Abstract
Purpose
The purpose of our study is to report our experience with the use of an ethylene vinyl alcohol copolymer (Onyx) in an off-label fashion for the treatment of type II endoleak after endovascular repair of the thoracic (TEVAR) and abdominal (EVAR) aorta.
Methods
A retrospective review of patients with type I and/or II endoleak treated with Onyx was performed. Data regarding the technical, clinical, and imaging outcomes were collected. Technical success was defined as decreased or eliminated endoleak on the first imaging follow-up. Clinical success was defined as unchanged or decreased aneurysm sac size on subsequent follow-up.
Results
Eighteen patients (15 male, 3 female) with a mean age of 79 years (range 69–92) met inclusion criteria (16 abdominal aortic aneurysm, 2 thoracic aortic aneurysm). Sixteen patients had type II endoleak, and 2 had complex type II endoleak with a type I component. The interval between endograft placement and treatment was a mean of 30 months. Direct sac treatment approach was used in 13 patients; transarterial approach was used in 3 patients. Seven patients required the use of coils, N-butyl cyanoacrylate glue, or Amplatzer vascular plugs. The average volume of Onyx used per treatment was 5.6 mL (range 2.5–13). Duration of imaging follow-up was 0.75–72.5 months (mean 32.8). Sixteen of 18 (88.9 %) patients had initial technical and clinical success. Two of 18 patients (11.1 %) were initial technical failures, and 1 remained a failure despite a second treatment and attempted surgical ligation. Eight of 18 (44.4 %) of patients eventually required a second intervention, 5 (27.8 %) of them due to delayed clinical failure. Complications included 1 psoas hematoma, 1 transient L2 nerve paresis, and 1 intraperitoneal Onyx leak; all of these were without clinical sequelae.
Conclusion
Onyx with or without coil/glue/Amplatzer plug embolization is safe and useful in the treatment of type II endoleak after TEVAR and EVAR. However, long-term clinical and imaging follow-up is needed for early detection and management of recurrence of the primary endoleak or the development of new, secondary endoleaks or enlargement of the aneurysm sac.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of stent-grafts for the treatment of abdominal aortic aneurysms (AAAs), thoracic aortic aneurysm (TAAs) with and without associated dissections, penetrating aortic ulcers, symptomatic intramural hematomas, and posttraumatic thoracic aortic pseudoaneurysms continues to grow [1, 2]. Endoleak, which is the persistent perfusion within the aneurysmal sac after endovascular repair, occurs in 10–20 % of cases after endograft repair and remains a significant issue in a subset of patients [3].
A variety of techniques, methods and agents have been used either in isolation or in combination for the treatment of type I and II endoleaks [4–10]. We report our experience with the use of an ethylene vinyl alcohol copolymer (Onyx; ev3 Neurovascular, Irvine, CA) in an off-label fashion for the treatment of type II endoleaks after endovascular repair of the thoracic (TEVAR) or abdominal (EVAR) aorta.
Materials and Methods
Institutional Review Board approval was obtained for this single-institution retrospective analysis. Patients with type II endoleak after endovascular repair of the thoracic or abdominal aorta who were treated with Onyx embolization between October 2005 and September 2010 were reviewed. Patients who were treated with coils, vascular plugs, or other liquid embolic agents in addition to Onyx were also included in this evaluation. Patients with primarily type I and type III endoleak at initial presentation were excluded (N = 3). Patient demographics, type(s) of endoleak(s), the indication for treatment, duration from endograft placement to Onyx embolization, access method for Onyx delivery, volume of Onyx used, complications, and duration of follow-up were assessed based on review of procedural and imaging reports, cross-sectional imaging studies themselves, and medical records for each patient.
