Abstract
Aim
To evaluate safety, technical and clinical success of embolization of type Ia endoleak (T1a EL) using ethylene–vinyl alcohol copolymer as embolic agent alone or in combination with other materials.
Materials and methods
Five patients presented T1a EL after endovascular repair of aortic aneurysms (EVAR) with radiological evidence of expanding sac size; in particular, three had contained rupture. In one patient, proximal cuff insertion was previously performed, in three patients proximal cuff was urgently inserted but T1a EL persisted; one patient, previously treated with Ovation Abdominal Stent Graft System, was directly proposed for endovascular treatment. In all cases, endovascular embolization was successfully performed and the transfemoral approach was always chosen; in one case it failed and translumbar approach by direct puncture of the sac was required. Used embolization agents were glue, ethylene–vinyl alcohol copolymer (Onyx) and coils in three cases, n-butyl cyanoacrylate and Onyx in one case, Onyx and coils in the last case.
Results
Technical success rate was 100% as well as clinical success. No major or minor complication, including non-target embolization, was registered. Clinical success was 100% until today and the sac diameter remained stable in four patients and decreased in one.
Conclusions
Onyx may be considered a suitable embolic agent in the treatment of patients with type Ia endoleaks after EVAR, after failure of conventional treatments such as prolonged balloon inflation of the aortic neck or deployment of large bare stent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endovascular aortic aneurysm repair (EVAR) has been accepted as a standard procedure for an anatomically suitable infrarenal abdominal aortic aneurysm (AAA) [1]. Although EVAR is less invasive and can be the treatment of choice for high-risk patients, it might lead to a great number of complications and reinterventions [2]. Endoleaks are one of the main reasons for reintervention, and Mehta et al. [2] reported that endoleaks accounted for 56.6% of all reintervention cases. Type I endoleaks occur because of inadequate sealing at the proximal and distal ends of the prosthesis [3]. Type I endoleaks are known to be associated with high sac pressure, aneurysmal dilatation, and aneurysm rupture. Therefore, treatment at the time of diagnosis is recommended [1]. The prognosis of type Ia endoleaks depends on the possibility of sealing the stent graft, proximally. Migration, malposition and inadequate sealing of the stent graft are the more frequent causes [4, 5]. Sealing of proximal type 1 endoleaks has traditionally been achieved by using an aortic cuff to extend graft coverage more proximally or by the placement of a large-caliber expandable balloon stent (e.g. Palmaz stent) [6]. An alternative approach, generally considered when the previously mentioned ones are unsuccessful or infeasible, is transcatheter embolization, which is a well-established treatment option for type 2 endoleaks [6]. In the literature, short reports have described this technique: embolic agents used are coils or liquids agents [N-butyl 2-cyanoacrylate (NBCA) or ethylene–vinyl alcohol copolymer (Onyx—Covidien, Irvine, California, USA)] [6,7,8,9].
We present eight cases of proximal type I endoleak with radiological evidence of expanding sac size, five of them had a contained rupture. They were treated with endovascular embolization performed with Onyx, alone or in combination with other embolic agents. In seven patients, a proximal cuff was deployed. The aim of this study was to evaluate the safety, technical success, and clinical success of embolization of type T1a EL after EVAR using ethylene–vinyl alcohol copolymer as an embolic agent alone or in combination with other materials.
Materials and methods
Our Internal Review Board approved the study.
Informed consent was obtained from all individual participants included in the study. From August 2013 to August 2015, eight patients who presented with a type Ia endoleak after EVAR [six men and two women, average age 72.5 years old (range 65–83 years old)] were studied (Table 1).
The treatment indications for exclusion of the EL were: expansion and rupture of the sac (five cases) and increasing of sac size (three cases). In all patients, balloon percutaneous angioplasty was the first attempted endovascular procedure, but it failed in all cases. Proximal cuff insertion was previously deployed in three patients, proximal cuff was urgently inserted but T1a EL persisted in four patients, and one patient was previously treated with the Ovation Abdominal Stent Graft System (TriVascular Inc., Santa Rosa, CA, USA) characterized by a suprarenal nitinol stent, which was directly proposed for endovascular treatment (Fig. 1a–f). In all cases, the transfemoral approach was chosen: a Simmons 1 (Cordis; Miami Lakes, Florida) angiographic catheter was used to catheterize endoleak and a microcatheter (Progreat, Terumo, Tokyo, Japan) was used to navigate in the sac; in one case, it failed and a translumbar approach by direct sac puncture was required (Fig. 2a–f). In this last case a 18 G (Biopsybell, Mirandola (MO), Italy) needle was used for the percutaneous puncture performed under cone beam computer tomography (CBCT) guidance with dedicate software (XperGuide, Philips Healthcare); the microcatheter was introduced through the needle. Used embolization agents were glue (Glubran II, N-butyl-2-cyanoacrylate (NBCA); GEM S.r.l., Viareggio, Italy), ethylene–vinyl alcohol copolymer (Onyx—Covidien, Irvine, California, USA), and coils (ConcertoTM Detachable Coil System; Irvine, CA, USA) in three cases, NBCA and Onyx in four cases, and Onyx and coils in the last case (Table 1). The total amount of Onyx used and its concentration is reported in Table 1. In all cases, embolization was performed with fluoroscopic guidance. Embolization was stopped when the sac was filled completely; final arteriogram confirmed complete embolization of the EL.
