F, Kunugiza Y, Tomita T, Yoshikawa H, Rakugi H, Ogihara T, Morishita R (2009) Prevention of osteoporosis by angiotensin-converting enzyme inhibitor in spontaneous hypertensive rats. Hypertens Res 32(9):786–790
Woo J, Leung SS, Ho SC et al (1998) Dietary intake and practices in the Hong Kong Chinese population. J Epidemiol Community Health 52(10):631–637
Chan JC, Cheung CK, Cockram CS et al (1994) Atrial natriuretic peptide and renin-angiotensin-aldosterone system in non-insulin-dependent diabetes mellitus. J Hum Hypertens 8(6):451–456
Azizi M, Chatellier G, Guyene TT et al (1995) Additive effects of combined angiotensin-converting enzyme inhibition and angiotensin II antagonism on blood pressure and renin release in sodium-depleted normotensives. Circulation 92(4):825–834
Koshida H, Takeda R, Miyamori I (1998) Lisinopril decreases plasma free testosterone in male hypertensive patients and increases sex hormone binding globulin in female hypertensive patients. Hypertens Res 21(4):279–282
Kwok T, Ohlsson C, Vandenput L, Tang N, Zhang YF, Tomlinson B, Leung PC (2010) ACE inhibitor use was associated with lower serum dehydroepiandrosterone concentrations in older men. Clin Chim Acta 411(15–16):1122–1125
Izu Y, Mizoguchi F, Kawamata A et al (2008) Angiotensin II type 2 receptor blockade increases bone mass. J Biol Chem 284(8):4857–4864
Asaba Y, Ito M, Fumoto T et al (2008) Activation of renin–angiotensin system induces osteoporosis independently of hypertension. J Bone Miner Res 24(2):241–250
Agata J, Ura N, Yoshida H et al (2006) Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzyme. Hypertens Res 29(11):865–874
Bandow K, Nishikawa Y, Ohnishi T, Kakimoto K, Soejima K, Iwabuchi S, Kuroe K, Matsuguchi T (2007) Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and MIP-1beta expression in osteoblasts through the angiotensin II type 1 receptor. J Cell Physiol 211(2):392–398
Zhang Y, Yeung B, Qin L, Kwok T (2009) The effect of angiotensin II type I receptor blocker (ARB) on bone loss in orchidectomized male hypertensive and normotensive rats. 36th European symposium on calcified tissues. Vienna, May 2009, p. 512
Musini VM, Fortin PM, Bassett K, Wright JM (2008) Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database Syst Rev (4):CD007066
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provided support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Center for Research Resources, and NIH Roadmap for Medical Research under the following grant numbers U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Conflicts of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Kwok, T., Leung, J., Zhang, Y.F. et al. Does the use of ACE inhibitors or angiotensin receptor blockers affect bone loss in older men?. Osteoporos Int 23, 2159–2167 (2012). https://doi.org/10.1007/s00198-011-1831-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1831-7