Abstract
Endovascular aneurysm repair (EVAR) is considered to be the treatment of choice for abdominal aortic aneurysms (AAA). Despite the initial technical success, EVAR is amenable to early and late complications, among which the migration of the endograft (EG) with subsequent proximal endoleak (Type Ia) leads to repressurization of the AAA sac, exposure to excessive wall stress, and, hence, to potential rupture. This article discusses the influence that certain geometrical factors, such as neck angulation, iliac bifurcation, EG curvature, neck-to-iliac diameter, and length ratios, as well as iliac limbs configuration can exert on the hemodynamic behavior of the EGs. The information provided could help both clinicians and EG manufacturers towards further development and improvement of EG designs and better operational planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endovascular aneurysm repair (EVAR) is considered to be the preferred treatment modality for abdominal aortic aneurysms (AAA) for over a decade. EVAR aims at the positioning of an endovascular graft (EVG) within the AAA sac using an over-the-wire technique either via surgical exposure of both common femoral arteries or totally percutaneously. Reduced perioperative morbidity and mortality comprise the major advantage of this minimally invasive technique compared with conventional open repair [1, 2]. However, despite the initial technical success and early discharge of the patient, this technique is amenable to early and late complications, such as EVG migration, endoleaks, and material failure, which may lead to reexposure of the AAA wall to pulsatile blood pressure and excessive wall stress and, thus, to potential rupture.
This short review discusses the geometrical factors that affect the hemodynamic behavior of the EVGs, providing both clinicians and EVG manufacturers with useful information for better planning of endovascular techniques and developing of EVG designs. In order to facilitate the understanding of the importance of geometric parameters, experimental data from our research group are displayed along with their potential clinical applications.
Geometrical Factors Affecting EVG Migration
According to the EUROSTAR study, 3.5 % of patients undergoing EVAR suffer from graft migration comprising a ≥5-mm movement of the stent-graft from its initial deployment site [3, 4]. Displacement of the EVG can cause loss of proper sealing at its proximal or distal landing zone (i.e., the aortic neck and the iliac sites, respectively), leading to Type Ia and Ib endoleaks, respectively and restoring the systemic pressure loading on the AAA sac, thus regenerating the risk of AAA rupture despite the preceded endovascular therapy. Many researchers have investigated the major determinants of EVG migration, depicting an association between the aortic-EVG geometric features and the displacement forces acting on the latter.
Modern computational analytical/numerical methods can estimate the displacement forces, the magnitude of which is strongly influenced by the diameter and angulation of the EVG neck or the angulation of iliac bifurcation [5–10]. The computational sequence requires fundamentally three distinct work steps: (i) geometry reconstruction of the study model from medical images, (ii) biomechanical simulation (Finite-Element or Fluid–Structure Interaction computation), and (iii) interpretation of biomechanical properties [8]. Molony et al. [11] used fluid–structure interaction (FSI) simulation for a group of patient-specific 3D reconstructed EVGs to show that the antero-posterior neck angulation and the large inlet-to-outlet area ratios were the greatest determinants of the magnitude of drag forces. Furthermore, the curvature of the iliac limbs can also create additional sideways forces that may predispose to displacement of the iliac limbs and peripheral endoleaks (Type Ib) [8]. Interestingly, the greatest part of the displacement forces seems to act on the EVG bifurcation site compared to the proximal (neck) and distal (iliac) counterparts [10].
It should be delineated that endovascular treatment of AAA is not an instant fixation of an EVG in the aortic lumen, but rather an ongoing process of conformational changes in the aortic endograft during the post-EVAR shrinkage process of the sac, changing its geometry and, thus, putting increased axial strain and altering the distribution stress patterns on the different components of an EVG [12–15]. Therefore, the importance of studying the aortic and EVGs geometry is not limited solely to preoperative proper size planning of the endovascular device but also may have predictive role on the hemodynamic behavior and the resultant adverse effects related to the EVG; furthermore, the information drawn from such studies may effectively modify the design of newer devices to improve their accommodation on challenging AAA anatomies, thus improving their hemodynamic performance. The significance of each geometric parameter is summarized in Table 1.