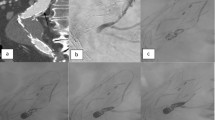
Contrast-enhanced CT revealed type Ia endoleak (a); angiogram performed with angiographic catheter located in the access to the aneurysmal sac (b); angiogram performed with the microcatheter in the sac (c); image acquired at the end of the procedure, shows ethylene–vinyl alcohol copolymer in the sac (d)
Ruptured abdominal aorta aneurysm (a); persistent type Ia endoleak after deployment of proximal cuff (b); angiogram performed after percutaneous puncture of the aneurysmal sac confirmed that there was an endoleak (c); embolization performed with coils, NBCA and ethylene–vinyl alcohol copolymer with the aim of filling remaining supplied gaps (d); minimum intensity projection (MIP) reconstruction confirmed complete embolization: embolic agents (coils, NBCA, and ethylene–vinyl alcohol copolymer) take the conformation of the endoleak (e)
Coils used presented a diameter of 20 mm and a length of 40 mm.
Patients were routinely monitored at our institution and follow-up included contrast-enhanced ultrasound (CEUS) before discharge, computed tomography angiography (CTA) at 30 days, CTA or CEUS at 6 months, and CTA at 12 months postoperatively, and annually thereafter. Technical success was defined as successful embolization of the endoleak and complete resolution of the endoleak on completion angiography. Clinical success was defined as complete resolution of the endoleak without enlargement of the aneurysm sac on follow-up CT. Safety was defined on the basis of minor or major complications related to the procedure [10].
Results
Technical success was 100%, with complete exclusion of the EL being reached in all cases. Follow-up did not show the reappearance of EL type Ia in any case; in particular, one patient had a recurrence-free follow-up of 24 months (death due to myocardial infarction), and another patient had a follow-up of 12 months (death due to stroke). For the remaining six patients, follow-up is underway (mean 16.5 months, range 12–30 months,) (Table 1). Diameter of the aneurysm sac decreased in three patients, whereas five patients had an unchanged aneurysm diameter. There were no procedure-related complications such as intraperitoneal bleeding, ischemic bowel injury, bowel perforation, or infection in the aneurysm sac or graft.
Discussion
The incidence of type I proximal endoleak is more frequent in anatomically difficult situations, such as short neck diameter (< 15 mm), large neck diameter (> 32 mm), tapered necks, increased angulations (> 60°), and landing zones with calcifications, thrombus, or uneven size [11]. Type I EL is associated with a significant pressure increase inside the aneurysm sac, and treatment should always be considered [12]. Conventional methods for the management of type IA endoleaks are cuffs or Palmaz stents. Cuffs can be applied only if a sufficient native aorta is available proximally to support the stent [10, 12]. If an endoleak persists despite these measures, definitive therapy may require conventional open surgery, visceral artery bypass combined with stent-graft extension, or the use of chimney or periscope grafts to extend proximal and distal landing zones.
Patients not eligible for these more complicated procedures because of severe comorbidities or adverse anatomical factors may be treated by transcatheter embolization of the endoleak itself [9]. There is limited published experience on type 1 endoleak embolization, and previous reports have involved coils and n-butyl 2-cyanoacrylate (NBCA) [8, 11]. Ethylene–vinyl alcohol copolymer (Onyx, ev3, Irvine, CA, USA) is a relatively novel nonadhesive liquid embolic agent, which is most commonly used to treat intracranial arteriovenous malformations [9, 12]. The use of Onyx for type 1 endoleaks was first described in 2010 [12]. Currently, the published experience of endoleak embolization with Onyx is very limited. The largest series reported eight patients that were treated with Onyx embolization of type 1 endoleaks following EVAR and TEVAR [13]. The authors described a reperfusion of the endoleak in one case that occurred 2 days after the procedure; in a second case, they showed an occluded endoleak but a small trace of contrast between the aortic wall and the stent-graft. Other studies of a small series of patients [7, 14] reported 100% technical success, although there were early occlusions of renal artery chimney grafts in one patient, and another patient experienced late stent-graft migration resulting in fatal aneurysm rupture at 18 months post-embolization [14]. Chun [9] reported no recurrent endoleaks at up to 10 months follow-up and no major complications. Our series presents a longer follow-up period, 16.5 months (range 12–30 months) without any complication related to the procedure or to the embolic agent used and no EL recurrences; moreover, all type Ia endoleaks of the series followed EVAR. The procedure itself is not technically challenging for operators with sufficient training and expertise in transcatheter embolization procedures. The vessels embolized with Onyx are completely filled by the embolic agent, and they are less fragile because of the lower inflammatory reaction and the absence of polymerization heat when compared with NBCA-embolized ones [15]. To obtain a complete and safe embolization, the association with other embolizing agents, like glue, should be preferred when the sac is large. In our experience, considering also endovascular embolization of Type II endoleak [16], Onyx could be useful to fill the remaining gaps of the sac filled with other embolic agents and to create a proximal cap.
