Abstract
The concentrations of total polycyclic aromatic hydrocarbons (ƩPAHs) and 22 individual PAH compounds in 42 surface sediments collected from the mangrove forest of Qeshm Island and Khamir Port (Persian Gulf) were analyzed. ƩPAHs concentrations ranged from 259 to 5,376 ng g−1 dry weight with mean and median values of 1,585 and 1,146 ng g−1, respectively. The mangrove sediments had higher percentages of lower molecular weight PAHs and the PAH profiles were dominated by naphthalene. Ratio values of specific PAH compounds were calculated to evaluate the possible source of PAH contamination. This ratios suggesting that the mangrove sediments have a petrogenic input of PAHs. Sediment quality guidelines were conducted to assess the toxicity of PAH compounds. The levels of total PAHs at all of stations except one station, namely Q6, were below the effects range low. Also, concentrations of naphthalene in some stations exceeded the effects range median.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persian Gulf is one of the most important water pathways in the world. This gulf has been heavily developed for crude oil production, transportation, and exportation. It is well established that such activities result in contamination of the marine environment by petroleum and petroleum productions. This gulf represents a highly stressful environment due to prevailing natural conditions and development pressures along its coastline. The Gulf has approximately two thirds of the world’s proven oil reserves and currently account for approximately one fourth of the world oil production (Khan et al. 2002). This gulf is greatly intensified by oil spills, and accidental discharges of various magnitudes have been reported in the region (Linden et al. 1990). Thus, it is expected that oil pollution is distributed on the Persian region and has seriously endangered the marine ecosystems (Behairy and Saad 1982; Rushdi et al. 1991). The enclosed and shallow nature of the Gulf makes it especially subject to accumulation of contaminants. Because of the narrow exchange through the Strait of Hormuz into the Gulf of Oman, the time needed for all Gulf water to come within the influence of the open sea is about 3–5.5 years (Sheppard 1993). The Gulf suffers from chronic oil pollution associated with local exploration, refining, discharge of tanker ballast water as well as from natural seeps and wars (Zarba et al. 1985).
One of the most valuable of Persian Gulf ecosystems are mangrove forests. Mangroves are perhaps the dominant and most important intertidal habitat along tropical and subtropical coastlines and estuaries. These rich ecosystems are located in areas of high risk of acute or chronic petroleum pollution. Further, high levels of hydrocarbons may be expected to remain in mangrove sediments after occurrence petroleum contamination because these environments are not favorable for hydrocarbon depletion by sediment transport or degradation by bacteria. These zones are critical areas where valuable biological resources and rich biodiversity are threatened. These regions are continually exposed to anthropogenic contamination of polycyclic aromatic hydrocarbons (PAHs) from different sources (Lu and Lin 1990; Klekowski et al. 1994). Mangrove’s unique features such as high productivity, abundant detritus, and rich organic carbon may make it an advantageous site for uptake and preservation of PAHs (Bernard et al. 1996).
PAHs are widespread contaminants throughout nature (Vilanova et al. 2001). Due to toxic, carcinogenic, and mutagenic properties of some these compounds, they are listed by the United States Environmental Protection Agency and the European Community as priority pollutants (Wenzel et al. 2006).
PAHs introduced to the environment are present in complex mixtures. Two classes of PAHs can be recognized based on their properties and molecular weight. First, PAHs with two or three aromatic rings are named low molecular weight, and secondly PAHs with four to six benzene rings are named high molecular weight. The first class has an acute toxicity while some of the high molecular weight PAHs shows high carcinogenic and mutagenic potential. Because of their hydrophobic and persistent nature, they accumulate in sediments. Therefore, many studies have examined PAHs contamination in coastal and oceanic sediments (Boonyatumanond et al. 2006; Zakaria and Mahat 2006; Ibrahim 2004).
The studies of distribution and concentration of PAHs were mainly performed in marine water and sediments (Boonyatumanond et al. 2006). Studies that have been carried out to assess the distribution and accumulation of PAHs in mangrove ecosystems are scarce. Therefore, the primary objective of this study is to investigate PAH contamination in the mangrove forests of Qeshm Island and Khamir Port (Persian Gulf), identify possible PAH sources in area, and evaluate potential toxicological impacts using biological thresholds.
Materials and methods
Study area
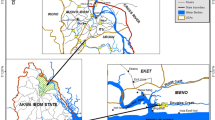
Qeshm is the biggest island in the Persian Gulf. This island is located in the Strait of Hormuz into the Gulf of Oman. This regain have high temperature and negligible precipitation. The sediment of these forests is muddy with high salinity water. Only two species of mangrove are found within Iranian mangrove forests, Avicennia marina and Rhizophora macrunata, and in the Hara Protected Area, A. marina species is dominant. Daily, many tankers and ships that transport oil production of Persian Gulf throughout the world cross near this Island. Therefore, the island and mangrove forests which are located in the northwest are continually subjected to oil contamination.