Data regarding technical, clinical, and imaging outcomes were also collected (elimination or decrease in the size of the endoleak, decrease or stability in the size of the aneurysm sac, persistence of the endoleak). Technical success was defined as a decrease in the size or elimination of the endoleak on first imaging follow-up. Clinical success was defined as an unchanged or decreased aneurysm sac size on imaging follow-up. Patients with inadequate imaging follow-up were excluded from the evaluation (N = 1). The standard imaging protocol at our institution is postendograft computed tomography angiogram (CTA) at 1, 3, 6, and 12 months. CTA is performed yearly after the first year if the endoleak is smaller and the aneurysm sac is stable or smaller in size. If a new or enlargement of the previous type II endoleak is detected, a subsequent CTA is performed within 30 days. If the endoleak or aneurysm sac is larger, the patient is treated. If the endoleak is unchanged or resolved, the patient is followed-up using the standard protocol. If the renal function of the patient is compromised (estimated glomerular filtration rate [eGFR] < 60), unenhanced CT is performed to evaluate the aneurysm sac size or any other complications.
Onyx is a liquid, cohesive, embolic agent with Food and Drug Administration approval for presurgical embolization of brain arteriovenous malformations (AVMs). It is an ethylene–vinyl alcohol (EVOH) copolymer dissolved in dimethyl sulfoxide (DMSO) [11]. Onyx is suspended in tantalum powder to provide radiopacity, thus allowing for its visualization during fluoroscopy. However, the tantalum power also results in streak artifacts, which may limit evaluation on cross-sectional imaging. Onyx is available in three formulations: 1.5-mL vials of Onyx-18 (6 % EVOH), Onyx-34 (8 % EVOH), and Onyx HD500 (20 % EVOH). Onyx-34 is more viscous than Onyx-18. Onyx HD500 was not used in this study. A vial of Onyx-18 costs approximately $2,000; however, the cost is dependent on volume discounts negotiated with the distributor. Onyx must be administered by way of DMSO-compatible microcatheters, such as the Echelon 14 or 18 or the Rebar (ev3 Neurovascular, Irvine, CA) because the integrity of the microcatheter might be affected by the DMSO [11]. It is also critical to know the dead space of the microcatheter so that the exact volume of the Onyx administered can be closely monitored.
Embolization Procedures
Access to the endoleaks was obtained by direct aneurysm sac puncture or transarterially by way of percutaneous transfemoral artery access similar to previous reports [4, 5]. Pulsed fluoroscopy at a rate of 1–7.5 pulses/s were employed depending on the body habitus of the patient. A variety of direct aneurysm sac–access techniques were employed, including the use of an Accustick system (Boston Scientific, Natick, MA), a 5F micropuncture kit (Cook, Bloomington, IN), or a 20-cm 4F sheath needle (Boston Scientific, Natick, MA). Once direct access into a portion of the nidus was confirmed by obtaining pulsatile blood flow, angiography of the aneurysm sac and endoleak was performed to evaluate the size and complexity of the nidus, the inflow and outflow vessels, the subjective dynamics of the endoleak, and in some cases, the intrasac pressures. Transarterial selective catheterization of the aneurysm sac was also performed by way of transfemoral arterial access in some patients using a coaxial microcatheter system by way of a 5F catheter placed into the parent branch artery, which included the inferior mesenteric, iliolumbar, lumbar, and intercostal arteries. Again, angiography of the aneurysm sac and endoleak was performed to evaluate the size and complexity of the nidus, the inflow, and outflow vessels, and the subjective dynamics of the endoleak. Intrasac pressures were not measured when the transarterial method for aneurysm sac catheterization was performed. Once the characteristics of the nidus of the endoleak were determined, Onyx was administered under direct fluoroscopic monitoring using the road-mapping technique. Injection of the Onyx was performed as previously described for brain AVMs [12].
Statistical Methods
All statistical analysis was performed using Spotfire S+software Version 8.2 (TIBCO, Palo Alto, CA). Percentage of patients without recurrence and secondary endoleak were assessed using Kaplan–Meier analysis and Fisher exact test.
Results
Fifteen men and 3 women with a mean age of 79 years (range 69–92) at presentation comprised the study population (see Table 1). Sixteen patients were initially treated with endovascular stent-grafts for AAAs and 2 for TAAs. Five of the 18 patients had their endografts placed at another institution. The interval between endograft placement and initial endoleak treatment was 2.3–70.5 months (mean 30). The indication for endoleak treatment included persistence of a type II endoleak after 6 months of imaging follow-up (N = 3), enlarging aneurysm sac size (N = 13), and referring clinician preference (N = 2). In one of the 2 cases in which treatment was performed due to clinician preference, the referring physician and patient requested treatment due a very large aneurysm sac at baseline and patient anxiety. In the second case for which treatment was performed due to clinician preference, the endoleak was found incidentally while imaging the patient for evaluation of claudication, where it was found that one of the iliac limbs of the endograft was stenosed. The referring clinician requested that the endoleak be treated while the stenotic limb was being treated.