In our opinion, the advantage to use Onyx in combination with other embolic agents is twofold: to limit expenditure, and most important, to exploit its characteristics, in particular the creation of a proximal cap is safer on the basis of the possibility to control injection. To this last point, the injection may be performed slowly and stopped when the desired embolization was reached.
In conclusion, Onyx may be considered a useful embolic agent in the treatment of patients with type 1 endoleaks after EVAR that are not suitable for standard therapeutic options.
Our results, in accordance with a few other publications, are promising. More numerous series with longer follow-up need to be conducted.
References
Walker TG, Kalva SP, Yeddula K, Wicky S, Kundu S, Drescher P, d’Othee BJ, Rose SC, Cardella JF (2010) Clinical practice guidelines for endovascular abdominal aortic aneurysm repair: written by the Standards of Practice Committee for the Society of Interventional Radiology and endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. J Vasc Interv Radiol 21(11):1632–1655
Mehta M, Sternbach Y, Taggert JB, Kreienberg PB, Roddy SP, Paty PS, Ozsvath KJ, Darling RC (2010) Long-term outcomes of secondary procedures after endovascular aneurysm repair. J Vasc Surg 52:1442–1449
Baum R, Stavropoulos W, Fairman RM, Carpenter JP (2003) Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol 14:1111–1117
Georgakarakos E, Argyriou C, Schoretsanitis N, Ioannou CV, Kontopodis N, Morgan R, Tsetis D (2014) Geometrical factors influencing the hemodynamic behavior of the AAA stent grafts: essentials for the clinician. Cardiovasc Interv Radiol 37(6):1420–1429
Venermo MA, Arko FR 3rd, Salenius JP, Saarinen JP, Zvaigzne A, Zarins CK (2011) EVAR may reduce the risk of aneurysm rupture despite persisting type Ia endoleaks. J Endovasc Ther 18:676–682
Khaja MS, Park AW, Swee W, Evans AJ, Angle JF, Turba UC, Sabri SS, Matsumoto AH (2014) Treatment of type II endoleak using Onyx with long-term imaging follow-up. Cardiovasc Interv Radiol 37(3):613–622
Grisafi JL, Boiteau G, Detschelt E, Potts J, Kiproff P, Muluk SC (2010) Endoluminal treatment of type IA endoleak with Onyx. J Vasc Surg 52(5):1346–1349
Maldonado TS, Rosen RJ, Rockman CB, Adelman MA, Bajakian D, Jacobowitz GR, Riles TS, Lamparello PJ (2003) Initial successful management of type I endoleak after endovascular aortic aneurysm repair with n-butyl cyanoacrylate adhesive. J Vasc Surg 38(4):664–670
Chun JY, Morgan R (2013) Transcatheter embolisation of type 1 endoleaks after endovascular aortic aneurysm repair with Onyx: when no other treatment option is feasible. Eur J Vasc Endovasc Surg 45(2):141–144
Rand T, Uberoi R, Cil B, Munneke G, Tsetis D (2013) Quality improvement guidelines for imaging detection and treatment of endoleaks following endovascular aneurysm repair (EVAR). Cardiovasc Intervent Radiol 36:35–45
Cao P, De Rango P, Verzini F, Parlani G (2010) Endoleak after endovascular aortic repair: classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J Cardiovasc Surg 51:53–69
Choi SY, Won JY, do Lee Y, Choi D, Shim WH, Lee KH (2010) Percutaneous transabdominal approach for the treatment of endoleaks after endovascular repair of infrarenal abdominal aortic aneurysm. Korean J Radiol 11:107–114
Eberhardt KM, Sadeghi-Azandaryani M, Worlicek S, Koeppel T, Reiser MF, Treitl M (2014) Treatment of type I endoleaks using transcatheter embolization with onyx. J Endovasc Ther 21(1):162–171
Henrikson O, Roos H, Falkenberg M (2011) Ethylene vinyl alcohol copolymer (Onyx) to seal type 1 endoleak. A new technique. Vascular 19(2):77e81
Guimaraes M, Wooster M (2011) Onyx (ethylene-vinyl alcohol copolymer) in peripheral applications. Semin Interv Radiol 28:350–356
Ierardi AM, Micieli C, Angileri SA, Rivolta N, Piffaretti G, Tonolini M, Fontana F, Miele V, Brunese L, Carrafiello G (2017) Ethylene-vinyl alcohol copolymer as embolic agent for treatment of type II endoleak: our experience. Radiol Med 122(2):154–159
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Rights and permissions
About this article
Cite this article
Ierardi, A.M., Franchin, M., Fontana, F. et al. The role of ethylene–vinyl alcohol copolymer in association with other embolic agents for the percutaneous and endovascular treatment of type Ia endoleak. Radiol med 123, 638–642 (2018). https://doi.org/10.1007/s11547-018-0885-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0885-4