Sample collection
During April 2010, 42 surface sediment samples (0–5 cm) were collected from the mangrove forest of Qeshm Island and Khamir Port (Fig. 1). The global position of each station is given in Table 1. Sampling was conducted during low tide and no water overlying the sediments. Surface sediment samples were collected by using a stainless steel spoon and then transferred into a stainless steel container to reduce any contamination. The containers were labeled and placed in an icebox at 4 °C then transported to the laboratory for further analysis. The samples were stored in a cold room (−20 °C) until further analysis.
Sample preparation
The collected sediment samples were freeze dried for 3 days to get rid of any water contained in the samples. After the samples were freeze dried, they were then stored frozen before proceeding with the analysis of the PAHs.
PAHs analysis
The samples were purified and fractionated by the method described elsewhere (Zakaria et al. 2002). Briefly, about 10 g of each sample (dry weight) was used for extraction. The samples were extracted with a Soxhlet extractor using 300 ml dichloromethane for 8 h. After Soxhlet extraction, 100 μl of deuterated PAH surrogate internal standard mixture (naphthalene-d8, anthracene-d10, chrysene-d12, and perylene-d12) was added to the samples for quality control of PAHs analyses. In order to eliminate any sulfur in the samples, a few copper chips were added into the samples and left overnight.
Volume of the solvents was reduced using a rotary evaporator to approximately 1 ml. In order to avoid loss of samples by volatilization, the temperature of the water bath kept at 40 °C.
The extracts were transferred onto the top of a 5 % H2O-deactivated silica gel column. The column internal diameter in this step was 0.9 mm and the 5 % H2O-deactivated silica gel was added until a height of 9 cm. The column was rinsed with 20 ml dichloromethane/hexane (1:3, v/v). In this step, most of the organic pollutants including n-alkanes, PCBs, LABs, and PAHs were separated from other polar compounds. The eluted sample was reduced in volume by a rotary evaporator for the second step of column chromatography.
Fully activated silica gel was used in the second step column chromatography. The column internal diameter in this step was 4.7 mm and the height of activated silica gel was 18 cm. To get PAHs fraction, 14 ml dichloromethane/hexane (1:3, v/v) was used. PAHs fraction was evaporated to approximately 1 ml then transferred to a glass ampoule and evaporated to dryness under gentle stream of nitrogen and re-dissolved into 100 μl p-terphenyl-d14 as an internal injection standard for PAHs.
PAHs was analyzed by gas chromatography–mass spectrometry using an Agilent Technologies 5975C quadrupole mass spectrometer coupled with an Agilent 7890A gas chromatograph. A 30-m fused silica capillary column was used with 0.25-μm internal diameter and 0.25-μm film thickness; helium was used as carrier gas in the analysis. The injection port was maintained at 310 °C and the sample was injected in the splitless mode followed by a purge 1 min after the injection. The column temperature was held at 70 °C for 2 min, programmed at 30 °C/min to 150 °C, and then 4 °C/min to 310 °C and held for 10 min (Zakaria et al. 2002; Bakhtiari et al. 2009).
After a delay of 3 min, a selected ion monitoring method was employed. PAHs were monitored at m/z = 128 (naphthalene), m/z = 152 (acenaphtylene), m/z = 154 (acenaphthene), m/z = 166 (fluorene), m/z = 178 (phenanthrene, anthracene), m/z = 192 (3-methylphenanthrene, 2-methylphenanthrene, 2-methylanthracene, 9-methylphenanthrene, 1-methylphenanthrene), m/z = 202 (fluoranthene, pyrene), m/z = 228 (chrysene, benz[a]anthracene), m/z = 252 (benzo[k]fluoranthene, benzo[b]fluoranthene, benzo[a]pyrene, perylene), m/z = 276 (indeno[1,2,3-cd]pyrene, benzo[ghi]perylene), and m/z = 278 (dibenz[a,h]anthracene).
Total organic carbon analysis
The total organic carbon (TOC) content of samples was measured following the wet digestion methods of Gao et al. (2005). Briefly, in this method 1–2 g dry weight of samples was oxidized with acidified (concentrated sulfuric acid) dichromate (K2Cr2O7) followed by titration with ferrous sulfate (FeSO4).
Analytical quality control
The losses of PAHs during processing the samples were estimated by deuterated PAHs that spiked to all samples. Average recovery ranged between 73 % and 95 %. Concentration of PAH compounds was corrected with the recoveries. In order to analytically quality control the procedure, blanks were run with each batch of samples. Limits of detection (0.2 ng/g) were estimated as three times the baseline of the measured chromatograms.
