Abstract
Anthropogenic activities are the main sources of soil, air, and water pollution by metals, including cadmium (Cd), lead (Pb), chromium (Cr), the metalloid arsenic (As), magnesium (Mg), zinc (Zn), and copper (Cu). The goal of this study was to assess the presence and concentration of toxic (As, Cd, Pb, and Cr) and essential metals (Mg, Zn, and Cu) in the liver and kidneys from 96 free-ranging rattlesnakes (Crotalus durissus) from Minas Gerais (Brazil). Bioaccumulation of Cd and Pb were significantly higher in males and heavier rattlesnakes (those with body weight above the average of the study population). Average ± standard deviations of Cd, Pb, Cr, Cu, Mg, Zn, and As in the general population (n = 96) were 3.19 ± 2.52; 5.98 ± 8.49; 0.66 ± 1.97; 3.27 ± 2.85; 776.14 ± 2982.92; 27.44 ± 29.55; and 0.32 ± 1.46; respectively. Bioaccumulation of some metals correlated positively with changes in hematologic and serum biochemical parameters. Results of this study were contrasted with previous studies assessing metal bioaccumulation in other species of terrestrial or aquatic snakes. Considering their position in the food chain and the broad range of bioaccumulation of both toxic and essential metals observed in this study, rattlesnakes may function as highly relevant biological sentinels for environmental pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination with metals is of increasing global concern due to their persistence in the environment, effects on biogeochemical recycling, and toxicological, and ecological risks (Quadra et al. 2019; Buch et al. 2021). Excessive natural and anthropogenic activities are the main sources of toxic metals, such as cadmium (Cd), lead (Pb), chromium (Cr), the metalloid arsenic (As), and essential metals, including magnesium (Mg), zinc (Zn), and copper (Cu), polluting the soil, air, and water. Consequently, plants, domestic animals, humans, and wildlife may be exposed to these toxicants with deleterious effects (Uluozlu et al. 2007; Gibbons et al. 2000; Guvvala et al. 2020; Shiek et al. 2021).
Although there have been considerable efforts to assess concentrations and deleterious effects of contaminant metals in birds, mammals, amphibians, and fish (Burger and Gochfeld 2016; Celik et al. 2021), as well as in some groups of reptiles, snakes have not been properly studied (Delany et al. 1988; Campbell and Campbell 2002; Marquez-Ferrando et al. 2009). Snakes are high trophic level predators, with increased risk of bioaccumulation of metals from their preys, and they are also in close contact with potentially contaminated soil and water. Furthermore, snakes are considered relevant environmental bio-indicators due to some of their biological features, such as long-life expectancy, low metabolic rate, non migrant, and feeding habits that predisposes to biomagnification (Burger 1992; Hopkins et al. 2001; Campbell and Campbell 2002; Burger et al. 2005; Jones and Holladay 2006).
Environmental contamination by trace elements is an important threat due to their high toxicity, long persistence, bioaccumulation, and biomagnification in the food chain (Mashroofeh et al. 2012). In addition, these trace elements may have cytotoxic, mutagenic, or carcinogenic effects in animals (Yadollahvand et al. 2014), being potentially toxic even in low concentrations when ingested over long periods of time. However, essential elements may also result in toxic effects when ingested in excess (Mashroofeh et al. 2013; Yadollahvand et al. 2014). Therefore, either essential or non-essential elements may be ingested and accumulate in animal tissues (Mashroofeh et al. 2013).
The genus Crotalus include poisonous snakes that belong to the Viperidae family. They are found all over South America in areas of open vegetation and dry climate (Bastos et al. 2005). This is not considered an endangered species. In fact, deforestation may favor territorial dispersion of this species (Bastos et al. 2005). In Brazil, Crotalus durissus is the only naturally occurring species, which is subdivided into five subspecies: C. durissus terrificus, C. durissus collilineatus, C. durissus cascavella, C. durissus ruruima, and C. durissus marajoensis (Pinho and Pereira 2001). This species adapts to areas employed for agriculture and livestock and may approach urban areas (Bastos et al. 2005). These animals may reach 1.5 m long and live for 15 years, which may favor bioaccumulation of heavy metals. In this study, the animals were captured in the State of Minas Gerais (Brazil) in a region named “Iron Quadrangle” where there is extensive mining activity, particularly extraction of iron ore, which may increase the risks of environmental contamination by heavy metals (Bosso and Enzweiler 2008; Buch et al. 2021; Davila et al. 2020).
This study aimed to assess the presence and concentration of toxic (As, Cd, Pb, and Cr) and essential metals (Mg, Zn, and Cu) in organs from 96 free-ranging rattlesnakes (Crotalus durissus) from Minas Gerais (Brazil). Bioaccumulation was evaluated according to many paramemters, including sex, body length, and body weight of these animals. Metal concentration in tissues were also correlated with hematological and biochemical parameters.
Material and methods
Site description and sample collection
This study has been approved by the Institutional Animal Care and Use Committee (Comitê de Ética no Uso de Animais—Fundação Ezequiel Dias/CEUA-FUNED) under protocol number 14/2020, and it has been registered and approved by the Sistema de Autorização e Informação em Biodiversidade (Sisbio) under protocol number 70233–1, according to the Brazilian environmental laws.
