Abstract
The giant garter snake (GGS; Thamnophis gigas) is a federally listed threatened species endemic to wetlands of the Central Valley of California. Habitat destruction has been the main factor in the decline of GGS populations, but the effects of contaminants on this species are unknown. To contribute to the recovery of these snakes, the U.S. Geological Survey (USGS) began studies of the life history and habitat use of GGSs in 1995. During a series of investigations conducted from 1995 to the present, specimens of dead GGSs were opportunistically collected from the Colusa National Wildlife Refuge (CNWR), the Natomas Basin, and other sites in northern California. Whole snakes were stored frozen for potential future analysis. As funding became available, we analyzed tissues of 23 GGSs to determine the concentrations of total mercury (Hg) and other trace elements in livers and concentrations of Hg in brains and tail clips. Mercury concentrations (μg/g, wet weight) ranged from 0.08 to 1.64 in livers, 0.01 to 0.18 in brains, and 0.02 to 0.32 in tail clips. In livers, geometric mean concentrations (μg/g, dry weight) of arsenic (25.7) and chromium (1.02) were higher than most values from studies of other snakes. Mercury concentrations in tail clips were positively correlated with concentrations in livers and brains, with the most significant correlations occurring at the Natomas Basin and when Natomas and CNWR were combined. Results indicate the value of using tail clips as a nonlethal bioindicator of contaminant concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The giant garter snake (GGS; Thamnophis gigas) is among the largest of the garter snakes endemic to California’s Central Valley. This highly aquatic species inhabits small ponds, sloughs, marshes, and rice fields, and forages mainly underwater for small fish, tadpoles, and frogs (Brode 1988). Once present in Central Valley wetlands from Butte County south to Kern County, GGS populations have declined, primarily due to habitat loss and fragmentation (U.S. Fish and Wildlife Service 1999). The State of California listed the GGS as threatened in 1971, and the U.S. Fish and Wildlife Service listed it as threatened in 1993 (U.S. Fish and Wildlife Service 1993). The decline of GGS populations may be related to their exposure to environmental contaminants, but virtually nothing is known about their contaminant burdens (U.S. Fish and Wildlife Service 1999).
Few studies have evaluated the adverse effects of contaminants on reptiles (Sparling et al. 2000) or the effects of metals and trace elements on snakes (Campbell and Campbell 2002). Even fewer studies have been conducted on rare and endangered snake species. Non-lethal tissue sampling techniques are essential for studies of contaminants in rare and endangered species, and such methods have been proposed for American alligators (Alligator mississippiensis) (Burger et al. 2000) and fish (Gremillion et al. 2005). Recent studies have also evaluated the use of shed skins, blood samples, and tail clips as nondestructive sampling techniques in snakes (Burger et al. 2005, 2006; Hopkins et al. 2001, 2005; Rainwater et al. 2005).
In 1995, the U.S. Geological Survey (USGS) began studying the biology and life history of GGSs (Wylie et al. 1997, 2003b; Wylie and Casazza 2000) to assist in recovery planning for this species. In the course of trapping, marking, and radiotracking snakes, dead snakes were opportunistically collected and were stored frozen for potential future chemical analyses. We used the GGSs collected during USGS field studies to examine snake contaminants burdens by sex, site, and tissue type. We focused on mercury (Hg), a major contaminant of concern in northern California (Rytuba 2000, 2003; Domagalski 2001). This study reports the relationships of Hg concentrations in tail clips, livers, and brains, as well as metals and trace elements in livers of GGSs, and evaluates the effectiveness of tail clips as nonlethal predictors of Hg contamination.
Methods
Study Area
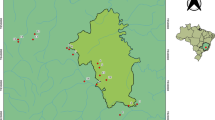
Between April 1997 and June 2004, we opportunistically collected snake carcasses from USGS study areas at Colusa National Wildlife Refuge (CNWR), Colusa County, California, and in the Natomas Basin of northern Sacramento and southern Sutter counties (Fig. 1, Table 1). The CNWR consists of 2000 ha of managed wetlands and associated uplands, managed primarily for waterfowl. The Natomas Basin is 25,000 ha of mostly agricultural land, predominantly rice fields, with water supply and drainage ditches and canals. We also opportunistically collected a few snakes from outside these two primary study areas in Butte County and in the Colusa Basin Drain in northern Yolo County (Fig. 1).