Sixteen of the 18 (88.9 %) patients had type II endoleaks. Two (11.1 %) patients had complex, primarily type II endoleaks with a type I component. Both of these patients with complex type I/II endoleaks were originally treated for AAA. Fourteen of the 16 patients with type II endoleak initially presented with AAA, and two were originally treated for TAA, 1 of whom who had an associated aortic dissection (Tables 1, 2).
A direct aneurysm sac access approach was used in 13 patients, whereas a transarterial access was employed in 5 patients. Direct aneurysm sac access was translumbar in all but 2 patients, in whom a transabdominal approach was employed. One patient who underwent direct aneurysm sac puncture was treated using CT guidance (by way of a transabdominal approach). The remaining 17 patients were treated using fluoroscopic guidance. Nine patients required the use of Onyx and other embolic materials, such as coils (N = 7) (Cook), N-butyl cyanoacrylate (NBCA) glue (N = 1) (Cordis Neurovascular, Warrenton, NJ), and Amplatzer vascular plug (N = 1; AGA Medical, Golden Valley, MN). In these patients, coils and vascular plugs served as barriers for nontarget embolization. Coils also aided in minimizing the total volume of Onyx necessary by taking up volume of the endoleak nidus. The average volume of Onyx used per initial treatment was 5.6 mL of (range 2.5–13).
Seventeen patients underwent initial imaging follow-up with CTA. One patient had initial imaging follow-up with unenhanced CT. All follow-up CT or CTA imaging was interpreted by a fellowship-trained cardiovascular radiologist. One patient underwent a subsequent follow-up duplex ultrasound, which was interpreted by a vascular surgeon; however, it was also reviewed by a cardiovascular radiologist. Follow-up imaging was performed at another institution in 1 of 18 patients. This patient was referred back to our institution for retreatment due to persistence of the endoleak. The duration of imaging follow-up ranged from 0.75 to 72.5 months (mean 32.8).
Initial technical and clinical successes were achieved in two of two (100 %) patients with complex type II endoleak having a type I component (see Table 2). One of these patients developed a new small type II endoleak at 24 months; however, there was no change in the aneurysm sac size, and no reintervention was performed (patient no. 1). The second patient did not require further intervention during the study period because the endoleak was decreased in size, and the aneurysm sac size was stable (patient no. 6).
Fourteen of 16 (87.5 %) patients with straightforward type II endoleaks had initial technical and clinical success (see Table 2). One technical failure occurred in a 92-year-old man who presented for treatment of type II endoleak after endograft placement for AAA. He was treated by way of direct aneurysm sac puncture. Six-week follow-up unenhanced CT (due to renal insufficiency) showed an enlarging aneurysm sac. The family of the patient chose not to pursue further treatment due to the interval onset of dementia in the patient (patient no. 4). The second technical-failure patient had persistent type II endoleak on initial follow-up imaging. The patient was treated with Onyx a second time 15 weeks later. Unenhanced CT scan (due to renal insufficiency) was performed 3 months later, which showed no change in aneurysm sac size; however, the size of the endoleak could not be determined. Unenhanced CT scan an additional 15 months later showed interval aneurysm sac size enlargement. The patient was referred for an attempt at laparoscopic ligation of the lumbar and middle sacral arteries, which subsequently also failed. This 86-year-old patient with an eGFR < 30 is still alive at 25 months of follow-up; however, this case is considered to be a delayed clinical failure (patient no. 5).