Diameter of the Neck
The significant influence of the inlet-to-outlet (i.e., neck-to-iliac limbs, d1/d2) diameter ratio has been recently studied by our study group and is depicted in Fig. 1. We estimated the maximum displacement forces over a cardiac cycle on two reconstructed EG models in the standard (BifG, Fig. 1A) or the cross-limbs (BalG, Fig. 1B) fashion using FSI with the validated ANSYS software (ANSYS version 12.1; Ansys Inc.). The calculations took place for two different inlet diameters, i.e., 36 and 24 mm, corresponding to the maximum and minimum inlet diameters of the commercially available aortic endografts respectively, with d1/d2 ranging from 1.5 to 3.0. The increase in d1/d2 caused a constant increase of the maximum total displacement force ranging from 2.6 to 14 and 1.2 to 7.1 N for d1 of 36 and 24 mm, respectively (Fig. 2A, B).
Reconstructed models used to computational fluid dynamics. A Customary bifurcated model (BifG). B, C Cross-limbs model (BalG). D Aortouniiliac model (UniG). The a 1, β, d1, and d2 in B represent the lateral neck angulation, the angle of endograft limb bifurcation, and the inlet and outlet diameter, respectively. The main body length up to the flow divider and the iliac limb length are depicted as L1 and L2, respectively (C)
Increase in inlet-to-outlet diameter ratio of an EVG is associated with an increase of the magnitude of total displacement forces (A, B). Ratio >2 predisposes to higher mechanical loading at the bifurcation site (C), whereas high or low main body-to-iliac length ratios enhance the instability of iliac limbs, creating greater forces exerted at this segment (D)
Clinical Relevance
The aforementioned findings come in accordance with previous studies, underscoring the role of inlet diameter as a major determinant of the hemodynamic behavior of an EVG, because their effect is coupled with an exponential raise of the displacement forces acting on the endograft. Compared with other geometrical parameters, such as the AAA neck angle, the endograft curvature, or angulation of the limb bifurcation, the neck diameter causes the most profound effect on the magnitude of displacement forces [12, 16]. Interestingly, these seem to confirm clinical observations regarding AVG migration and loss of adequate sealing, because proximal EVG fixation failure seems to be determined to a greater degree by an aortic neck dilatation exceeding EVG oversizing (i.e., increase in d1) rather than simple migration distance, according to Litwinski et al. [17]. The importance of the (in)sufficient AAA neck length also is questioned by Hager et al. [18], who compared clinical performance of EVG with supra- and infrarenal fixation in short but straight proximal necks and reported equal and reported freedom intervals from early and late type 1a endoleaks.
Taking into consideration that a cut-off value of AAA neck diameter >28 mm is considered to represent a high risk for EVAR according to the Society for Vascular Surgery/American Association for Vascular Surgery (SVS/AAVS) [19], many researchers conducted studies comparing the clinical performance of EVGs in the treatment of AAA with large and smaller neck diameters. Jim et al. [20] reported a higher rate of major adverse effects within the first year and higher migration rates at 5 years when treating AAAs with large (>28 mm) versus smaller (<28 mm) using the Talent device (a bimodular device with suprarenal fixation), whereas Stanley et al. [21] reported a migration incidence of 4.2 % in a series of 238 AAA treated with the Zenith device, identifying a neck diameter >28 mm (p = 0.0024) as the sole determinant of this complication. Generally, an oversizing of the EVG central fixation segment by 10–25 % is suggested to ensure an adequate radial force and proper sealing in AAA necks of >28 mm, providing an acceptable low migration rate and incidence of proximal endoleak (Type Ia) [22]. On the other hand, the continuous radial force exerted by self-expanding stent-grafts has been associated with progressive dilatation of the aortic neck postoperatively, predisposing to generation of higher displacement forces to migration (i.e., increase of d1/d2) and loss of proximal sealing [23–26]. Therefore, newer EVG aiming at proximal sealing with alternative modes, such as polymer-filled sealing rings merit greater attention [27–29].
Role of Diameter of Iliac Vessels
While clinical studies focus mainly on the geometrical characteristics of the AAA and endograft neck diameter, a computational evaluation of the influence of inlet diameter on the hemodynamic performance of an EVG unveils an important role for the iliac (outlet) diameters, as well. The computational estimation of the displacement forces acting on the bifurcation of an EVG with large (36 mm) neck diameter exemplifies this (Fig. 2C). As can be seen, an inlet-to-outlet diameter ratio of 3 (i.e., iliac arteries of 12 mm) renders a higher bifurcation force compared with an AAA of the same inlet diameter but with ectatic iliacs of 18 mm (ratio of 2). In such instances, adapting an accommodation mode of a main body actually sitting on the aortic bifurcation, such as the Powerlink XL EVG system [30] or of a long mainbody available with the Cook Zenith AAA EVG [31] renders the AAA-EVG conjugation theoretically more stable.