This study included 96 free-ranging rattlesnakes from peri-urban and rural areas in the State of Minas Gerais (Brazil), which were captured due to the risk of ophidic accidents (mostly by fire department personnel or police officers) and referred to the Fundação Ezequiel Dias (FUNED; Belo Horizonte, Brazil) between August 2019 and February 2020. The study areas covered the following mesoregions of the State of Minas Gerais: Sul/Sudoeste, Zona da Mata, Metropolitana de Belo Horizonte, Oeste de Minas and Vale do Mucuri. The animals included in this study were euthanized in accordance with all applicable laws and regulations, as indicated by the Conselho Nacional de Controle de Experimentação Animal (CONCEA).
Prior to euthanasia, all rattlesnakes were weighed and measured, sex was identified, and age estimated. Blood was collected by puncturing the caudal vein and kept in tubes with heparin for hematological and biochemical analysis. Biological data including sex (male or female), estimated age (adult or young – snakes were considered young when they had less than 300 g of body weight), body weight, and body length were registered. Rattlesnakes were then exposed to vapor of dry ice to promote hypothermia and hypoxia, followed by intracoelomic injection of 100 mg/kg of thiopental. The animals were then necropsied, and 20 g of liver and kidney were collected. Sampling equipment was rinsed in 10% nitric acid solution and deionized water prior to each use. Tissue samples were immediately stored at -20 °C until further processing.
Hematological and biochemical analysis
Hematological and biochemical analyses were performed soon after sampling. Manual methods were applied for cell counting (Neubauer chamber using saline as diluter), packed cell volume measurement (microcapillary centrifugation 10,000 rpm/10 min), and morphological differentiation of leukocytes (blood smear stained by Romanosky´s). Cobas Mira plus was elected for the biochemical profile, executed using commercial kits already validated for mammal species. By color enzymatic reaction were determined total protein, albumin, creatinine, glucose, bilirubin and triglycerides, by ultraviolet methods urea and by kinetic reaction the GGT activity, according to the manufacture instructions. Pooled tissue samples (liver and kidney) from each animal were rinsed in deionized water and then dried until reaching a constant weight. Approximately 100 mg of dried matter were mineralized in 1.5 mL of a mixture of nitric acid-perchloric acid (2:1) and the extract was used for measuring the concentration of Mg, Zn, Cu, Cr, Cd, and Pb by atomic absorption spectrometry (Agilent) as described by Marin et al. (1993). Measurements were expressed as parts per million (ppm), which is equivalent to μg/g of dry matter, and minimum detection limits (mg/L) were 0.1 (Pb), 0.02 (Cd), 0.03 (Cu), 0.06 (Cr), 0.003 (Mg), and 0.01 (Zn).
Heavy metal analysis
For measuring concentrations of As, 0.4 mL of the mineral extract (described above) was added to 1.8 mL of 6 N HCl, 0.2 mL of 50% potassium iodide, and 7.6 mL of deionized water. Tubes were vortexed and kept under room temperature for 50 min under dark. Samples were then analyzed using a hydride vapor generator (HVG-1, Shimadzu Corporation, Tokio, Japan) coupled to an atomic absorption spectrometrometer (AA-6701F, Shimadzu). Blank and standard controls were measured every 10 samples. Detection limit for As was 0.02 mg/L. Recovery ranged from 88 to 102%, and the coefficient of variation in replicates was up to 10%.
Statistical analysis
Mineral concentration data did not have a normal distribution, therefore nonparametric tests, namely Mann–Whitney and Kruskal–Wallis, were used for comparisons between two or three groups, respectively. When there were significant differences based on Kruskal–Wallis test, the Dunn’s test was used for pared comparisons. Values were considered significantly different when p < 0.05. Data were analyzed using the software Pandas version 1.3.3, Scipy version 1.7.1, and Scikit_posthocs version 0.6.7. Graphs were generated using the software Matplotlib version 3.4.3 and Seaborn version 0.11.0. Data is expressed as mean and standard deviation. Spearman correlation test was performed to evaluate heavy metals concentration and hematological/biochemical parameters using Graphpad Prism 7.00.
Results
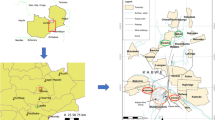
Considering their origin, 56.2% of the rattlesnakes included in this study were from the mesoregion “Metropolitana de Belo Horizonte”, followed by the mesoregion “Oeste de Minas” with 24.0% of the rattlesnakes, 9.4% from “Sul de Minas”, 6.2% from “Vale do Mucuri”, and 2.1% from “Zona da Mata” (Fig. 1). Two rattlesnakes (2.1%) did not have their origin recorded. Considering all 96 rattlesnakes, 54 (56%) were female and 42 (44%) male (Fig. 1), whereas 89 (93%) were considered adults, and seven (7%) young. Body weights ranged from 40 to 1700 g, with an average of 596 ± 315 g. Males had an average body weight significantly higher than females: 687 ± 345 g and 525 ± 269 g (p = 0.0153), respectively.
Origin of 94 out of 96 rattlesnakes (Crotalus durissus) captured from five mesoregions in the State of Minas Gerais: “Vale do Mucuri”, “Metropolitana de Belo Horizonte”, “Oeste de Minas”, “Sul/Sudoeste de Minas”, and “Zona da Mata”. Two rattlesnakes did not have their origin recorded. The number of males and females are indicated between parenthesis. Source of the map: Instituto Brasileiro de Geografia e Estatística (IBGE)
Concentrations of trace elements in tissues (liver and kidney) of rattlesnakes are described in Table 1. In the case of As, samples from only 16 out of the 96 rattlesnakes allowed quantification, with concentrations ranging from 0.05 to 9.42 ppm. Concentrations of trace elements had a high coefficient of variation, particularly for Mg (Table 1).