Result and discussions
Table 2 shows the composition of PAH compounds and total PAHs concentration of mangrove forest sediment samples in the Qeshm Island and Khamir Port (Persian Gulf). Total PAHs concentration ranged from 259 to 5,376 ng g−1 dry weight. Most paired stations such as 36–39, 3–5, 20–21, 31–32, etc. appear to have similar total PAHs because these stations were very near together (about 500 m), but total PAHs in Q4 and Q6 were magnitudes apart because in Q6 we observed a point source of oil pollution.
Correlation of PAHs with TOC
It has been demonstrated that the chemical composition of the sediment such as organic matter and clay content influences the distribution and concentration of PAHs (Kim et al. 1999; Witt 1995; Wang et al. 2001). In other words, sediments with high organic carbon content were characterized with high values of PAHs (Witt 1995; Yang 2000). However, in the present study, no correlation was found between ƩPAHs and organic matter concentration (Fig. 2). This non-affinity between organic matter and concentration of PAHs has also been reported by previous workers (Raoux and Garrigues 1991; Coakley et al. 1993). These studies suggested that the distribution and concentrations of PAHs in sediments would be determined more by direct input, rather than by the type of sediment. Moreover, Simpson et al. (1996) demonstrated that the relationship between total PAHs and organic carbon was only significant for highly polluted places where total PAHs concentration was greater than 2,000 ng g−1.
Assessment of sediment quality using biological thresholds
In order to make an assessment of aquatic sediment with a ranking of low to high impact values, the effects range low (ERL) and the effects range median (ERM) values were used (Long et al. 1995). The measured concentrations of PAHs were compared with the ERL and ERM levels. Table 3 lists the thresholds for sediments quality guideline and values obtained from this study. Total PAH concentrations at all stations, except one that is located in the harbor (Q6), had total concentrations lower than the ERL value (4,000 ng g−1) suggested by Long et al. (1995). They reported that the concentrations below the ERL value represent a negligible effect range, i.e., adverse biological effect would seldom be discovered below the ERL. On the other hand, adverse effects on biological systems will frequently occur if the concentration is higher than the ERM value (4,4792 ng g−1). The concentrations of individual PAH recorded in the present study ranged from non-detected level to 4,753 ng/g. Results showed that all of individual PAH except naphthalene had values below ERL, but concentrations of naphthalene in some stations such as Q2, Q6, Q10, Q29, Q30, Q31, Q32, Q33, Q35, Q39, and Q42 exceeded the ERM, which might need a more detailed study. Most of the stations in which the concentration of naphthalene exceeded the thresholds are located in the main mangrove channels where boat traffic occurs. Naphthalene and its alkyl derivatives are known as the most toxic petroleum compounds for marine organisms because of their medium to low boiling point (Anderson et al. 1974; Moore and Dwyer 1974), and in high concentrations they are known to provoke behavioral responses in organisms (Hargrave and Newcome 1973; Linden 1977; Nagarajah et al. 1985). Also, the low to moderate octanol–water partition coefficients of naphthalene (log K ow 3.01–3.45) suggest that this compound is moderately hydrophobic and may thus have a tendency to adsorb particulate matter and accumulate in biota (Bates et al. 1997). PAHs with log K ow below 5.2 such as naphthalene are considered to be directly toxic for marine environments due to their high water solubility (Sverdrup et al. 2002).
Uncertainty of sediment quality guidelines (SQGs)
There are many uncertainties in the application of biological threshold levels in this type of environmental assessment. The threshold levels set in this guideline has been based on one or a few species, which raises concerns when the results are extrapolated to diverse communities. This suggests that a complete set of SQGs with a comprehensive set of biological data should be used at each site. Furthermore, sample collection, storage, and manipulation may alter the natural concentration of contaminants (Dennis et al. 2000). Also, a wide variety of factors influenced the bioavailability of sediment contaminants that are not considered in the current SQGs. These factors include pH, hardness, organic matter content, particle surface area and size, hardness, redox gradients, chemical time-of-contact (spatial and temporal dynamics), pore water convection, resuspension and deposition (flux), organism acclimation, and ingestion (Allen Burton 2002). In order to reduce these uncertainties, these impacts will be further evaluated and addressed as part of the ongoing research for this regain and the values used in combination with other sediment quality assessment tools including effects-based testing.