Investigating the Role of EVG Limb Length
The iliac diameter can affect the displacement forces and the stability of the peripheral fixation with respect to endoleaks Type Ib. A low bifurcation, as determined by the presence of long EVG main body accommodating at the aortoiliac bifurcation and/or short iliac limbs, renders the EG less prone to proximal migration [32]. Indeed, Benharash et al. [33] and Heikkinen et al. [34] underscored the migration-preventive role of long iliac fixation, especially in cases of suboptimal or inadequate proximal fixation. This finding also was supported by Waasdorp et al. [35], who suggested that the shorter the proximal fixation, the longer the iliac fixation has to be to prevent migration. In other words, an EVG of long proximal and short iliac fixation could bear the same migration risk compared to an EG of short proximal and long iliac fixation. The aforementioned indicate that the hemodynamic effect of the relative limb length with respect to the EVG main body should not be considered negligible. While the relative lengths influence the magnitude of the displacement forces at different EVG parts, the stabilization provided by the iliac fixation lengths contributes to resistance to migration.
Our computational example suggests that a high ratio of main body-to-iliac limb length (i.e., either a long main body or short limbs, both coinciding with a low bifurcation) favors hemodynamically EVGs with low bifurcation, such as the AFX® stent-graft (Endologix Inc., Irvine, CA, USA), the COOK Zenith EVG (available in five different lengths), or the Treovance® (Bolton Medical Inc., Sunrise, FL, USA) [36] but may theoretically attenuate the iliac limb stability (Fig. 2D). Indeed, while our model shows the beneficial role of an iliac-limb length twice as long the main-body, it seems that higher or lower length ratios beyond that point lead to increased forces along the iliac limbs, thereby reflecting a higher predisposition for the development of Type III endoleaks, due to modular disconnection. To counteract this problem when the patient’s individualized geometry leads to an endograft configuration as previously described, certain mechanisms have been evolved to enhance limb’s stability, such as the unique Lock-stent mechanism of five rounded bards for fixation within stent modules [37].
Influence of Iliac Configuration
Unfavourable iliac geometry comprises one of the commonest reasons that render AAA unsuitable for EVAR, with extreme iliac tortuosity accounting for 10 % of the exclusion criteria [38]. While iliac angulation and tortuosity have been implicated in endograft limb kinking and thrombosis [39, 40], it is worth mentioning that excessive iliac tortuosity (Fig. 3) may generate hemodynamic forces with a cephalad direction leading to upward migration of the endograft. Although EVG upward migration constitutes a very rare entity with only few cases reported in the immediate peri- or postoperative period, attributed either to surgical manipulating errors or material failure [41–43], there seems that iliac geometry may predispose to such events in the long-term, as demonstrated above. In routine practice, this tendency is counteracted by various proximal fixation mechanisms (hooks, pins, and barbs) available in newer generation endografts [44, 45].
Computational reconstruction of an endograft model (grey color) used to treat an AAA with iliacs (red color) of excessive tortuosity. Because the vertical z-axis is headed caudally, the estimated negative values of displacement forces (shown in diagram) corresponding to tendency for upward instability can be attributed to excessive iliac tortuosity
Furthermore, one also should bear in mind that the upward movement of an EVG associated with angulation/tortuosity of the device’s iliac EVG limbs either following the native iliac anatomy or secondarily caused by aneurysm sac shrinkage can lead to disconnection of the EVG’s components and consequent endoleak Type III [46]. This complication occurs usually in the third to the sixth postoperative year [47].
Comparison of the Bifurcated EVG with the “Crossed-Limbs” and the Aortouniiliac Configuration
While the customary bifurcated EVG configuration lies in the center of both clinical and computational studies, clinicians may occasionally come along challenging anatomies or clinical circumstances where the successful sealing of the AAA is accomplished by adapting the cross-limb accommodation of the EVG or using an aortouniiliac configuration [48, 49]. A comparison of the displacement forces between the cross-limbs and customary bifurcated EVG configurations was recently performed [16, 50–52]. It has been shown recently that these configurations sustain similar displacement forces, irrespective of any variability in the EVG curvature, in the angulation or in the relative lengths and diameters of the proximal and distal EVG segments (Fig. 2A–D). Moreover, the similar oscillatory shear index pattern (expressing the shear stress vector deflection from blood flow’s predominant direction during a cardiac cycle), expressed in both endograft configurations, suggest that thrombosis may occur similarly between the two configurations [52]. Indeed, the only-to-date clinical study comparing the clinical performance between the two configurations in terms 12 and 36 months freedom from of migrations, any type of endoleak and need for reintervention and limb thrombosis yielded similar clinical outcomes, with no statistical significance [53].