According to sex, significant differences between males and females were observed for Cd and Pb, which had higher bioaccumulation in males than in females (p < 0.05; Fig. 2). Rattlesnakes were also divided according to their weight (above or below 596 g, which was the overall average body weight), and concentrations of Cd and Pb were significantly higher in animals with more than 596 g (Fig. 2).
Bioaccumulation of heavy metals in tissues (liver and kidney) of free-ranging rattlesnakes (Crotalus durissus) from the State of Minas Gerais (Brazil) according to sex and body weight. A Cadmium (Cd) levels according to sex (p = 0.04). B Lead (Pb) levels according to sex (p = 0.04). C Cd levels according to body weight (p = 0.04). D Pb levels according to body weight (p = 0.03). Data were compared by the Mann–Whitney test. Dots represent individual rattlesnakes and boxes indicate 1st percentile (p25), median (p50) and 3rd percentile (p75), while the whiskers were calculated over the interquantile range (IQR), as follows: p25 – 1.5 * IQR (lower whisker), p75 + 1.5 * IQR (higher whisker). Abbreviation: ppm = parts per million (μg/g)
Concentrations of trace elements in rattlesnakes according to the geographic location also indicated some significant differences. Mg and Zn were significantly higher in rattlesnakes from the “Oeste de Minas”, whereas rattlesnakes from other regions had significantly higher concentrations of Cr when compared to animals from the mesoregion “Metropolitana de Belo Horizonte” (Fig. 3).
Bioaccumulation of heavy metals in tissues (liver and kidney) of free-ranging rattlesnakes (Crotalus durissus) from the State of Minas (Brazil) according to their geographic origin. A Magnesium (Mg); B Zinc (Zn); and (C) Chromium (Cr). Data were compared by the Kruskal–Wallis test followed by pairwise comparisons using the Dunn’s post-hoc test. Dots represent individual rattlesnakes and boxes indicate 1st percentile (p25), median (p50) and 3rd percentile (p75), while the whiskers point to the interquantile range (IQR), calculated as follows: p25 – 1.5 * IQR (lower whisker), p75 + 1.5 * IQR (higher whisker). Abbreviation: ppm = parts per million (μg/g)
Statistically significant correlations between heavy metal concentrations and hematological and biochemical parameters are described on Tables 2 and 3. All non-significant correlations are described in the Online Resources 1 and 2. Metalloid As concentration had a negative correlation with total leucocytes (Fig. 4a), lymphocytes and monocytes counts (r values of − 0.3436, − 0.3152 and -0.2611 respectively). Cd had a positive correlation with mean corpuscular volume (0.2525). Cr had significant correlations with total plasma protein (0.3647), erythrocytes (0.3974), and mean corpuscular volume (− 0.3274). Mg had a negative correlation with progranulocytes (− 0.2356) and heterophils (− 0.2927). Pb had significant correlations with erythrocytes (− 0.3225) (Fig. 4b), mean corpuscular volume (0.3565), heterophils (− 0.2515), and monocytes (− 0.3062). Zn and Cu had no significant correlations with any of the hematological parameters evaluated.
Spearman correlations between concentration of metals in tissues (liver and kidney) of free-ranging rattlesnakes (Crotallus durissus) and hematologic or blood biochemistry parameters. a total leucocytes and metalloid arsenic concentration in tissues. b erythrocytes and lead concentration in tissues. c total bilirubin and copper concentration in tissues. d total urea and lead concentration in tissues
Cd had positive correlations with gamma glutamil transferase (GGT) (0.3018) and glucose (0.2848). Cu had significant correlations with albumin (− 0.2438) and total bilirubin (0.8824) (Fig. 4c). Mg had a positive correlation with urea (0.2791). Pb had positive correlations with urea (0.3206) (Fig. 4d), glucose (0.3774), and triglycerides (0.3466). Zn had positive correlation with total bilirubin (0.8986). Metalloid As and Cr had no significant correlations with any of the biochemical parameters evaluated.
Discussion
In this study we investigated the presence and concentration of metals in tissue samples (pool of liver and kidney) from 96 free-ranging rattlesnakes captured in various mesoregions of the State of Minas Gerais (Brazil). Metals may be transported by the wind contaminating the soil and water, contaminating both terrestrial and aquatic food chains. Plants absorb metals, accumulating them in their leaves and fruits, which are consumed by invertebrates, which are eaten by small animals, which are prey for many predators, including snakes. However, predators that are at the top of the food chain are more vulnerable because they accumulate higher concentrations of metals when compared to species at lower trophic levels. We evaluated the association between bioaccumulation of metals with sex, weight, and geographic origin of these animals. Cd and Pb were detected in significantly higher concentrations in males and animals with body weight above 596 g. Higher concentrations of Zn and Mg were detected in animals from the region “Oeste de Minas Gerais”, whereas As was detected in only 18 rattlesnakes. Interestingly, this study demonstrated significant differences in bioaccumulation among various geographic regions, particularly tissue concentrations of Mg, Zn, and Cr. The State of Minas Gerais has an intense mining activity as well as areas of intensive agriculture (Cabral-Pinto et al. 2020; Davila et al. 2020; Buch et al. 2021). Therefore, future studies should consider potential risks of environmental contamination and bioaccumulation in rattlesnakes.