Profile PAHs
The dominant PAH compound in all stations was naphthalene (Fig. 3). Naphthalene, the simplest lower molecular weight PAH, accounted for 70–80 % of total PAHs in the present study. This is in good agreement with the finding of Wu et al. (1999) who examined the PAH profile in sediments of the northern part of the South China Sea. Naphthalene was also found at relatively high levels (Yang 2000) in the Beaufort Sea and Cook Inlet sediments (Venkatesan and Kaplan 1982); these previous studies indicated that naphthalene was derived mainly from anthropogenic activities especially from petrogenic sources. Naphthalene constitutes an important fraction of crude oils and petroleum products with lighter fractions. This compound can be used as an indicator of petrogenic source of PAH contamination (Sporstol et al. 1983).
Petrogenic versus pyrogenic sources
The sources of PAHs, whether from pyrolytic or from petrogenic origins, may be determined by ratios of individual PAH compounds based on properties in PAH composition and distribution pattern as a function of the emission source (Gschwend and Hites 1981; Colombo et al. 1989). The molecular patterns produced by each source, however, are like fingerprints, which make it possible to hypothesize which processes produce PAHs. Pyrolytic sources are distinguished by the presence of PAHs over a wide range of molecular weights, while petrogenic sources are dominated by the lowest molecular weight PAH compounds (Culotta et al. 2006). If PAH compounds are separated into different classes depending on the number of rings present in their structure, it can be observed that the PAHs with two or three aromatic rings in the present study were found at high levels.
In order to source identification of PAHs, ratio values such as fluoranthene/pyrene (Flu/Pyr) and phenanthrene/anthracene (Phe/Ant) are widely employed as characteristic tools (Benlahcen et al. 1997; Baumard et al. 1998; Tam et al. 2001; Yim et al. 2005). These ratios had been employed by previous workers (Sicre et al. 1987; Budzinski et al. 1997; Soclo et al. 2000). Due to differences in the kinetic characteristics and thermodynamic properties of some PAHs, it is possible to exactly establish the source of the compounds in the environment (Benlahcen et al. 1997; Baumard et al. 1998). Processes involving high temperatures, such as the combustion of organic material (pyrogenic process), usually release PAHs with Phe/Ant ratios <10, while the slow maturation of the organic material during catagenesis (petrogenic process) leads to Phe/Ant ratios >10 (Benlahcen et al. 1997; Baumard et al. 1998). Motor vehicle exhaust had a Phe/Ant ratio of around four, and crude oil had a ratio of around 50 (Yang et al. 1991). Low Phe/Ant ratio values (range from 4 to 10) suggested that the main PAHs source were from combustion of fossil fuel (Gschwend and Hites 1981; Colombo et al. 1989).
Budzinski et al. (1997) suggested that sediments with Phe/Ant >10 were mainly contaminated by petrogenic sources and Phe/Ant <10 was typical of pyrolytic inputs. In the present study, large Phe/Ant ratios (>10) were found in sediments, indicating that the major source in this regain is petrogenic.
Similarly, Flr/Pyr ratios >1 and <1 indicate pyrogenic and petrogenic sources, respectively (Benlahcen et al. 1997; Baumard et al. 1998). Crude oil and fuel oil had Flu/Pyr ratios of 0.6–0.9, while combustion of coal and wood gave values of 1.4 and 1, respectively (Gschwend and Hites 1981). Mangrove sediments from this study had values less than 1. The small Flu/Pyr ratio values suggest strong petrogenic input. In order to provide a good estimate of PAH sources, Budzinski et al. (1997) plotted Phe/Ant against Flu/Pyr and defined two zones from the plot: one is characteristic of pyrolytic PAHs and the other of a petrogenic origin. In this study, sediment samples with Phe/Ant >10 and Flu/Pyr <1 showed strong petrogenic origin (Fig. 4).
The benzo[a]anthracene/chrysene (BaA/Chr) ratio has also been suggested to identify PAH origins; this ratio tended to increase as petrogenic contribution decreased (Colombo et al. 1989). The ratio values for crude and fuel oil ranged from 0.24 to 0.40 (Gschwend and Hites 1981). The mangrove sediments in this study had very small values, around 0.05 to 0.36, suggesting that the main source of PAH contamination in mangrove sediment came from crude and fuel oil. Also, Getter et al. (1981) stated that mangrove is a coastal ecosystem regularly polluted by accidental oil spills.
PAHs of molecular mass 178 and 202 are widely used to identify between petrogenic and pyrogenic sources (Gschwend and Hites 1981; Sicre et al. 1987; Budzinski et al. 1997; Soclo et al. 2000). For mass 178, an anthracene to anthracene plus phenanthrene (An/An+Ph) ratio >0.10 usually is taken as an indication of combustion while a ratio <0.10 indicates a dominance of petroleum (Budzinski et al. 1997).