The use of aortomonoiliac EVG facilitates the management of AAA in cases of narrow terminal aorta, tortuous, kinked, small, calcified, or occluded contralateral iliac artery, emergent treatment of ruptured AAAs or treatment of endoleaks of previously implanted endoprostheses [45, 54, 55]. Therefore, limited data exist to compare directly the performance and hemodynamic profile of aortomonoiliac (Fig. 1D) versus bifurcated EVG (Fig. 1A). Our laboratory results suggest higher displacement forces over the entire EVG or, specifically, at the iliac area (Fig. 4) for the aortouniiliac configuration, predisposing to higher migration rates. Therefore, this mode of AAA treatment should be reserved only in selected cases with the aforementioned indications, rather than as an alternative to bifurcated endografts.
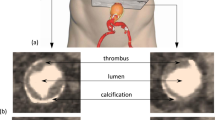
Thrombus Formation in Aortic Endografts
A frequent observation considering the endovascular repair of an AAA is the deposition of thrombus inside the stent-graft lumen (Fig. 5). Several studies have reported intragraft mural thrombus formation starting from the first month after the operation up to nearly 5 months [56, 57]. Mestres et al. [56] estimated the postoperative progression of intragraft thrombus using CT angiography and concluded that the presence of thrombus in the native aorta and the presence of the aortouniiliac configuration were independent predictive factors for the progression of EVG mural thrombus. Additional evidence was provided by Wegener et al. [57], who noted that more than one-fifth of patients developed intraluminal deposits of thrombotic material identifying; however, no potential risk factors associated with thrombus formation. Noteworthy, there was evidence that some of the thrombotic depositions resolved completely without any specific therapy and interestingly enough in these patients no episode of thromboembolism was noticed. Consistent with previous reports, intragraft thrombus deposition after postimplantation of endograft in EVAR patients was observed by Wu et al. [58]. who further reported that the incidence of intraprosthetic thrombosis increased in endografts with longer mainbody and in those with larger mainbody diameter compared with the iliac graft diameter (i.e., d1/d2), whereas no correlation was found between the preoperative presence of thrombus or the postoperative antiplatelet/anticoagulant treatment and the deposition of thrombus in the stent-graft. Finally, investigating the flow patterns in a bifurcated stent-graft deployed in a AAA model, Chong et al. reported that the geometry of the arterial vessel and the configuration of the stentgraft could have an impact on the formation of thrombus, with aortic neck angulation, iliac tortuosity, and configuration of the endograft identified as important parameters for the deposition of intra-stent thrombus [8, 59, 60].
Flow Patterns in AAA
Local geometric factors play a role in the determination of velocity values and flow patterns (recirculating zones, flow separation, skewed flow) [60, 61]. Chong et al. [60] described the flow patterns in several regions of a bifurcated, nonplanar stented-AAA model under pulsatile blood flow throughout the cardiac cycle and found a region of flow separation and recirculation at the anterior wall of the proximal stent, which increases with increasing angle and is most predominant during the diastolic phase. As the anteroposterior neck angle increases, the flow patterns present greater asymmetry with flow separation and recirculation zones at the posterior region of the graft main trunk, while most of the flow diverting towards the anterior wall. These phenomena occur mainly in the late deceleration and early systolic phase. Additionally, the flow patterns in the two EVG iliac limbs present quite similar, with skewing of the flow during the presystolic acceleration/peak systolic phase and with subsequent flow separation at the outer wall surface of the EVG limbs. Frauenfelder et al. [62] demonstrated a reduction of turbulence after placement of a stent-graft, with equal blood flow volume through both the stented iliac arteries coupled with a reduction of wall pressure and wall shear stress. They also concluded that high shear stress values develop at the junction site between the stump (iliac gate) of the main body and the contralateral limb (docking area), predisposing to type III endoleak, as also supported by Juchems et al. [63] and Kramer et al. [15].
Clilical Application of Hemodynamic Principles: from Theory to Practice
Computational simulation provides useful data to understand basic pathophysiological aspects and delineate the behavior of AAA and EVG. The demonstration presented above show that the combination of geometrical aspects provides more information to predict the postimplantation EVG performance rather than single geometric features. The advantages and disadvantages associated with certain conformational features of various EVGs show that there is no ideal EVG that serves better than others the purpose of AAA endovascular treatment; rather, each AAA possesses a unique anatomy in which some EVGs accommodate better than others, so that no single EVG pattern emerges as the best [64].