Snakes in this study had high concentrations of Cd in their pool of tissue samples (liver and kidney). Values detected in this study were approximately 25-fold higher than those reported by Burger et al. (2017) in Pituophis melanoleucus (pine snakes) from New Jersey (USA). However, samples in that study were obtained from an area of environmental protection with no houses, paved roads, or industries (Burger et al. 2017), which contrasts with the conditions of this study that included areas with heavy anthropic activity. Increased Cd concentrations in soil are often associated with the use of sewage sludge or manure as fertilizers since these may contain high Cd concentrations (Bergkvist et al. 2003). In addition, soil contamination with Cd may result from dispersion of mining residues due to industrial processing of metals such as Zn or Pb (Koh and Judson 1986; Spierenburg et al. 1988; Kubier et al. 2019). Cd is retained in the soil so concentrations may increase over time if the source of contamination remains. Therefore, older and heavier animals have higher risk of bioaccumulation of this metal as demonstrated in this study. Its bioaccumulation is due to stable ligation of Cd and proteins such as in Cd-metallothionein complexes, which prevents renal excretion of Cd (Himeno et al. 2019). Cd is one of the most toxic metals, and it is not an essential element for either humans or animals. Some studies have employed snakes are indicators of environmental pollution by comparing levels of metals in snakes from a contaminated area in comparison to animals from a non-contaminated reference area (Hopkins et al. 1999; Burger et al. 2006, 2007), or by comparing bioaccumulation in different snake species from a contaminated area (Drewett et al. 2013).
Interestingly, Cd concentrations found in terrestrial snakes in this study were lower than those detected in the kidney, liver, skin, and muscle of marine snakes (Lapemis curtus) from the Strait of Hormuz in the Persian Gulf (Sereshk and Bakhtiari 2015). Marine snakes are usually found in 4 to 40 m-deep waters and they spend their entire life in the water feeding on a wide range of prey including fish, mollusks, and crustaceans. Importantly, in that region of the Persian Gulf there are reports of illegal disposal of ship hold water, leakage from oil wells, release of swage and industrial wastewater (Emara 1990; Shirani et al. 2012) as well as residues from desalinization and energy plants (Al-Yousuf et al. 2000). The region also suffered from release of millions of barrels due to military conflicts (Eghtesadi-Araghi and Farzadnia 2011) so oil pollution is one of the most important environmental threats in the Persian Gulf (Ebrahimi-Sirizi and Riyahi-Bakhtiyari 2013).
Male rattlesnakes had significantly higher concentrations of Cd when compared to females in this study, which is in good agreement with a previous report (Frossard et al. 2017). Considering that weight and age correlates positively with bioaccumulation, males may have higher concentrations of Cd since they have higher average weight when compared to females. However, Burger et al. (2017) reported levels of Cd of 15.0 ± 5.2 and 22.0 ± 4.3 ppm (p > 0.05) in male and female pine snakes (Pituophis melanoleucus), respectively. Presumably, differences in bioaccumulation between males and females may be due to differences in body size and weight, reproductive activity such as transfer of metals to the eggs in the case of females (Burger 1992; Hopkins et al. 2002, 2004).
Pb concentrations were higher in male rattlesnakes and animals heavier than 596 g in this study. Frossard et al. (2017) reported low levels of Pb in Bothrops jararaca and Boa constrictor captured in the Southeastern region in Brazil. Burger et al. (2005), studying northern water snakes (Nerodia sipedon) from Tennessee (USA), found lower levels of Pb in tissue samples. In contrast, another study found a concentration of 31 ppm in the kidney of one northern water snake (Nerodia sipedon) also from Tennessee (USA). High levels of Pb were also detected in cotton mouth snakes (Akistrodon piscivorous) from North Carolina (USA) (Burger et al. 2006). Kinetics models have been proposed to explain the distribution of Pb in tissues considering three compartments: blood, soft tissues, and bones (Rabinowitz et al. 1976; Rabinowitz 1991). Half life of Pb in these compartments varies, being estimated in 36 days, 40 days, and 27 years for blood, soft tissues, and bones, respectively. After binding to metallothionein, Pb accumulates in the liver and kidney (particularly in the cortex). However, after long term exposure Pb also accumulates in the bones by co-precipitating with calcium. It accumulates predominantly in the cortical bone, where it persists for years without substantially change concentrations in the blood or other tissues (EFSA 2004). Importantly, Pb is highly toxic and may be associated with lethality in wild animal species (Mateo et al. 1998) or with sub-lethal toxicity (Souza et al. 2023), but there are no previous studies on Pb accumulation in rattlesnakes in Brazil. Environmental contamination with Pb is a consequence of its broad industrial use, including the oil industry, paint and dyes, ceramics, print, and military supplies (World Health Organization 2023). Therefore, the level of contamination is usually associated with human activity (Cabral-Pinto et al. 2020). Indeed, the levels of Pb in the soils tend to be higher close to highways in comparison to isolated areas, particularly where there is intensive mining, industrial activity, or use of sewage sludge as fertilizer (EFSA 2004).