Another molecular ratio that has been suggested to characterize the source of PAHs is fluoranthene to fluoranthene plus pyrene (Fl/Fl+Py). The value is 0.40 for this ratio, specified as the petrogenic/pyrogenic transition point (Yunker et al. 2002). Most petroleum samples have Fl/Fl+Pyr ratio below 0.40 while those of most combustion produced PAHs are above 0.40. In addition, the ratio is further parted into two regains: a ratio between 0.40 and 0.50 is more characteristic of liquid fossil fuel combustion, while a ratio greater than 0.50 is characteristic of biomass and coal combustion (Zhang et al. 2004).
Among PAHs with molecular mass of 228, the ratio of benzo[a]anthracene to the sum of benzo[a]anthracene and chrysene, BaA/BaA+Chr is also declarative of the PAHs sources. Values lower than 0.20 for this ratio suggest a petrogenic source, whereas values from 0.20 to 0.35 indicate a petroleum or combustion source, and values higher than 0.35 signify a combustion source. Likewise, petrogenic sources may be identified by a ratio of indeno[1,2,3-cd] pyrene to the sum of indeno[1,2,3-cd] pyrene and benzo[g,h,i] perylene, IP/(IP+Bghi), lower than 0.20. A ratio between 0.20 and 0.50 may suggest liquid fossil fuel combustion, and a ratio higher than 0.50 indicates biomass and coal combustion (Chen et al. 2005).
The samples were also calculated using methylphenanthrene/phenanthrene (MP/P) ratio to determine the source of PAHs. The value of <1 is the combustion sources and >1 consists of petroleum sources (Zakaria and Mahat 2006). In the present study, most of the samples had MP/P ratio high than 1; this indicates that sources of PAHs in this mangrove forest are from petrogenic input.
In order to provide a good estimate of PAH sources, Yunker et al. (2002) plotted some of ratios against another and defined petrogenic and combustion zone for each plot. Table 4 and Fig. 5 show some of these ratios.
PAH cross-plots for the ratios of a Flu/(Flu+Pyr) vs. An/(An+Ph), b Flu/(Flu+Pyr) vs. IP/(IP+Bghi), c Flu/(Flu+Pyr) vs. BaA/(BaA+Chr), d An/(An+Ph) vs. IP/(IP+Bghi), e An/(An+Ph) vs. BaA/(BaA+Chr), and f BaA/(BaA+Chr) vs. IP/(IP+Bghi) in mangrove forest of Qeshm Island and Khamir Port (Persian Gulf)
Perylene (Per) is a PAH with five benzene rings and isomers of BbF, BkF, and BaP (C20H12). The origin of this compound is still debatable (Silliman et al. 1998, 2000, 2001; Jiang et al. 2000). Low concentrations of this compound are generated during the burning of fossil fuel and released into the atmosphere (Silliman et al. 1998). Furthermore, Per is absent in the water column and only found in sediments (Silliman et al. 1998).
Generally, PAH levels in contaminated sites are highest in sediments that were recently deposited. However, for Per, the levels increase toward the deep sediments. This suggests that perylene is not transported and deposited in the sediment like other PAHs, but created after deposition from anaerobic diagenesis of organic matter (Silliman et al. 1998, 2000, 2001; Jiang et al. 2000). Silliman et al. (2000) described that Per concentrations in a sediment core from Lake Ontario (Canada) significantly increased under the bioturbation zone. Anoxic conditions are the principal obligatory for the formation of Per in the sediments (Silliman et al. 1998, 2000, 2001; Jiang et al. 2000). Furthermore, some of the organisms can produce perylene in high levels. For example, Bakhtiari et al. (2009) reported that termites on woody plants in tropical environment produce large amounts of this compound.
The %Per is the percentage of Per concentration relative to the total PAH concentrations. Values >10 % indicate natural sources for this compound (Baumard et al. 1998), while ratios of %Per to total concentration of PAHs with five benzene rings (%Per/ƩPAHs five rings) higher than 10 % suggest diagenetic processes (natural). For ratios lower than 10 %, an anthropogenic input is more probable (Tolosa et al. 2004). The %Per versus %Per/ƩPAHs five rings ratio for this study are plotted in Fig. 6. All sites showed anthropogenic inputs for this compound.