Accordingly, identification of certain AAA geometrical challenges, such as iliac tortuosity and severe angulation or large diameter of the neck, led to development of enhanced suprarenal fixation modes, supported iliac legs and modified stent designs. A recent study based on Finite Element Analysis estimated the stresses on different designs of iliac limb stents, showing differences between them, with spiral and circular stents providing greater flexibility and lower stress values than Z-stents [65]. This comes in accordance with the hybrid concept of combining components (main body, limb extensions) from different endografts, not only in emergent but also in selective clinical setting, treating challenging anatomies with components of different mechanical properties [65–67].
Finally, computational data showed that the displacement forces acting on an EVG are directed sideways rather than downward [11, 13, 14]. These were confirmed by clinical studies that identified a frequent late occurrence of postimplantation EVG sideway movements (27–35 % Rafii et al. [68] and Waasdorp et al. [69], respectively) [68–71].
Although interesting as computational simulations may seem, it should be stressed out that reproducibility and comparison between results of different studies should be cautiously approached, because these models carry certain limitations associated with pressure and flow parameters (boundary conditions) applied to these models; therefore, detailed information about preconditions and model assumptions should always be provided. Admittedly, future clinical studies are needed to validate clinically of computational results and expand further the practical applications of the latter.
In conclusion, this article discusses the influence that certain geometrical factors can exert on the hemodynamic behavior of the EVGs. No EVG design emerges as the best; rather, every AAA has a unique anatomy served better by some EVG than others and vice versa. The information derived seems to be in accordance with clinical observations and comprises a useful adjunct for both clinicians and manufacturers to further development and improvement of EVG designs and better operational planning.
References
Greenhalgh RM, Brown LC, Kwong GPS, Powell JT, Thompson SG (2004) Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results; randomized controlled trial. Lancet 364:843–848
Prinssen M, Buskens E, Blakensteijn JD (2004) Quality of life after endovascular and open AAA repair: results of a randomized trial. Eur J Vasc Surg 27:121–127
Laheij RJ, Buth J, Harris PL, Moll FL, Stelter WJ, Verhoeven EL (2000) Need for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg 87:1666–1673
Conners MS, Sternbergh WC, Carter G, Tonnessen BH (2002) Endograft migration one to four years after endovascular abdominal aortic aneurysm repair with the AneuRx device: a cautionary note. J Vasc Surg 36:476–482
Mohan IV, Harris HL, Van Marrewijk JC, Laheij RJ, How TV (2002) Factors and forces influencing stent-graft migration after endovascular aortic aneurysm repair. J Endovasc Ther 9:748–755
Sternbergh W, Carter G, York J, Yoselevitz M, Money S (2002) Aortic neck angulation predicts adverse outcome with endovascular abdominal aortic aneurysm repair. J Vasc Surg 35:482–486
Morris LG, Delassus P, Walsh M, McGloughlin TM (2004) A mathematical model to predict the in vivo pulsatile drag forces acting on bifurcated stent grafts in endovascular treatment of abdominal aortic aneurysms (AAA). J Biomech 37:1087–1095
Liffman K, Lawrence-Brown MMD, Semmens JB, Semmens JB, Bui A, Rudman M, Hartley DE (2001) Analytical modeling and numerical simulation of forces in an endoluminal graft. J Endovasc Ther 8:358–371
Li Z, Kleinstreuer C (2006) Analysis of biomechanical factors affecting stent-graft migration in an abdominal aortic aneurysm model. J Biomech 39:2264–2273
Howell BA, Kim TK, Cheer A, Dwyer H, Saloner D, Chuter TAM (2007) Computational fluid dynamics within bifurcated abdominal aortic stent-grafts. J Endovasc Ther 14:138–143
Molony DS, Kavanagh EG, Madhavan P, Walsh MT, McGloughlin TM (2010) A computational study of the magnitude and direction of migration forces in patient-specific abdominal aortic aneurysm stent-grafts. Eur J Vasc Endovasc Surg 40:332–339