Eighteen out of the 96 rattlesnakes included in this study had detectable levels of As (above the detection limit of 0.1 ppm). Two of those animals had high concentrations (9.4248 and 5.0208 ppm), whereas the remaining rattlesnakes (n = 16) had concentrations of As ranging from 0.0168 and 0.8152 ppm. There are many sources of As, including burning fossil fuels, metal casting, semiconductor and glass industries, and As may be an ingredient of many materials including wood preservatives, pigments, and herbicides (Hathaway et al. 1991). The use of As in agriculture (herbicides and fertilizers), use of sewage sludge as fertilizer, mining, and metal casting industries may lead to heavy contamination of soil, superficial and underground water, and plants (O’Neill 1990; Smedley et al. 1996; Smedley and Kinniburgh 2002; Postma et al. 2007; EFSA 2009). Hopkins et al. (1999) studied concentrations of As in the liver of water snakes (Nerodia fasciata) from the Savannah River (USA) in an area close to a coal combustion plant, and found levels of 135 ppm in average, which are much higher than those detected in this study. In contrast, Burger et al. (2007) measured bioaccumulation of As in a different species of water snake (Nerodia sipedon) from the states of New Jersey, Tennessee and South Carolina, and found much lower levels in the kidney and liver: 0.089 ± 0.019 ppm and 0.093 ± 0.028 ppm, respectively.
Cr levels in tissues of rattlesnakes were 0.66 ± 2.14 ppm in this study. Campbell et al. (2005) measured concentrations of Cr in water snakes (Nerodia sipedon) from two different areas in the State of Tennessee (USA) and found concentrations of 0.0314 ± 0.0112 and 0.0273 ± 0.0086 ppm in the liver and kidneys, respectively, in one of the areas, and 0.0843 ± 0.0248 and 0.0530 ± 0.0249 ppm in the other area. Hopkins et al. (1999) found hepatic levels of Cr ranging from 0.3 to 0.7 ppm in banded water snakes (N. fasciata) from an area near to a coal combustion plant.
Average concentration of Cu in rattlesnakes in this study was 3.27 ± 3.14 ppm, which is similar to levels previously reported in marine snakes (Lapemis curtus) (Sereshk and Bakhtiari 2015). It is unlikely that the levels of Cu found in rattlesnakes in this study are associated to pollution since this is an essential element, required for good health and for a normal immune system (Yadollahvand et al. 2014). However, the effects of Cu on the immune system of rattlesnakes are poorly known, but there are a few studies assessing accumulation of Cu in tissues. Hopkins et al. (2001) reported higher levels of Cu in the liver, whereas Sereshk and Bakhtiari (2015) found higher levels in the kidney.
Concentration of Zn in the liver and kidneys of rattlesnakes in this study were 27.44 ± 20.75 ppm, which is lower than that reported in marine snakes (L. curtus) as reported by Sereshk and Bakhtiari (2015). Frossard et al. (2017) detected Zn concentrations ranging from 12.773 to 28.418 ppm and 10.395 to 74.778 ppm in Boa constrictor and Bothrops jararaca from Brazil, respectively, which are levels that are similar to the ones detected in rattlesnakes in this study. There are several sources of Zn, including mineral or organic fertilizers (Kiekens 1990), which may result in increased concentrations in the soil (Ramalho et al. 2000), favoring transfer of this element through the food chain (Oliveira et al. 1999).
Rattlesnakes had average levels of manganese of 776.14 ± 3338.00 ppm. Lower levels of manganese (1.39 ppm) have been reported in giant snakes (Thamnophis gigas) from Sacramento Valley (California, USA) (Wylie et al. 2009). Lower manganese levels have also been reported in water snakes from Tennessee (1.70 ppm) and from the Savannah River in New Jersey, Tennessee and South Carolina (2.17 ppm) (Campbell et al. 2005; Burger et al. 2007).
Hematological and biochemical analyses demonstrated that bioaccumulation of metals assessed in this study, particularly As, Mg, and Pb, had significant negative correlations with blood cells. Furthermore, tissue concentrations of Cd, Cu, Mg, Pb, and Zn correlated with various blood biochemistry parameters. Although these findings do not support any conclusion in terms of cause and effect relationship, these results support the hypothesis that toxic levels of these metals may interfere with hematopoiesis in rattlesnakes. Previous studies established hematological and biochemical reference values for captive rattlesnakes (Troiano et al. 1997, 2001). However, due to the numerous factors that could interfere with those parameters in free-ranging rattlesnakes, including infectious and parasitic diseases (Toledo et al. 2022), as well as the difficulty for establishing thresholds for toxic concentrations of these metals in this species, the analysis of absolute hematological and biochemical values were considered out of the scope of this study.
Intense anthropic activities currently affects most if not all wild environments, which favors contamination of soil, water, plants, and animals with heavy metals. Therefore, monitoring bioaccumulation in areas with variable environmental features is becoming increasingly relevant. In this context, considering their position in the food chain, rattlesnakes may represent highly relevant biological sentinels for environmental pollution, particularly by heavy metals. The use of rattlesnakes as exposure biomarkers may be an important tool for monitoring anthropic actions in the terrestrial environment. Monitoring the bioaccumulation of heavy metals in these animals can serve as a reference for comparative studies between different habitats or species, or even as a starting point for monitoring the progression of contamination in an ecosystem at different time intervals. In addition, further studies are needed to understand the effects of heavy metal bioaccumulation on the health and well-being of these animals, which under pathological conditions can compromise the entire cycle of the food chain, consequently affecting environmental and human health.