Collection sites for giant garter snakes, 1997–2004: seven collected at the Colusa National Wildlife Refuge (CNWR) and 12 collected at the Natomas Basin. Four other snakes (triangles) were collected: two in Butte County, one in Glenn County, and one in Yolo County (Colusa Drain) (see Table 1)
Field Methods
Some dead snakes were found during trapping and tracking studies, some died from complications associated with surgery to implant radio transmitters, and some snakes were collected as roadkills. Because fieldwork was conducted daily, snakes found dead in traps or during tracking studies likely died less than 24 h before their discovery. Only freshly dead snakes (not more than 2 days) were collected as mortalities from the roads. All snakes collected in the field were placed in plastic bags, transported on wet ice, and stored frozen as soon as possible after collection. Snakes that died in the laboratory were frozen at −20°C immediately after death. Although contaminant concentrations may be related to the degree of sample decomposition, only snakes that had not been subject to significant autolysis were included in the samples for analysis, thus minimizing this potential bias. We compared Hg concentrations using 12 snakes from the Natomas Basin (5 males, 5 females, and 2 of undetermined sex) and 7 snakes from the CNWR (5 males and 2 females). Three additional adult females were collected from outlying areas of Butte County and one female was collected from the Colusa Basin Drain.
Laboratory Methods
Prior to dissection, snake specimens were allowed to thaw to ambient temperatures. Each specimen was rinsed with tap water to remove any debris, then rinsed with deionized water, patted dry with clean paper towels, measured for snout-vent length (SVL; ±1 mm), and weighed on an electronic balance (±0.1 g). We used chemically cleaned instruments and disposable latex gloves while dissecting snakes and changed instruments between snakes to avoid cross-contamination. Whole livers were removed from all 23 snakes and placed in individually labeled chemically clean jars (VWR TraceClean), sealed with Parafilm, and frozen at −20°C until chemical analysis. Tail clips, the distal portions (46–100 mm) of 18 snakes, were surgically removed and stored frozen similar to the livers. For comparisons among snakes, and to ensure that a minimum of 1 g of tissue was available for chemical analysis, we attempted to collect a standardized tail clip 100 mm long. In seven snakes, primarily roadkills, less than 100 mm of tail was available to sample. Tails of five of the snakes either were missing or were unsuitable for analysis, usually because they had been crushed by vehicles. Heads of 22 snakes were removed and shipped frozen to the Trace Element Research Laboratory (TERL, College Station, TX) where the brains were extracted using similar clean techniques. One snake had a crushed skull, rendering the brain unsuitable for analysis.
Chemical Analyses
All 23 livers, 22 brains, and 18 tail clips were analyzed for total Hg. Due to cost considerations, other metals and trace elements were determined only in liver tissue, and no sample was analyzed for methyl mercury (MeHg). All chemical analyses were conducted at TERL. Tissue samples were freeze-dried and homogenized into a fine powder. Three milliliters of HNO3 was added to 0.20–0.25 g of powdered tissue, and digestion was completed in a Teflon reaction vessel heated to 130°C. Samples were then diluted to 20 ml with quartz-distilled water and stored in polyethylene bottles until analysis. Metals were measured by inductively coupled plasma optical emission spectroscopy. Due to lower detection limits, arsenic (As), cadmium (Cd), and lead (Pb) were analyzed by inductively coupled plasma-mass spectroscopy, and selenium (Se) was determined by atomic fluorescence spectroscopy. Mercury concentrations were analyzed by cold vapor atomic absorption spectroscopy. Results for Hg are presented as micrograms per gram on a wet-weight (ww) basis, because literature values are commonly presented on a wet-weight basis. Other elements are presented on a dry-weight (dw) basis.