Li Z, Kleinstreuer C, Farber M (2005) Computational analysis of biomechanical contributors to possible endovascular graft failure. Biomech Model Mechanobiol 4:221–234
Georgakarakos E, Georgiadis GS, Ioannou CV, Kapoulas KC, Trellopoulos G, Lazarides M (2012) Aneurysm sac shrinkage after endovascular treatment of the aorta: beyond sac pressure and endoleaks. Vasc Med 17:168–173
Harris P, Brennan J, Martin J et al (1999) Longitudinal aneurysm shrinkage following endovascular aortic aneurysm repair: a source of intermediate and late complications. J Endovasc Surg 6:11–16
Kramer SC, Seifarth H, Pamler R, Fleiter T, Gorich J (2001) Geometric changes in aortic endografts over a 2-year observation period. J Endovasc Ther 8:34–38
Georgakarakos E, Xenakis A, Manopoulos C, Georgiadis GS, Tsangaris S, Lazarides MK (2013) Geometric factors affecting the displacement forces in an aortic endograft with crossed-limbs: a computational study. J Endovasc Ther 20:191–199
Litwinski RA, Donayre CE, Chow SL et al (2006) The role of aortic neck dilation and elongation in the etiology of stent graft migration after endovascular abdominal aortic aneurysm repair with a passive fixation device. J Vasc Surg 44:1176–1181
Hager ES, Cho JS, Makaroun MS et al (2012) Endografts with suprarenal fixation do not perform better than those with infrarenal fixation in the treatment of patients with short straight proximal aortic necks. J Vasc Surg 55:1242–1246
Chaikof EL, Fillinger MF, Matsumura JS et al (2002) Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg 35:1061–1066
Jim J, Rubin BG, Geraghty PJ, Criado FJ, Fajardo A, Sanchez LA (2010) A 5-year comparison of EVAR for large and small aortic necks. J Endovasc Ther 17:575–584
Stanley BM, Semmens JB, Mai Q et al (2001) Evaluation of patient selection guidelines for endoluminal AAA repair with the Zenith stent-graft: the Australasian experience. J Endovasc Ther 8:457–464
Gawenda M (2010) Commentary: Endovascular treatment of abdominal aortic aneurysms with large diameter proximal necks: a European experience. J Endovasc Ther 17:585–588
Arthurs ZM, Lyden SP, Rajani RR, Eagleton MJ, Clair DG (2011) Long-term outcomes of Palmaz stent placement for intraoperative type Ia endoleak during endovascular aneurysm repair. Ann Vasc Surg 25:120–126
Dalainas I, Nano G, Bianchi P et al (2007) Aortic neck dilatation and endograft migration are correlated with self-expanding endografts. J Endovasc Ther 14:318–323
Malas MB, Ohki T, Veith FJ et al (2005) Absence of proximal neck dilatation and graft migration after endovascular aneurysm repair with balloon-expandable stent-based endografts. J Vasc Surg 42:639–644
Monahan TS, Chuter TA, Reilly LM, Rapp JH, Hiramoto JS (2010) Long-term follow-up of neck expansion after endovascular aortic aneurysm repair. J Vasc Surg 52:303–307
Mehta M, Valdés FE, Nolte T, A Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the Ovation Abdominal Stent Graft System Investigators et al (2013) One-year outcomes from an international study of the Ovation Abdominal Stent Graft System for endovascular aneurysm repair. J Vasc Surg 59(1):65-73.e1–65-73.e3. doi:10.1016/j.jvs.2013.06.065
Mangialardi N, Ronchey S, Kasemi H, Alberti V, Fazzini S, Serrao E (2013) Percutaneous endovascular aneurysm repair with the ultra-low profile Ovation Abdominal Stent-Graft System. J Cardiovasc Surg (Torino) 54:581–587
Moulakakis KG, Dalainas I, Kakisis J, Giannakopoulos TG, Liapis CD (2012) Current knowledge on EVAR with the ultra-low profile Ovation Abdominal Stent-graft System. J Cardiovasc Surg (Torino) 53:427–432
Jordan WD, Moore WM, Melton JG, Brown OW, Carpenter JP (2009) Secure fixation following EVAR with the Powerlink XL system in wide aortic necks: results of a prospective, multicenter trial. J Vasc Surg 50:979–986
Ricotta JJ 2nd, Oderich GS (2008) The Cook Zenith AAA endovascular graft. Perspect Vasc Surg Endovasc Ther 20:167–173
Avgerinos ED, Dalainas I, Kakisis J, Moulakakis K, Giannakopoulos T, Liapis CD (2011) Endograft accommodation on the aortic bifurcation: an overview of anatomical fixation and implications for long-term stent-graft stability. J Endovasc Ther 18:462–470