Conclusion
The available data for PAHs in many regions of the Persian Gulf are relatively distributed and generally indicate medium levels of PAH contamination comparative to other marine areas. This is in spite of heavy introduction of oil hydrocarbons to the Persian Gulf marine environment. In this study, no significant differences were observed between PAHs concentration in Qeshm Island and Khamir Port. This matter refers that oil pollution in Hara Protected Area came from the same origin and is not localized. The PAH profile reveals that the dominant PAHs were lower molecular weight and approximately all of the ratios used for source identification confirmed that the dominant origin in this regain is petrogenic input (Fig. 5). However, some stations showed a mixed source. This behavior was observed in other ecosystems especially near urban centers. Thus, when a primary source of PAHs was confirmed in one environment, another source cannot be completely discarded. In this study, some stations showed a mixed source; these stations that were outliers from the remainder were the same stations such as Q13, Q29, Q35, Q41, etc., but we did not observe any potential PAHs sources such as roads, ports, and so on in these stations.
In this study, the concentrations of total PAH and most individual PAH compounds were below the ERL guidelines, suggesting that PAHs accumulated in mangrove sediments were unlikely to cause any serious toxicity. However, periodic monitoring of persistent sediment-associated contaminations such as PAHs should be instituted as an indicator of environmental quality in this region.
References
Allen Burton, G. (2002). Sediment quality criteria in use around the world. Limnology, 3, 65–75.
Anderson, J. W., Neff, J. M., Cox, B. A., Tatem, N. E., & Hightower, C. M. (1974). Characteristics of dispersions and water soluble extracts of crude and refined oils and their toxicity to estuarine crustaceans and fish. Marine Biology, 27, 75–88.
Bakhtiari, A. R., Zakaria, M., Yaziz, M. I., Lajis, M. N. H., Bi, H., & Rahim, M. C. A. (2009). Vertical distribution and source identification of polycyclic aromatic hydrocarbons in anoxic sediment cores of Chini Lake, Malaysia: perylene as indicator of land plant-derived hydrocarbons. Applied Geochemistry, 24, 1777–1877.
Bates, K., Young, W., Sutton, A.. (1997). Proposed Environmental Quality Standards for Naphthalene in Water. Environment Agency R&D Technical Report P54.
Baumard, P., Budzinski, H., Michon, Q., Garrigues, P., Burgeot, T., & Bellocq, J. (1998). Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment records. Estuarine, Coastal and Shelf Science, 47, 77–90.
Behairy, A. K. A., & Saad, M. A. H. (1982). Effect of pollution on the coastal water of the Red Sea from Jeddah, Saudi Arabia. 1. Environmental conditions. Tethys, 2, 111–117.
Benlahcen, K. T., Chaoui, A., Budzinski, H., Bellocq, J., & Garrigues, P. (1997). Distribution and sources of polycyclic aromatic hydrocarbons in some Mediterranean coastal sediments. Marine Pollution Bulletin, 34, 298–316.
Bernard, D., Pascaline, H., & Jeremie, J. J. (1996). Distribution and origin of hydrocarbons in sediments from lagoons with fringing mangrove communities. Marine Pollution Bulletin, 32, 734–739.
Boonyatumanond, R., Wattayakorn, G., Togo, A., & Takada, H. (2006). Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine and marine sediments in Thailand. Marine Pollution Bulletin, 52, 942–950.
Budzinski, H., Jones, I., Bellocq, J., Pierard, C., & Garrigues, P. (1997). Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in The Gironde Estuary. Marine Chemistry, 58, 85–97.
Chen, L. G., Ran, Y., & Xing, B. S. (2005). Contents and sources of polycyclic aromatic hydrocarbons and organochlorine pesticides in vegetable soils of Guangzhou, China. Chemosphere, 60, 879–890.
Coakley, J., Nagy, E., & Serodes, J. (1993). Spatial and vertical trends in sediment-phase contaminants in the upper Estuary of the St Lawrence Rivers. Estuaries, 16, 653–669.
Colombo, j., Pelletier, E., Brochu, C., & Khalil, M. (1989). Determination of hydrocarbon sources using n-alkane and polyaromatic hydrocarbon distribution indexes. Case study: Rio de La Plata Estuary, Argentina. Environmental Science and Technology, 23, 888–894.
Culotta, L., Stefano, C. D., Gianguzza, A., Mannino, M. R., & Orecchio, S. (2006). The PAH composition of surface sediments from Stagnone coastal lagoon, Marsala (Italy). Marine Chemistry, 99, 117–127.
Dennis, J. M., DeGraeve, G. M., & Linton, T. K. (2000). Sediment quality guidelines and assessment: overview and research needs. Environmental Science and Policy, 3, 133–144.
Gao, Q. F., Cheung, K. L., Cheung, S. G., & Shin, P. (2005). Effects of nutrient enrichment derived from fish farming activities on macroinvertebrate assemblages in a subtropical region of Hong Kong. Marine Pollution Bulletin, 51, 994–1002.