One EVAR patient with persistent type II endoleak underwent initially successful embolization with Onyx, which resulted in a significant decrease in the size of the type II endoleak and a stable aneurysm sac size for 30 months. However, subsequent follow-up imaging showed enlargement of the aneurysm sac, and a second treatment session with Onyx was successfully performed (patient no. 6). The patient developed a type III endoleak 12 months later due to endograft migration secondary to aneurysm remodeling. This patient underwent subsequent successful endovascular repair by placement of a new aorto-uni-iliac endograft and a femoral–femoral artery bypass.
Three other patients required reinterventions for the primary type II endoleak, two of whom were treated with additional Onyx (patients no. 7 and 8) and one with glue and coils (patient no. 9). One patient developed a new type II endoleak at a different site, which was successfully treated with coils (patient no. 10). One of the patients who required retreatment of a primary type II endoleak with Onyx developed a new, secondary type II endoleak 13 months later, which was successfully (technically and clinically) treated with a combination of Onyx and coils (patient no. 11).
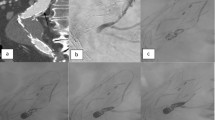
Three patients (patients no. 6–8) who required reintervention for their primary endoleak were approached from a different access during their subsequent treatment session. Therefore, these three patients underwent Onyx embolization from both a transarterial and direct sac–access approach. An example of a pretreatment CTA, an angiogram during an embolization procedure, and a postembolization follow-up CTA is provided (see Figs. 1A through F).
A 71 year-old man with persistent type II endoleak for 16 months after EVAR. A Axial CTA at L4 level shows aortoiliac stent-graft with large type II endoleak (arrow). B Curved multiplanar reconstruction (MPR) of CTA shows type II endoleak involving an L4 lumbar artery (arrow), inferior mesenteric artery (IMA) (arrowhead), and nidus (*). C Cone beam noncontrast axial CT image immediately after treatment shows Onyx embolization within endoleak (arrows) and significant streak artifact. D Oblique aneurysm sac angiogram shows stent-graft with complex type II endoleak involving L4 lumbar artery (white arrow), IMA (black arrow), nidus (*), and multiple feeder vessels (arrowheads). E Right anterior oblique view postembolization image shows Onyx filling endoleak nidus (*), L4 lumbar artery (arrowhead), and IMA as well as coils within the IMA (arrow). F Sagittal MPR of CTA 45.5 months after coil and Onyx embolization shows coils within the IMA (arrowhead), Onyx at previous site of endoleak (white arrows), including a lumbar artery, and resulting streak artifact
In summary, 16 of 18 (88.9 %) treated patients were initial technical successes (see Table 2). Two of 18 patients (11.1 %) were initial technical failures (Table 3), 1 of whom remained a failure despite a second treatment session and attempted surgical ligation of the lumbar collaterals. A total of 44.4 % (8 of 18) of the patients eventually required a second intervention. Six of the reinterventions were required for persistence of the primary endoleak. One patient, who required reintervention on the primary endoleak with Onyx, developed a new type III endoleak 12 months later, requiring placement of a new endovascular graft (patient no. 6). Four patients were retreated with Onyx, with the fifth patient being treated with NBCA glue and coils (patients no. 5, 7, 8, 9, and 11, respectively). Reintervention for a new, secondary endoleak was performed in a total of 3 patients (16.7 %), 1 with an additional endograft as mentioned previously (patient no. 6), 1 with coils (patient no. 10), and 1 with Onyx and coils (patient no. 11). Five of 18 (27.8 %) patients were delayed clinical failures (apparent improvement or stabilization of the endoleak and/or aneurysm sac size with subsequent enlargement of the endoleak and/or aneurysm sac size) requiring further treatment.
Four patients were anticoagulated with warfarin and an additional 10 with aspirin (ASA) or clopidogrel. As listed in Tables 4 and 5, there was no significant difference in the frequency of recurrence or development of secondary endoleaks between the two groups (Figs. 2, 3). Eight of the 18 (44.4 %) patients with either recurrent endoleak or development of new endoleak were anticoagulated with warfarin, ASA, or clopidogrel. Five of 6 (83.3 %) patients requiring a second treatment of the initial endoleak and 75 % (3 of 4) of patients who developed a secondary endoleak were taking one of these medications. Conversely, 7 of 18 (38.9 %) patients were taking one of the above-mentioned medications; however, they did not further endoleak or recurrence.