Benharash P, Lee JT, Abilez OJ, Crabtree T, Bloch DA, Zarins CK (2007) Iliac fixation inhibits migration of both suprarenal and infrarenal aortic endografts. J Vasc Surg 45:250–257
Heikkinen MA, Alsac JM, Arko FR, Metsänoja R, Zvaigzne A, Zarins CK (2006) The importance of iliac fixation in prevention of stent graft migration. J Vasc Surg 43:1130–1137
Waasdorp EJ, de Vries JP, Sterkenburg A et al (2009) The association between iliac fixation and proximal stent-graft migration during EVAR follow-up: mid-term results of 154 Talent devices. Eur J Vasc Endovasc Surg 37:681–687
Donas KP, Torsello G, Bisdas T (2012) New EVAR devices: pros and cons. J Cardiovasc Surg (Torino) 53:559–569
Chiesa R, Riambau V, Coppi G et al (2012) ADVANCE Investigational Study Investigators. The Bolton Treovance abdominal stent-graft: European clinical trial design. J Cardiovasc Surg (Torino) 53:595–604
Carpenter JP, Baum RA, Barker CF et al (2001) Impact of exclusion criteria on patient selection for endovascular abdominal aortic aneurysm repair. J Vasc Surg 34:1050–1054
Carroccio A, Faries PL, Morrissey NJ et al (2002) Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg 36:679–684
Cochennec F, Becquemin JP, Desgranges P, Allaire E, Kobeiter H, Roudot-Thoraval F (2007) Limb graft occlusion following EVAR: clinical pattern, outcomes and predictive factors of occurrence. Eur J Vasc Endovasc Surg 34:59–65
Katzen BT, MacLean AA, Katzman HE (2005) Retrograde migration of an abdominal aortic aneurysm endograft leading to postoperative renal failure. J Vasc Surg 42:784–787
Inan K, Ucak A, Onan B, Temizkan V, Ugur M, Yilmaz AT (2010) Bilateral renal artery occlusion due to intraoperative retrograde migration of an abdominal aortic aneurysm endograft. J Vasc Surg 51:720–724
Katsargyris A, Oikonomou K, Bracale UM, Verhoeven EL (2013) Unexpected complication with the new C3 excluder: cause and treatment. Cardiovasc Intervent Radiol 36:536–539
Melas N, Saratzis A, Saratzis N et al (2010) Aortic and iliac fixation of seven endografts for abdominal-aortic aneurysm repair in an experimental model using human cadaveric aortas. Eur J Vasc Endovasc Surg 40:429–435
Bosman WM, Steenhoven TJ, Suárez DR, Hinnen JW, Valstar ER, Hamming JF (2010) The proximal fixation strength of modern EVAR grafts in a short aneurysm neck. An in vitro study. Eur J Vasc Endovasc Surg 39:187–192
Schlösser FJ, Muhs BE (2012) Endoleaks after endovascular abdominal aortic aneurysm repair: what one needs to know. Curr Opin Cardiol 27:598–603
Steingruber IE, Neuhauser B, Seiler R et al (2006) Technical and clinical success of infrarenal endovascular abdominal aortic aneurysm repair: a 10-year single-center experience. Eur J Radiol 59:384–392
Ramaiah VG, Thompson CS, Shafique S et al (2002) Crossing the limbs: a useful adjunct for successful deployment of the AneuRx stent-graft. J Endovasc Ther 9:583–586
Katsikas VC, Dalainas I, Martinakis VG, Xiromeritis K (2012) The role of aortouniiliac devices in the treatment of aneurysmal disease. Eur Rev Med Pharmacol Sci 16:1061–1071
Georgakarakos E, Xenakis A, Manopoulos C, Georgiadis GS, Tsangaris S, Lazarides MK (2012) Modeling and computational analysis of the hemodynamic effects of crossing the limbs in an aortic endograft (“Ballerina” position). J Endovasc Ther 19:549–557
Stefanov F, McGloughlin T, Delassus P, Morris L (2013) Hemodynamic variations due to spiral blood flow through four patient-specific bifurcated stent graft configurations for the treatment of abdominal aortic aneurysms. Int J Numer Method Biomed Eng 29:179–196
Shek TL, Tse LW, Nabovati A, Amon CH (2012) Computational fluid dynamics evaluation of the cross-limb stent graft configuration for endovascular aneurysm repair. J Biomech Eng 134:121002
Georgiadis GS, Georgakarakos EI, Antoniou GA (2013) Clinical outcomes after crossed-limb vs. conventional endograft configuration in endovascular AAA repair. J Endovasc Ther 20(6):853–862
Lazaridis J, Melas N, Saratzis A et al (2009) Reporting mid- and long-term results of endovascular grafting for abdominal aortic aneurysms using the aortomonoiliac configuration. J Vasc Surg 50:8–14