The mean detection limits for metals and trace elements (μg/g, dw) were as follows: aluminum (Al), 6.9; (As), 0.09; boron (B), 1.4; barium (Ba), 0.14; beryllium (Be), 0.07; calcium (Ca), 2.7; (Cd), 0.02; chromium (Cr), 0.69; copper (Cu), 0.69; iron (Fe), 1.4; Hg (brain), 0.0077; Hg (liver), 0.014; Hg (tail), 0.0059; potassium (K), 14.0; magnesium (Mg), 1.4; manganese (Mn), 0.27; molybdenum (Mo), 1.4; nickel (Ni), 0.69; Pb, 0.05; Se, 0.030; strontium (Sr), 0.070; vanadium (V), 1.4; and zinc (Zn), 0.69. Sample analyses included the use of procedural blanks, spiked samples, standard reference materials (NIST 1577b, NRCC DOLT 2, DORM 2), and duplicate samples. Recoveries from spikes and reference materials ranged from 74% to 105% and from 75% to 124%, respectively. Duplicate analyses were within an acceptable range of ±15%.
Statistical Analysis
Data analyses were performed using SigmaStat statistical software (ver. 3.1; Systat Software Inc, Point Richmond, CA, USA). Data were log-transformed to achieve homogeneity of variance. Because of our limited number of samples, for site comparisons we used only data from CNWR and Natomas. Where data were normally distributed (based on Kolmogorov-Smirnov test), we compared contaminant results by site, gender, and tissue using one-way analysis of variance (ANOVA) and Tukey’s all-pairwise multiple-comparison procedure. We used the Kruskal-Wallis one-way ANOVA on ranks if the distributions were not normal. Relationships between contaminant levels in different tissues, by gender and sample site, were examined using linear regression analysis. The significance level for all tests was α = 0.05. All mean concentrations for contaminants in GGS tissues are presented as geometric means.
Results
Mercury
We detected Hg in every GGS tissue analyzed in this study (Table 1). The overall geometric mean concentration in livers from all sites (0.393 μg/g, ww) was significantly higher (p < 0.001) than in brains (0.062 μg/g) and tails (0.082 μg/g), but concentrations of Hg did not differ between brains and tails (p = 0.586). The sites with the most samples, CNWR and Natomas, were compared by tissue and gender. At CNWR, geometric mean concentrations of Hg were higher (p = 0.004) in livers of the two female snakes (1.612 μg/g) than in the five males (0.360 μg/g). Differences in mean Hg concentrations between female brains (0.127 μg/g) and male brains (0.053 μg/g) were not significant (p = 0.087). Too few tails were analyzed to allow intersex comparisons at CNWR. At Natomas, mean concentrations of Hg did not differ by gender for liver (p = 0.396) or brain (p = 0.115), but males had a higher mean Hg concentration in tails (p = 0.044).
Using only snakes of known gender from CNWR and Natomas combined, there were no differences based on gender for any of the three tissues (p = 0.567–0.768). When data from CNWR and Natomas were compared, regardless of gender, we found no differences between sites in mean Hg concentrations in liver (0.553 and 0.482 μg/g, respectively; p = 0.704) or brain (0.068 and 0.088 μg/g, respectively; p = 0.333). However, the mean Hg concentration in GGS tail clips was higher in snakes from Natomas (0.140 μg/g) than in those from CNWR (0.075 μg/g; p = 0.035). For males alone, mean Hg concentrations in livers were not different between the two sites (p = 0.249), but concentrations in both brain (p = 0.037) and tail clips (p = 0.008) were higher at Natomas (0.103 and 0.203 μg/g, respectively) than at CNWR (0.053 and 0.069 μg/g, respectively). For females alone, the mean Hg concentration in livers from CNWR (1.612 μg/g) was not different from that in livers from Natomas (1.451 μg/g; p = 0.095). The brains were also not different between sites (p = 0.153), and there were too few tails collected at CNWR to compare between sites.
Female GGSs from CNWR and Natomas were larger on average than males, based on both mean (±standard error) SVL (808.1 ± 66.3 and 625.2 ± 47.4 mm, respectively; p = 0.017) and mean mass (253.7 ± 40.5 and 105.7 ± 14.7 g; p = 0.008). Of 18 correlations between snake size and Hg concentrations in tissues, only 4 were significant. For female snakes, both body mass and SVL were significantly correlated (p ≤ 0.007) with the Hg concentration in tail clips, while only the SVL of females was correlated with Hg concentrations in liver and brain (Table 2). Mercury concentrations in tissues of males and of both sexes combined were not correlated with mass or SVL.