Complications that occurred included one psoas hematoma (patient no. 7), 1 transient L2 nerve paresis (patient no. 18) (which was likely due to the local anesthetic due to the time course and rapid resolution of symptoms), and 1 intraperitoneal Onyx leak (patient no. 8); all were without clinical sequelae. The three complications occurred in patients treated with direct aneurysm sac puncture. DMSO-related odor emanating from the patients was reported and lasted up to several days; however, this caused no apparent clinically significant side effects.
Discussion
Embolization of endoleaks has been performed with many different embolic agents. Among these agents, NBCA glue, coils, thrombin, and Onyx are the agents most commonly used [4–10]. This current retrospective study shows 88.9 % initial clinical and technical success using Onyx embolization for treatment of type II endoleak. During a mean imaging follow-up of >2.5 years (maximum of > 6), 44.4 % of the patients required reintervention, 27.8 % (5 of 18) due to a delayed clinical failure. The reinterventions were either surgical or endovascular for persistence of the primary endoleak or development of a new, secondary endoleak.
Nearly all of the patients who developed a new endoleak (75 %) or had recurrence of their primary leak (83.3 %) were on some form of anticoagulation or antiplatelet therapy. However, the small sample size does not allow for determining if this difference is significant. Given the limited sample size and lack of significance, it should be noted that a recent report found an increased risk of endoleak development in patients being anticoagulated with warfarin [13].
The long-term follow of our patients, i.e., ≤72.5 months (mean 32.8) provides significance to this report because much of the previous literature regarding use of Onyx for endoleak treatment has been presented in case reports, very small case series, or with limited and/or inconsistent follow-up [14–18]. Massis et al. reported their experience in treating 101 patients with Onyx. The investigators described an overall 73.6 % clinical success at a median follow-up of 15 weeks and a residual endoleak rate of 34 %. However, no details were provided regarding reinterventions [19]. In comparison, our overall clinical success rate of 66.7 % accounts for any delayed clinical failures that occurred during a mean follow-up period of 32.8 months. In addition, Abularrage et al. described their results with secondary interventions for persistent type II endoleak. However, the number of patients treated with Onyx was 17, and their entire patient group had a median follow-up of 13.7 months compared with our mean of 32.8 months [20].
A number of other series have been reported mixed results with the treatment of type II endoleak using a variety of agents and approaches. One report described the treatment of 14 patients with type II endoleak using a variety of embolic agents with a mean follow-up of 1.9 years (13 patients had some type of follow-up) [21]. Of these 14 patients, 4 (29 %) required reintervention. Gorich et al. treated 11 endoleaks with coils successfully. No repeat interventions were required during a mean follow-up of 6.8 months [22]. Mansueto et al. [9] reported embolization of 12 patients with type II endoleak using thrombin administered by way of a transcaval approach with an 80 % success rate at a follow-up of 1 year. Baum and Stavropoulos reported their experience with the use of coils and NBCA for the treatment of type II endoleak and showed a significantly increased failure rate with the transarterial (80 %) versus translumbar (8 %) approach with a mean follow-up of 13.2, and 8.5 months, respectively [4, 23]. Two of 9 (22 %) patients treated with NBCA and coils required reintervention during a mean follow-up of 83 days [24]. As a result of the relatively high rate of persistence of the primary endoleak or the development of new endoleaks during follow-up, there is a need for life-long follow-up imaging in this patient population [25].
The treatment of type I and II endoleak has received much discussion and debate in the literature with many investigators describing their experience with various embolic agents [5–9, 14, 15, 17, 18, 23, 24, 26]. Type I endoleaks have historically been corrected at the time of stent-graft deployment or have required open surgical repair, although there are case reports regarding other treatment methods [18, 27]. Typically patients who present with type II endoleak have close clinical follow-up and CT imaging with attention to aneurysm sac size as well as the characteristics of the persistent endoleak. If changes in aneurysm sac size are seen, there is a general agreement and understanding that an intervention may be warranted [2, 5, 27, 28]. In addition, if the type II endoleak shows a large nidus, > 3 feeding/draining arteries, diameter of the feeding collateral artery > 4 mm, and high flow velocities within the aneurysm sac on duplex ultrasound imaging, some reports have suggested a greater likelihood for aneurysm sac enlargement or persistence or enlargement of the endoleak [29–34].