Getter, C.D., Scott, G.I., Michel, J. (1981). The effects of oil spills on mangrove forests: a comparison on five oil spill sites in the Gulf of Mexico and the Caribbean Sea. In: Proceedings of the 1981 Oil Spill Conference. American Petroleum Institute, Washington DC, pp. 535–540.
Gschwend, P. M., & Hites, R. A. (1981). Fluxes of polycyclic aromatic hydrocarbons to marine and lacustrine sediments in the northeastern United States. Geochimica et Cosmochimica Acta, 45, 2359–2367.
Hargrave, B. T., & Newcome, B. T. (1973). Crawling and respiration as indices of sublethal effects of oil and dispersant on an intertidal snail Littorina litorea. Journal of the Fisheries Research Board of Canada, 30, 1789–1792.
Ibrahim, M. B. M. (2004). Levels and sources of polycyclic aromatic hydrocarbons in sediments from the Gulf of Suez. Marine Pollution Bulletin, 49, 356–367.
Jiang, C., Alexander, R., Kagi, R. I., & Murray, A. P. (2000). Origin of perylene in ancient sediments and its geological significance. Organic Geochemistry, 31, 1545–1559.
Khan, N. Y., Munawar, M., & Price, A. R. G. (2002). Physical and human geography in the gulf ecosystem. Health and sustainability (pp. 3–21). Leiden: Bakhuys.
Kim, G. B., Maruya, K. A., Lee, R. F., Lee, J. H., Koh, C. H., & Tanabe, S. S. (1999). Distribution and source of polycyclic aromatic hydrocarbons in sediments from Kyeonggi Bay, Korea. Marine Pollution Bulletin, 28, 7–15.
Klekowski, E. J., Jr., Corredor, J. E., Morell, J. M., & DelCastello, C. A. (1994). Petroleum pollution and mutation in mangroves. Marine Pollution Bulletin, 28, 166–169.
Linden, O. (1977). Sublethal effects of oil on mollusk species from the Baltic Sea. Water, Air, and Soil Pollution, 8, 305–313.
Linden, O., Abdulraheem, M., Gerges, M. A., Alam, I., Behbehani, M., Borhan, M. A., et al. (1990). Status of the marine environment in the ROPME sea area. UNEP Regional Sea Reports and Studies, 112(Rev. 1), 34.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Lu, C. Y., & Lin, P. (1990). Preliminary study on the method of monitoring coastal oil pollution by mangrove plants. Chinese Journal of Ecology, 9, 57–59.
Moore, S. F., & Dwyer, R. L. (1974). Effects of oil on marine organisms: a critical assessment of the published data. Water Research, 8, 819–828.
Nagarajah, R., Antonette-Sophia, A. J., & Balasubramanian, T. (1985). Behavior of some intertidal molluscs exposed to water soluble fractions of diesel. Marine Pollution Bulletin, 16, 67–271.
Raoux, C. Y., & Garrigues, P. (1991). Mechanics model of polycyclic aromatic hydrocarbons contamination of marine coastal sediments from the Mediterranean Sea. Polycyclic Aromatic Compounds Supplement, 3, 443–450.
Rushdi, A.I., Ba-lssa, A.A., Ba-Bagi, A. (1991). Preliminary investigations of oil pollution along the Red Sea Coast of Yemen. In Proceedings of the Seminar on the Status of the Environment in the Republic of Yemen, Sana’a, pp. 1–5.
Sheppard, C. R. C. (1993). Physical environment of the Gulf relevant to marine pollution: an overview. Marine Pollution Bulletin, 27, 3–8.
Sicre, M. A., Marty, J. C., Saliot, A., Aparicio, X., Grimalt, J., & Albaiges, J. (1987). Aliphatic and aromatic hydrocarbons in different sized aerosols over the Mediterranean Sea: occurrence and origin. Atmospheric Environment, 21, 2247–2259.
Silliman, J. E., Meyers, P. A., & Eadie, B. J. (1998). Perylene: an indicator of alteration processes or precursor materials? Organic Geochemistry, 29, 1737–1744.
Silliman, J. E., Meyers, P. A., Ostrom, P. H., Ostrom, N. E., & Eadie, B. J. (2000). Insights into the origin of perylene from isotopic analyses of sediments from Saanich Inlet, British Columbia. Organic Geochemistry, 31, 1133–1142.
Silliman, J. E., Meyers, P. A., Eadie, B. J., & Klump, J. V. (2001). A hypothesis for the origin of perylene based on its low abundance in sediments of Green Bay, Wisconsin. Chemical Geology, 177, 309–322.
Simpson, C. D., Mosi, A. A., Cullen, W. R., & Reimer, K. J. (1996). Composition and distribution of polycyclic aromatic hydrocarbons in surficial marine sediments from Kitimat Harbour, Canada. The Science of the Total Environment, 181, 265–278.