In conclusion, this study demonstrated a broad range of bioaccumulation of both toxic and essential metals in free-ranging rattlesnakes. Predisposition for bioaccumulation according to age, size, and sex were demonstrated as well as correlations between levels of metals in tissues and hematological or blood biochemical changes. Therefore, considering the position of rattlesnakes in the wildlife food chain this study provided relevant baseline data that will support their function as highly relevant biological sentinels for environmental pollution.
Data availability
Raw data are available from the corresponding author on reasonable request.
References
Al-Yousuf MH, El-Shahawi MS, Al-Ghais SM (2000) Trace elements in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci Total Environ 256:87–94
Bastos EGM, Araújo AFB, Silva HR (2005) Records of the rattlesnakes Crotalus durissus terrificus (Laurenti) (Serpentes, Viperidae) in the State of Rio de Janeiro, Brazil: a possible case of invasion facilitated by deforestation. Rev Bras Zool 22:812–815
Bergkvist P, Jarvis N, Berggren D, Carlgren K (2003) Long-term effects of sewage sludge applications on soil properties, cadmium availability and distribution in arable soil. Agric Ecosyst Environm 97:167–179
Bosso ST, Enzweiler J (2008) Ensaios para determinar a (Bio)disponibilidade de chumbo em solos contaminados: revisão. Quim Nova 31(2):394–400
Buch AC, Niemeyer JC, Marques ED, Silva-Filho EV (2021) Ecological risk assessment of trace metals in soils affected by mine tailings. J Hazard Mater 403:123852
Burger J (1992) Trace element levels in pine snake hatchlings: tissue and temporal differences. Arch Environ Contam Toxicol 22:209–213
Burger J, Gochfeld M (2016) Habitat, population dynamics and metal concentrations in colonial waterbirds. CRC Press, Boca Raton
Burger J, Campbell KR, Campbell TS, Shukla T, Jeitner C, Ghochfeld M (2005) Use of skin and blood as nonlethal indicators of heavy metal contamination in northern water snake (Nerodia sipedon). Arch Environ Contam Toxicol 49:232–238
Burger J, Murray S, Gaines KF, Novak JM, Punshon T, Dixon C, Gochfeld M (2006) Element concentrations in snakes in South Carolina: differences between a control and exposed site on the Savannah River Site. Environ Monitor Assess 112:35052
Burger J, Campbell KR, Murray S, Campbell TS, Gaines KF, Jeitner C, Shukla T, Burke S, Gochfeld M (2007) Metal concentrations in blood, muscle, and liver of Water Snakes (Nerodia spp.) from New Jersey, Tennessee and South Carolina. Sci Total Environ 373:556–563
Burger J, Gochfeld M, Jeitner C, Zappalorti R, Pittfield T, Devito E (2017) Arsenic, cadmium, chromium, lead, mercury and selenium concentrations in Pine Snakes (Pituophis melanoleucus) from the New Jersey Pine Barrens. Arch Environ Contam Toxicol 72(4):586–595
Cabral-Pinto MMS, Inácio M, Neves O, Almeida AA, Pinto E, Oliveiros B, Ferreira da Silva EAF (2020) Human health risk assessment due to agricultural activities and crop consumption in the surroundings of an industrial area. Expo Health 12:629–640
Campbell KR, Campbell TS (2002) A logical starting point for developing priorities for lizard and snake ecotoxicology: a review of available data. Environ Toxicol Chem 21:894–898
Campbell KR, Campbell TS, Burger J (2005) Heavy metal concentrations in northern water snakes (Nerodia sipedon) from East Fork Poplar Creek and the Little River, East Tennessee, USA. Arch Environ Contam Toxicol 49(2):239–248
Celik E, Durmus A, Adizel O, Nergiz Uyar H (2021) A bibliometric analysis: what do we know about metals(loids) accumulation in wild birds? Environ Sci Pollut Res Int 28(8):10302–10334
Davila RB, Fontes MPF, Pacheco AA, Ferreira MDS (2020) Heavy metals in iron ore tailings and floodplain soils affected by the Samarco dam collapse in Brazil. Sci Total Environ 709:136151
Delany MR, Bell JU, Sundlof SR (1988) Concentrations of contaminants in muscle of the American alligator in Florida. Wildl Dis 24:62–66
Drewett DV, Wilson JD, Cristol DA, Chin SY, Hopkins WA (2013) Inter- and intraspecific variation in mercury bioaccumulation by snakes inhabiting a contaminated river floodplain. Environ Toxicol Chem 32:1178–1186
Ebrahimi-Sirizi Z, Riyahi-Bakhtiyari A (2013) Petroleum pollution in mangrove forests sediments from Qeshm Island and Khamir PortPersian Gulf, Iran. Environ Monit Assess 185(5):4019–4032
EFSA (European Food Safety Authority) (2004) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to lead as undesirable substance in animal feed. EFSA J 71:20
EFSA (European Food Safety Authority) (2009) Scientific opinion on arsenic in food. Panel on contaminants in the food chain (CONTAM). EFSA J 1351:198