Treatment of type II endoleak has been given much attention due to the complexity of many type II endoleaks and their similarities to AVMs. Many of the embolic agents, including Onyx, have previously been investigated for their use in nidus reduction before neurosurgery or for palliative embolization of intracranial AVMs. In those studies, Onyx has shown that it can be used for the treatment of intracranial AVMs with more control than glue, thus leading to more precise nidus penetration and obliteration [35]. Like AVMs, complex type II endoleaks have feeding and draining vessels as well as a nidus [5, 14, 23]. Successful treatment of the nidus and the inflow and outflow vessels is necessary for the optimum treatment and complete elimination of the endoleak. Onyx seems to be suitable for effective management of these endoleaks because it has more predictable behavior and excellent visibility during fluoroscopy. The nonadhesive character of Onyx allows for a slower, more deliberate delivery of the agent, with less concern for injury to the vessel during catheter removal, distal migration of the Onyx, and catheter adherence to the vessel wall. The slow administration of Onyx and propagation of the polymeric cast allows for a better chance to embolize the endoleak nidus and the feeding/draining vessels. In addition, previous laboratory investigation has shown that exposure of a variety of endografts to Onyx and DMSO was not associated with structural compromise of the endograft materials [36]. Finally, the soft texture of Onyx should not compromise any future surgical intervention if it becomes necessary [37].
Onyx, like many other embolic agents, also has its disadvantages. The DMSO solvent may cause vasospasm or pain in a conscious patient if the DMSO is administered too rapidly. The formation of a polymeric cast “plug” and a continuous column of material are important to adequately treat the endoleak nidus and the feeding/draining vessels. Onyx also requires the use of DMSO-compatible microcatheters. In addition, a 1.5-cc vial of Onyx is quite expensive, nearly $2,000. Because of these disadvantages, the use of Onyx has some drawbacks and a significant learning curve and requires skilled operators. As the DMSO diffuses out of solution from the Onyx, patients may experience a transient foul odor from their breath and/or sweat; however, this has not been associated with any known clinical sequelae [18]. None of our patients experienced a clinically significant side effect from the odor. Finally, the tantalum powder necessary for visualization of the Onyx during fluoroscopy causes significant streak artifacts on follow-up CT imaging, which may limit evaluation for persistence of subtle endoleaks. In these cases, evaluation for a change in aneurysm sac size plays an increased role.
Although many of these patients are elderly and at low risk for developing radiation-induced malignancy, radiation hygiene should be optimized. Because of the slow and controlled delivery of Onyx and to minimize radiation exposure to the patient, the embolization procedure should be monitored using pulsed fluoroscopy, tight collimation, and optimal positioning of the image intensifier. With the availability of improved fluoroscopic technology, these processes may be further improved.
Our study has the common limitations of retrospective research, including the lack of randomization and variations in technique and follow-up due to having several primary operators. In addition, the total number of patients included in this series is small, although it is relatively large compared with other published reports. In addition, our study does not include a direct comparison to other endoleak embolization techniques. Although our study included a mean follow-up of >2.5 years, longer follow-up is necessary to further evaluate the durability of the embolization procedure and the necessity for reintervention.
Conclusion
Onyx with or without coil/glue/vascular plug embolization is safe and useful in the treatment of type II endoleaks after TEVAR and EVAR with 66.7 % of treated patients showing an on-going benefit of endoleak control ≥72.5 months (mean = 32.8) after treatment. However, even with successful immediate control or decrease in the primary endoleak, long-term clinical and imaging follow-up is needed for early detection and management of recurrence of the primary endoleak, development of new secondary endoleak, or enlargement of the aneurysm sac.