Soclo, H. H., Garrigues, P., & Ewald, M. (2000). Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal Marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Marine Pollution Bulletin, 40, 387–396.
Sporstol, S., Gjos, N., Lichtenthaler, R. G., Gustavsen, K. O., Urdal, k., Oreld, F., et al. (1983). Source identification of aromatic hydrocarbons in sediments using GC/MS. Environmental Science and Technology, 17, 282–286.
Sverdrup, L. E., Nielson, T., & Krogh, P. H. (2002). Soil ecotoxicity of polycyclic aromatic hydrocarbons in relation to soil sorption, lipophilicity, and water solubility. Environmental Science and Technology, 36, 2429–2435.
Tam, N. F. Y., Ke, L., Wang, X. H., & Wong, Y. S. (2001). Contamination of polycyclic aromatic hydrocarbons in surface sediments of mangrove swamps. Environmental Pollution, 114, 255–263.
Tolosa, I., de Mora, S., Sheikholeslami, M. R., Villeneuve, J. P., Bartocci, J., & Cattini, C. (2004). Aliphatic and aromatic hydrocarbons in coastal Caspian Sea sediments. Marine Pollution Bulletin, 48, 44–60.
Venkatesan, M. I., & Kaplan, I. R. (1982). Distribution and transport of hydrocarbons in surface sediments of the Alaskan outer continental shelf. Geochimica et Cosmochimica Acta, 46, 2135–2149.
Vilanova, R. M., Fernandez, P., Martinaz, C., & Grimalt, J. O. (2001). Polycyclic aromatic hydrocarbons in remote mountain lake waters. Water Research, 35(16), 3916–3926.
Wang, X. C., Zhang, Y. X., & Robert, F. C. (2001). Distribution and Partitioning of polycyclic aromatic hydrocarbons (PAHs) in different size fractions in sediments from Boston Harbor, United States. Marine Pollution Bulletin, 42, 1139–1149.
Wenzel, T., Simon, R., Kleiner, J., & Anklam, E. (2006). Analytical methods for polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. Analytical Chemistry, 25, 716–726.
Witt, G. (1995). Polycyclic aromatic hydrocarbons in water and sediment of the Baltic Sea. Marine Pollution Bulletin, 31, 237–248.
Wu, Y., Zhang, J., & Tang, Y. (1999). Geochemistry of n-alkanes and polycyclic aromatic hydrocarbons in the sediments from the South China Sea. Journal of Ocean University of Qingdao, 29, 112–120.
Yang, G. P. (2000). Polycyclic aromatic hydrocarbons in the sediments of the South China Sea. Environmental Pollution, 108, 163–171.
Yang, S. Y. N., Connell, D. W., Hawker, D. W., & Kayal, S. I. (1991). Polycyclic aromatic hydrocarbons in air, soil and vegetation in the vicinity of an urban roadway. The Science of the Total Environment, 102, 229–240.
Yim, U. H., Hong, S. H., Shim, W. J., Oh, J. R., & Chang, M. (2005). Spatio-temporal distribution and characteristics of PAHs in sediments from Masan Bay, Korea. Marine Pollution Bulletin, 50, 319–326.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33, 489–515.
Zakaria, M. P., & Mahat, A. A. (2006). Distribution of polycyclic aromatic hydrocarbons (PAHs) in sediments in the Langat Estuary. Coastal Marine Science, 30(1), 387–395.
Zakaria, M. P., Takada, H., Tsutsumi, S., Ohno, K., Yamada, J., Kouno, E., et al. (2002). Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environmental Science and Technology, 36, 1907–1918.
Zarba, M. A., Mohammad, O. S., Anderlini, V. C., Literathy, P., & Shunbo, F. (1985). Petroleum residues in surface sediments of Kuwait. Marine Pollution Bulletin, 16(5), 209–211.
Zhang, Z. L., Hong, H. S., Zhou, J. L., & Yu, G. (2004). Phase association of polycyclic aromatic hydrocarbons in the Minjiang River Estuary, China. The Science of the Total Environment, 323, 71–86.
Acknowledgments
The authors wish to thank Dr. Nader Bahramifar, Sanaz Ghaffari, Golshan Shirneshan, Samar Mortazavi, and Manzar Haghdoost who cooperated in all stages of this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebrahimi-Sirizi, Z., Riyahi-Bakhtiyari, A. Petroleum pollution in mangrove forests sediments from Qeshm Island and Khamir Port—Persian Gulf, Iran. Environ Monit Assess 185, 4019–4032 (2013). https://doi.org/10.1007/s10661-012-2846-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2846-z