Correlations between Hg in tail clips and Hg in livers and brains at CNWR were not significant. Because only one female tail was available, gender-dependent differences could not be examined. At Natomas, all correlations of Hg concentrations in tails with those in livers and brains were significant, except for brains of the males (p = 0.823) (Table 3). When snakes from both CNWR and Natomas were analyzed together by gender, correlations between Hg concentrations in tails and both brains and livers were significant, with the exception of livers of females (p = 0.081) (Table 3).
Metals and Trace Elements
Results of the liver analyses for metals and trace elements are presented in Table 4. The number of snakes that had values below the detection limit for various elements was as follows: Al, 1; B, 18; Be, 23 (all snakes); Cr, 5; Mo, 4; Ni, 2; Pb, 8; and V, 7. Geometric mean concentrations were not calculated for boron, which was detected in only 5 of the 23 samples, or beryllium, which was not detected in any samples. Between sites, snakes from the CNWR were significantly higher in As, Fe, Mo, Ni, and Se compared to those from Natomas (p = 0.0039, 0.001, 0.003, 0.008, and 0.017 respectively).
Discussion
Mercury
Relatively few studies have examined total Hg in snakes, and even fewer have reported concentrations of MeHg in snakes. However, total Hg should be a good indicator for MeHg; according to Bazar (2002), 95%–99% of the Hg in blood and liver of corn snakes was in the organic form. Our data show that GGSs have lower concentrations of total Hg in livers compared to snakes from most other geographic areas (Table 5). The geometric mean Hg concentration in livers of GGS (0.393 μg/g, ww) was lower than that in livers of cottonmouths (Agkistrodon piscivorus) from two sites in Texas (1.19 and 0.86 μg/g); northern water snakes (Nerodia sipedon) from two sites in Tennessee, Little River (0.750 μg/g) and East Fork Poplar Creek (1.403 μg/g) (Campbell et al. 2005); and banded water snakes (Nerodia fasciata) from the Savannah River in South Carolina (arithmetic mean, 1.86 μg/g) (Burger et al. 2007). The mean Hg in GGS livers was similar to that in cottonmouths from Harrison Bayou, Texas (0.408 μg/g) (Rainwater et al. 2005), and was higher than that in banded water snakes from New Jersey (0.182 μg/g) (Burger et al. 2007).
Rainwater et al. (2005) found that male cottonmouths had higher concentrations of Hg in both liver and kidney than females. Overall, mean Hg concentrations in all analyzed tissues of GGS did not differ by sex. While Hg concentrations increased with body size in both male and female cottonmouths (Rainwater et al. 2005), the correlation between tissue Hg and body size was more often significant for female GGSs in this study (Table 2).
The mean Hg concentrations in tail clips of cottonmouths from three Texas sites (Rainwater et al. 2005) were 1.6 to 2.5 times higher, and the tail clips from banded water snakes from two sites in South Carolina (Burger et al. 2006) were 2 and 3.6 times higher, than the mean Hg concentrations in GGS tail clips. In cottonmouths, the correlations between tail clip Hg and liver and kidney Hg were better for males than for females (Rainwater 2005). In this study, when GGS genders were combined, the correlations between Hg in tail clips and Hg in both brains and livers were significant.
Little information exists in the literature on contaminant effects in reptiles (e.g., Hopkins et al. 2002; Campbell and Campbell 2002). However, in one study, Bazar (2002) documented behavioral and toxic effects of dietary MeHg on juvenile corn snakes (Elaphe guttata). Similar behavioral effects could reduce survival in wild populations, by increasing snakes’ susceptibility to predation or mortality caused by vehicles, as was observed in this study of GGSs.
Metals and Trace Elements
We compared the results of our study (Table 4) with metals and trace elements (on a ww basis) commonly analyzed in livers of aquatic snakes from reference and contaminated sites in the United States (Table 5). The mean As concentration in GGS livers (6.48 μg/g) was higher than those in aquatic snakes from Tennessee, South Carolina, and New Jersey (Table 5), but lower than the mean concentration in banded water snake livers from a coal ash settling basin (33.5 μg/g) in South Carolina (Hopkins et al. 1999).