Eghtesadi-Araghi P, Farzadnia S (2011) Petroleum inputs to the Persian Gulf. Res J Environ Sci 5:134–141
Emara HI (1990) Oil pollution in the southern Persian Gulf and Gulf of Oman. Mar Pollut Bull 21:399–401
Frossard A, Carneiro MTWD, Silva ELF, Camargo Filho CB, Rossi Júnior JL (2017) Concentração de elementos traços em serpentes do litoral e da região serrana do Espírito Santo. Pesq Vet Bras 37(10):1146–1152
Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville T, Metts BS, Greene JL, Leiden Y, Poppy S, Winne CT (2000) The global decline of reptiles, déjà vu amphibians. Bio Sci 50(8):653–666
Guvvala PR, Ravindra JP, Selvaraju S (2020) Impact of environmental contaminants on reproductive health of male domestic ruminants: a review. Environ Sci Pollut Res Int 27(4):3819–3836
Hathaway GJ, Proctor NH, Hughes JP et al (1991) Arsenic and Arsine. In: Proctor NH, Hughe SJP (eds) Chemical hazards of the workplace, 3rd edn. Van Nostrand Reinhold, New York, pp 92–96
Himeno S, Sumi D, Fujishiro H (2019) Toxicometallomics of cadmium, manganese and arsenic with special reference to the roles of metal transporters. Toxicol Res 35(4):311–317
Hopkins WA, Rowe CL, Congdon JS (1999) Elevated trace element concentrations and standard metabolic rate in banded Water Snake (Nerodia fusciata) exposed to coal combustion wastes. Environ Toxicol Chem 18:1258–1263
Hopkins WA, Roe JH, Snodgrass JW, Jackson BP, Kling DE, Rowe CL, Congdon JD (2001) Nondestructive indices of trace element exposure in squamata reptiles. Environ Pollut 115:1–7
Hopkins WA, Roe JH, Snodgrass JW, Staub BP, Jackson BP, Congdon JD (2002) Effects of chronic dietary exposure to trace elements on banded water snakes (Nerodia fasciata). Environ Toxicol Chem 21:906–913
Hopkins WA, Staub BP, Baionno JA, Jackson BP, Roe JH, Ford NB (2004) Trophic and maternal transfer of selenium in brown house snakes (Lamprophis fuliginosus). Ecotoxicol Environ Safety 58:285–293
Jones DE, Holladay SD (2006) Excretion of three heavy metals in the shed skins of exposed corn snakes (Elaphe guttata). Ecotoxicol Environ Saf 64:221–225
Kiekens L (1990) Zinc, p.261–277. In: Alloway BJ (ed) Heavy metals in soils. Blackie and Son, Glasgow
Koh TS, Judson GJ (1986) Trace elements in sheep grazing near a lead-zinc smelting complex at Port Pirie, South Australia. Bull Envirom Contam Toxicol 37:87–95
Kubier A, Wilkin RT, Pichler T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:1–16
Marquez-Ferrando R, Pleguezuelos JM, Ontiveros D (2009) Bioaccumulation of heavy metals in lizard Psammodromus algirus after a tailing-dam collapse in Axnalcollar (southwest Spain). Arch Environ Contamin Toxicol 56:276–285
Marin AR, Pezeshki SR, Masschelen PH, Choi HS (1993) Effect of dimethy larsenic acid (DMAA) on growth, tissue arsenic, and photosynthesis of rice plants. J Plant Nutr 16(5):865–880. https://doi.org/10.1080/01904169309364580
Mashroofeh A, Riyahi BA, Pourkazemi M (2012) Bioaccumulation of Zn, Cu and Mn in the caviar and muscle of Persian sturgeon (Acipenser persicus) from the Caspian Sea, Iran. Bull Environ Contam Toxicol 89:1201–1204
Mashroofeh A, Riyahi BA, Pourkazemi M, Rasouli S (2013) Bioaccumulation of Cd, Pb and Zn in the edible and inedible tissues of three sturgeon species in the Iranian coastline of the Caspian Sea. Chemosphere 90:573–580
Mateo R, Belliure J, Dolz JC, Aguilar Serrano JM, Guitart R (1998) High prevalences of lead poisoning in wintering waterfowl in Spain. Arch Environ Contam Toxicol 35(2):342–347
O’Neill P (1990) Arsenic. In: Alloway BJ (ed) Heavy metals in soils. Blackie and Sons, Glasgow, pp 83–99
Oliveira TS, Costa LM, Cruz CD, Horn HA (1999) Metais pesados como indicadores de materiais de origem em uma topolitosequência do triângulo mineiro, estado de Minas Gerais. Pesq Agropec Bras 34:1451–1465
Pinho FMO, Pereira ID (2001) Ofidismo. Rev Assoc Méd Bras 47(1):24–29
Postma D, Larse F, Hue NTM, Duc MT, Viet PH, Nhan PQ, Jessen S (2007) Arsenic in groundwater of the Red River floodplain, Vietnam: controlling geochemical processes and reactive transport modeling. Geochim Cosmochim Acta 71:5054–5071
Quadra GR, Lino A, Sobek A, Malm O, Barros N, Guida Y, Thomaz J, Mendonça R, Cardoso S, Estrada C, Rust F, Roland F (2019) Environmental risk of metal contamination in sediments of tropical reservoirs. Bull Environ Contam Toxicol 103(2):292–301
Rabinowitz MB (1991) Toxicokinetics of bone lead. Environ Health Perspect 91:33–37
Rabinowitz MB, Wetherill GW, Kopple JD (1976) Kinetic analysis of lead metabolism in healthy humans. J Clin Invest 58(2):260–270