Hinchliffe RJ, Braithwaite BD; European Bifab Study Collaborators (2007) A modular aortouniiliac endovascular stent-graft is a useful device for the treatment of symptomatic and ruptured infrarenal abdominal aortic aneurysms: one-year results from a multicentre study. Eur J Vasc Endovasc Surg 34:291–298
Mestres G, Maeso J, Fernandez V, Allegue N, Constenla I, Matas M (2009) Incidence and evolution of mural thrombus in abdominal aortic endografts. Ann Vasc Surg 23:627–633
Wegener M, Gorich J, Kramer S et al (2001) Thrombus formation in aortic endografts. J Endovasc Ther 8:372–379
Wu IH, Liang PC, Huang SC, Chi NS, Lin FY, Wang SS (2009) The significance of endograft geometry on the incidence of intraprosthetic thrombus deposits after abdominal endovascular grafting. Eur J Endovasc Surg 39:741–747
Chong CK, How TV (2004) Flow patterns in an endovascular stent-graft for abdominal aortic aneurysm repair. J Biomech 37:89–97
Chong CK, How TV, Harris PL (2005) Flow visualization in a model of a bifurcated stent-graft. J Endovasc Ther 12:435–445
Morris L, Delassus P, Grace P, Wallis F, Walsh M, McGloughlin T (2006) Effects of flat, parabolic and realistic steady flow inlet profiles on idealised and realistic stent graft fits through Abdominal Aortic Aneurysms (AAA). Med Eng Phys 28:19–26
Frauenfelder T, Lotfey M, Boehm T, Wildermuth S (2006) Computational fluid dynamics: hemodynamic changes in abdominal aortic aneurysm after stent-graft implantation. Cardiovasc Intervent Radiol 29:613–623
Juchems MS, Pless D, Fleiter TR et al (2004) Non invasive, multi detector row (MDR) CT based computational fluid dynamics (CFD) analysis of hemodynamics in infrarenal abdominal aortic aneurysm (AAA) before and after endovascular repair. Rofo 176:56–61
van Marrewijk CJ, Leurs LJ, Vallabhaneni SR, Harris PL, Buth J, Laheij RJ, EUROSTAR collaborators (2005) Risk-adjusted outcome analysis of endovascular abdominal aortic aneurysm repair in a large population: how do stent-grafts compare? J Endovasc Ther 12:417–429
Demanget N, Duprey A, Badel P et al (2013) Finite element analysis of the mechanical performances of 8 marketed aortic stent-grafts. J Endovasc Ther 20:523–535
Bos WT, Tielliu IF, Sondakh AO, Vourliotakis G, Bracale UM, Verhoeven EL (2010) Hybrid endograft solution for complex iliac anatomy: zenith body and excluder limbs. Vascular 18:136–140
Georgiadis GS, Trellopoulos G, Antoniou GA et al (2013) Hybrid endografts combinations for the treatment of endoleak in endovascular abdominal aortic aneurysm repair. Int J Artif Organs 36:28–38
Rafii BY, Abilez OJ, Benharash P, Zarins CK (2008) Lateral movement of endografts within the aneurysm sac is an indicator of stent-graft instability. J Endovasc Ther 15:335–343
Waasdorp EJ, Gorrepati ML, Rafii BY, de Vries JP, Zarins CK (2012) Sideways displacement of the endograft within the aneurysm sac is associated with late adverse effects after endovascular aneurysm repair. J Vasc Surg 55:947–955
Figueroa CA, Taylor CA, Yeh V, Chiou AJ, Gorrepati ML, Zarins CK (2010) Preliminary 3D computational analysis of the relationship between aortic displacement force and direction of endograft movement. J Vasc Surg 51:1488–1497
Figueroa CA, Taylor CAT, Yeh V, Chiou JA, Zarins CK (2009) Effect of curvature on displacement forces acting on aortic endografts: a 3-dimensional computational analysis. J Endovasc Ther 16:284–294
Acknowledgments
The authors thank Prof. S. Tsangaris, MEng, PhD and Antonios Xenakis, MEng (Fluids Section, School of Mechanical Engineering, National Technical University of Athens, Athens, Greece) for their technical support.
Conflict of interest
Efstratios Georgakarakos, Christos Argyriou, Nikolaos Schoretsanitis, Chris V. Ioannou, Nikolaos Kontopodis, Robert Morgan declare that they have no conflict of interest. Dimitrios Tsetis; Dr. Tsetis gives lectures for Trivascular outside the scope of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Georgakarakos, E., Argyriou, C., Schoretsanitis, N. et al. Geometrical Factors Influencing the Hemodynamic Behavior of the AAA Stent Grafts: Essentials for the Clinician. Cardiovasc Intervent Radiol 37, 1420–1429 (2014). https://doi.org/10.1007/s00270-014-0927-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-0927-9