References
Coady MA, Ikonomidis JS, Cheung AT, Matsumoto AH, Dake MD, Chaikof EL, et al (2010) AHA council on Cardiovascular Surgery and Anesthesia and Council on Peripheral Vascular Disease Surgical Management of descending thoracic aortic disease: open and endovascular approaches. A scientific statement from the American Heart Association. Circulation 121(25):2780–2804
Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA et al (2009) The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 50(4):S2–S49
Jones JE, Atkins MD, Brewster DC, Chung TK, Kwolek CJ, LaMuraglia GM et al (2007) Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg 46(1):1–8
Baum RA, Carpenter JP, Golden MA, Velazques OC, Clark TW, Stavropoulos SW et al (2002) Treatment of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms: comparison of transarterial and translumbar techniques. J Vasc Surg 35(1):23–29
Golzarian J, Maes EB, Sun S (2005) Endoleak: treatment options. Tech Vasc Interv Radiol 8(1):41–49
Maldonado TS, Rosen RJ, Rockman CB, Adelman MA, Bajakian D, Jacobowitz GR et al (2003) Initial successful management of type I endoleak after endovascular aortic aneurysm repair with n-butyl cyanoacrylate adhesive. J Vasc Surg 38(4):664–670
Peynircioğlu B, Türkbey B, Ozkan M, Cil BE (2008) Use of glue and microcoils for transarterial catheter embolization of a type 1 endoleak. Diagn Interv Radiol 14(2):111–115
Martin ML, Dolmatch BL, Fry PD, Machan LS (2001) Treatment of type II endoleaks with Onyx. J Vasc Interv Radiol 12(5):629–632
Mansueto G, Cenzi D, Scuro A, Gottin L, Griso A, Gumbs AA et al (2007) Treatment of type II endoleak with a transcatheter transcaval approach: results at 1-year follow-up. J Vasc Surg 45(6):1120–1127
White S, Stavropoulos S (2009) Management of endoleaks following endovascular aneurysm repair. Semin Intervent Radiol 26(1):33–38
Onyx liquid embolic system [package insert] (2007) Neurovascular, eV3. Irvine, CA
Weber W, Kis B, Siekmann R, Kuehne D (2007) Endovascular treatment of intracranial arteriovenous malformations with onyx: technical aspects. AJNR Am J Neuroradiol 28(2):371–377
Bobadilla JL, Hoch JR, Leverson GE, Tefera G (2010) The effect of warfarin therapy on endoleak development after endovascular aneurysm repair (EVAR) of the abdominal aorta. J Vasc Surg 52(2):267–271
Fanelli F (2013) ONYX: A liquid embolic material for the treatment of type II endoleaks and arterio-venous fistulas and malformations, p 1–4. Available from: http://www.aimsymposium.org/pdf/vei/2046.pdf. Accessed 28 March 2013
Massis K, Zwiebel B, Carson WG (2010) Abstract no. 289: transarterial embolization of type II endoleaks using ethylene-vinyl-alcohol copolymer. J Vasc Interv Radiol 21:S109–S110
Nevala T, Biancari F, Manninen H, Aho PS, Matsi P, Makinen K et al (2010) Type II endoleak after endovascular repair of abdominal aortic aneurysm: effectiveness of embolization. Cardiovasc Interv Radiol 33(2):278–284
Ling AJ, Pathak R, Garbowski M, Nadkarni S (2007) Treatment of a large type II endoleak via extraperitoneal dissection and embolization of a collateral vessel using ethylene vinyl alcohol copolymer (Onyx). J Vasc Interv Radiol 18(5):659–662
Grisafi JL, Boiteau G, Detschelt E, Potts J, Kiproff P, Muluk SC (2010) Endoluminal treatment of type IA endoleak with Onyx. J Vasc Surg 52(5):1346–1349
Massis K, Carson WG, Rozas A, Patel V, Zwiebel B (2012) Treatment of type II endoleaks with ethylene-vinyl-alcohol copolymer (Onyx). Vasc Endovasc Surg 46(3):251–257
Abularrage CJ, Patel VI, Conrad MF, Schneider EB, Cambria RP, Kwolek CJ (2012) Improved results using Onyx glue for the treatment of persistent type 2 endoleak after endovascular aneurysm repair. J Vasc Surg 56(3):630–636
Nevala T (2010) Endovascular treatment of an abdominal aortic aneurysm. Dissertation. Acta Universitas, Oulu