The mean Cd concentration in GGSs (0.02 μg/g) was lower than the means at two contaminated sites (0.12 μg/g at East Fork Poplar Creek, TN, site and 0.13 μg/g at the coal ash settling basin, SC, site), but they were also lower than those at reference sites in both studies (Hopkins et al. 1999; Campbell et al. 2005) and at a site in New Jersey (Burger et al. 2007) (Table 5). The mean Cr concentration in our study (0.26 μg/g) was higher than those at all sites except the two contaminated sites in South Carolina (Table 5).
The mean Mn level in GGSs (1.39 μg/g) fell between the concentrations found in northern water snakes at reference (1.01 μg/g) and contaminated sites (1.70 μg/g) in Tennessee (Campbell et al. 2005). The mean was lower than that in banded water snakes at the Savannah River site (arithmetic mean, 2.17 μg/g) and was similar to the concentration in banded water snakes in New Jersey (1.36 μg/g) (Burger et al. 2007). The mean Pb concentration in GGSs (0.02 μg/g) was lower than all comparable values in the literature (Table 5) but was most similar to that in northern water snakes (0.026 μg/g) at the reference site in Tennessee (Campbell et al. 2005).
The mean Se concentration in livers of GGSs (0.773 μg/g) was most similar to the mean observed at the South Carolina reference site (0.91 μg/g) (Hopkins et al. 1999). All other reported values were higher, with the coal ash settling basin being the highest (35.5 μg/g) (Table 5). The mean concentration of Se was lower in GGSs from our study than in livers of gopher snakes (Pituophis melanoleucus; 2.61 μg/g) from the highly contaminated Kesterson Reservoir (Merced County, CA) (Ohlendorf et al. 1988). The mean Se concentrations in livers of gopher snakes from two reference sites (0.48 and 0.50 μg/g), however, were lower than those in the GGSs in this study.
In recent studies, investigators have begun to focus on the biological effects of trace element contaminants in reptiles. For example, Hopkins et al. (1999) demonstrated that elevated concentrations of a suite of trace elements (As, Cd, and Se) in field-collected adult banded water snakes from a site contaminated by coal ash disposal resulted in a 32% increase in standard metabolic rate over snakes from a reference site. Following this, however, captive-reared offspring from wild adults were fed contaminated prey items from this site over 2 years, with no observed biological effects (Hopkins et al. 2002), despite demonstrable uptake of As, Cd, Se, Sr, and V by various tissues. The effects on reptiles of metals and other elements as well as potential synergistic effects require further investigation in both the field and the laboratory.
Numerous studies have evaluated the utility of snake tail clips as a nondestructive sampling technique for assessing contaminant exposure and risk (Burger et al. 2005; Hopkins et al. 2001, 2005; Rainwater et al. 2005). Tail clips, which are comprised of many tissue types, including bone, skin, muscle, and blood, are well suited for detecting contaminants that may not be present in one tissue alone (Hopkins et al. 2001). In addition, tail clips may serve as better indicators of ecosystem health over a longer temporal scale than other nondestructive samples such as blood (Burger et al. 2006). Metals for which tail clips may be especially well suited include As, Sr, and Se (Hopkins et al. 2001, 2005) as well as Cr, Mn, and Pb (Burger et al. 2007). However, Hg was found to be the element with the best correlation between Hg concentrations in tail clips and those in liver, kidney, and muscle (Burger et al. 2007). We found significant correlations between Hg concentrations in tail clips and livers and suggest that further study using nonthreatened species is needed to document this relationship for both Hg and other environmental contaminants.
Tail clips used in this study were 46–100 mm long, much longer than the 2- to 3-cm clips used by most other investigators. Willis et al. (1982) found that natural tail loss, usually related to the effects of predation, adversely affected survivability, especially in first-year snakes, and while locomotor speed may not be reduced by tail loss in garter snakes (Jayne and Bennett 1989), it may reduce mating success (Shine et al. 1999). Therefore, in studies of threatened or endangered snakes or of snakes that are sampled and then released back to the wild, we recommend removing the least possible amount of tail tissue that will permit contaminant analyses.