Ramalho JF, Garcia P, Amaral S, Nelson MBV (2000) Contaminação da microbacia de Caetés com metais pesados pelo uso de agroquímicos. Pesq Agropec Bras 35(7):1289–1303
Sereshk ZH, Bakhtiari AR (2015) Concentrations of trace elements in the kidney, liver, muscle, and skin of short sea snake (Lapemis curtus) from the Strait of Hormuz Persian Gulf. Environ Sci Pollut Res 22:15781–15787
Shiek SS, Mani MS, Kabekkodu SP, Dsouza HS (2021) Health repercussions of environmental exposure to lead: methylation perspective. Toxicology 461:152927
Shirani M, Mirvaghefi A, Farahmand H, Abdollahi M (2012) Biomarker responses in mudskipper (Periophthalmus waltoni) from the coastal areas of the Persian Gulf with oil pollution. Environ Toxicol Pharm 34(3):705–713
Smedley PL, Kinniburgh DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smedley PL, Edmunds WM, Pelig-Ba KB (1996) Mobility of arsenic in groundwater in the Obuasi gold-mining area of Ghana: some implications for human health. In: Appleton JD, Fuge R, Mccall GJH (eds) Environmental geochemistry and health. Geological Society special publication. Chapman and Hall, New York, pp 163–181
Souza LR, Oliveira AR, Santos DO, Carvalho TP, Santana CH, Pimentel SP, Vasconcelos IMA, Coelho CM, Tinoco HP, Ribeiro ATPG, Silva ROS, Costa EA, Melo MM, Paixão TA, Santos RL (2023) Chronic lead intoxication in a jaguar (Panthera onca) shot with round lead pellets - case report. Arq Bras Med Vet Zootec 75:696–702
Spierenburg TJ, De Graaf GJ, Baars AJ, Brus DH, Tielen MJ, Arts BJ (1988) Cadmium, zinc, lead and copper in livers and kidneys of cattle in the neighborhood of zinc refineries. Environ Monit Assess 11:107–114
Toledo FAO, Alves PV, Vasconcelos IMA, Oliveira AR, Santos DO, Cabral JAG, Toledo RAR, Pinto HA, Cunha PHH, PaesLeme FO, Carvalho MPN, Paixão TA, Santos RL (2022) Parasitologic and pathologic study of free-ranging South American rattlesnakes (Crotalus durissus terrificus) in Brazil. J Zoo Wildl Med 53:515–527
Troiano JC, Vidal JC, Gould J, Gould E (1997) Haematological reference intervals of the South American rattlesnake (Crotalus durissus terrificus, Laurenti, 1768) in captivity. Comp Haematol Int 7:109–112
Troiano JC, Gould EG, Althaus R, Malinskas G, Gould JA, Heker J, Vidal JC, Amantini E, Simoncini C (2001) Blood biochemical profile of the South American rattlesnake (Crotalus durissus terrificus) in captivity. J Venom Anim Toxins 7:183–189
Uluozlu OD, Tuzen M, Mendil D, Soylak M (2007) Trace metal content in nine species of fish from the Black and Aegean seas, Turkey. Food Chem 104:835–840
World Health Organization (2023) Exposure to lead: a major public health concern, 3 edn. WHO. Available online: https://iris.who.int/bitstream/handle/10665/372293/9789240078130-eng.pdf?sequence=1. Accessed 20 Apr 2024
Wylie GD, Hothem RL, Bergen DR, Martin LL, Taylor RJ, Brussee BE (2009) Metals and trace elements in giant garter snakes (Thamnophis gigas) from the Sacramento Valley, California, USA. Arch Environ Contam Toxicol 56(3):577–587
Yadollahvand R, Kami HG, Mashroofeh A, Riyahi Bakhtiari A (2014) Assessment trace elements concentrations in tissues in Caspian Pond Turtle (Mauremys caspica) from Golestan Province, Iran. Ecotoxicol Environ Saf 101:191–195
Acknowledgements
Work in RLS lab is supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil), FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais, Brazil), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil). MM-N was funded by CNPQ (grant 313524/2021-1).
Funding
Work in RLS lab is supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil), FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais, Brazil), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil). MM-N was funded by CNPQ (grant 313524/2021–1).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Frank August de Oliveira Toledo, Daniel Oliveira dos Santos, Izabela Magalhães Arthuso Vasconcelos, Ayisa Rodrigues Oliveira, Juliana Araújo Gomes Cabral, Rômulo Antônio Righi de Toledo, Pedro Hugo Henriques Cunha, Diego Felipe Alves Batista. The first draft of the manuscript was written by Frank August de Oliveira Toledo and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study protocol was reviewed and approved by the institutional Ethics Committee on the Use of Animals of the Fundação Ezequiel Dias (FUNED, protocol 14/2019).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toledo, F.A.d.O., Santos, D.O., Vasconcelos, I.M.A. et al. Heavy metals bioaccumulation in free-ranging South American rattlesnakes (Crotalus durissus) in Southeastern Brazil. Environ Sci Pollut Res 31, 32339–32349 (2024). https://doi.org/10.1007/s11356-024-33432-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33432-5