Görich J, Rilinger N, Sokiranski R, Kramer SC, Erms C, Schutz A et al (2000) Treatment of leaks after endovascular repair of aortic aneurysms. Radiology 215(2):414–420
Baum RA, Stavropoulos SW, Fairman RM, Carpenter JP (2003) Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol 14(9):1111–1117
Stavropoulos SW, Kim H, Clark TWI, Fairman RM, Velazquez O, Carpenter JP (2005) Embolization of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms with use of cyanoacrylate with or without coils. J Vasc Interv Radiol 16(6):857–861
Stavropoulos SW, Charagundla SR (2007) Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology 243(3):641–655
Stavropoulos SW, Park J, Fairman R, Carpenter J (2009) Type 2 endoleak embolization comparison: translumbar embolization versus modified transarterial embolization. J Vasc Interv Radiol 20(10):1299–1302
Rosen RJ, Green RM (2008) Endoleak management following endovascular aneurysm repair. J Vasc Interv Radiol 19(6):S37–S43
Jonker FHW, Aruny J, Muhs BE (2009) Management of type II endoleaks: preoperative versus postoperative versus expectant management. Semin Vasc Surg 22(3):165–171
Keedy AW, Yeh BM, Kohr JR, Hiramoto JS, Schneider DB, Breiman RS (2011) Evaluation of potential outcome predictors in type II endoleak: a retrospective study with CT angiography feature analysis. AJR Am J Roentgenol 197(1):234–240
Noel Parent F, Meier GH, Godziachvili V, LeSar CJ, Parker FM, Carter KA et al (2002) The incidence and natural history of type I and II endoleak: a 5-year follow-up assessment with 1 color duplex ultrasound scan. J Vasc Surg 35(3):474–481
Fairman RM, Carpenter JP, Baum RA, Larson RA, Golden MA, Barker CF et al (2002) Potential impact of therapeutic warfarin treatment on type II endoleaks and sac shrinkage rates on midterm follow-up examination. J Vasc Surg 35(4):679–685
Timaran CH, Ohki T, Rhee SJ, Veith FJ, Gargiuo NJ 3rd, Toriumi H et al (2004) Predicting aneurysm enlargement in patients with persistent type II endoleaks. J Vasc Surg 39(6):1157–1162
Arko FR, Filis KA, Siedel SA, Johnson BL, Drake AR, Fogarty TJ et al (2003) Intrasac flow velocities predict sealing of type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Surg 37(1):8–15
Bargellini I, Napoli V, Petruzzi P, Cioni R, Vignali C, Sardella SG et al (2005) Type II lumbar endoleaks: hemodynamic differentiation by contrast-enhanced ultrasound scanning and influence on aneurysm enlargement after endovascular aneurysm repair. J Vasc Surg 41(1):10–18
Panagiotopoulos V, Gizewski E, Asgari S, Regel J, Forsting M, Wanke I (2009) Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol 30(1):99–106
Stone JR, Evans AJ, Angle JF, Arslan B, Turba UC, Matsumoto AH (2009) In vitro assessment of aortic stent-graft integrity following exposure to onyx liquid embolic agent. J Vasc Interv Radiol 20(1):107–112
Akin ED, Perkins E, Ross IB (2003) Surgical handling characteristics of an ethylene vinyl alcohol copolymer compared with N-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurg 98(2):366–370
Conflict of interest
Avery J. Evans is a consultant and proctor for eV3 Neurovascular. J. Fritz Angle is a consultant for Terumo Medical and received a grant from Siemens Medical. Alan H. Matsumoto sits on the Data Safety Monitoring Board for the Trivascular, Bolton Medical, Boston Scientific, and Medicines companies; has received institutional grants from W. L. Gore, Medtronic, Cook Medical, and InSightec; holds personal stock or stock options of Crux Medical; and has received other travel/accommodations/meeting expenses from Siemens Medical. None of these are relevant to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khaja, M.S., Park, A.W., Swee, W. et al. Treatment of Type II Endoleak Using Onyx With Long-Term Imaging Follow-Up. Cardiovasc Intervent Radiol 37, 613–622 (2014). https://doi.org/10.1007/s00270-013-0706-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-013-0706-z