This study demonstrates that GGSs in California’s Central Valley are chronically exposed to Hg and other metals. The GGS, like other upper trophic-level carnivores, are at risk of environmental contaminants that biomagnify (Hopkins 2006). In turn, their predators, which include wading birds such as herons (Campbell and Campbell 2001) and bullfrogs (Lithobates catesbeianus) (Wylie et al. 2003a), are at risk of contaminant biomagnification. Mercury tissue burdens in GGS were comparable to those in juvenile corn snakes that were experimentally dosed with MeHg (Bazar 2002). The corn snakes exhibited a decrease in behavioral and physiological performance due to MeHg exposure. Wild populations of GGSs may be at risk of similar effects, including decreased predator-avoidance ability, reduced success in the capture of prey items, and difficulty shedding normally (Bazar 2002). Reduced neuromuscular and locomotor performance due to Hg exposure could potentially affect the daily activities and overall survival of GGSs in the wild by adversely affecting their ability to move between shelter and forage areas (including across roads), as well as the ability to perform daily movements required for thermoregulation. These potential effects, as well as the effects of Hg and other elements on snake reproductive success, should be investigated.
References
Bazar MA (2002) Effects of dietary methylmercury in juvenile corn snakes. MS thesis. Towson University, Towson, MD
Brode J (1988) Natural history of the giant garter snake (Thamnophis couchi gigas). In: DeListe HF, Brown PR, Kaufman B, McGurty BM (eds) Proceedings of the Conference on California Herpetology, Southwestern Herpetologists Society. Special Publication No. 4, pp 25–28
Burger J, Gochfeld M, Rooney AA, Orlando EF, Woodward AR, Guillette LJ Jr (2000) Metals and metalloids in tissues of American alligators in three Florida lakes. Arch Environ Contam Toxicol 38:501–508. doi:10.1007/s002449910066
Burger J, Campbell KR, Campbell TS, Shukla T, Jeitner C, Gochfeld M (2005) Use of skin and blood as nonlethal indicators of heavy metal contamination in northern water snakes (Nerodia sipedon). Arch Environ Contam Toxicol 49:232–238. doi:10.1007/s00244-004-0098-9
Burger J, Murray S, Gaines KF, Novak JM, Punshon T, Dixon C, Gochfeld M (2006) Element levels in snakes in South Carolina: differences between a control site and exposed site on the Savannah River site. Environ Monitor Assess 112:35–52. doi:10.1007/s10661-006-0695-3
Burger J, Campbell KR, Murray S, Campbell TS, Gaines KF, Jeitner C, Shukla T, Burke S, Gochfeld M (2007) Metal levels in blood, muscle and liver of water snakes (Nerodia spp.) from New Jersey, Tennessee and South Carolina. Sci Total Environ 373:556–563. doi:10.1016/j.scitotenv.2006.06.018
Campbell KR, Campbell TS (2001) The accumulation and effects of environmental contaminants on snakes: a review. Environ Monit Assess 70:253–301. doi:10.1023/A:1010731409732
Campbell KR, Campbell TS (2002) A logical starting point for developing priorities for lizard and snake Ecotoxicology: a review of available data. Environ Toxicol Chem 21:894–898. doi: 10.1897/1551-5028(2002)021<0894:ALSPFD>2.0.CO;2
Campbell KR, Campbell TS, Burger J (2005) Heavy metal concentrations in northern water snakes (Nerodia sipedon) from East Fork Poplar Creek and the Little River, east Tennessee, USA. Arch Environ Contam Toxicol 49:239–248. doi:10.1007/s00244-004-0200-3
Domagalski JL (2001) Mercury and methylmercury in water and sediment of the Sacramento River Basin, California. Appl Geochem 16:1677–1691. doi:10.1016/S0883-2927(01)00068-3
Gremillion PT, Cizdziel JV, Cody NR (2005) Caudal fin mercury as a non-lethal predictor of fish-muscle mercury. Environ Chem 2:96–99. doi:10.1071/EN05018
Hopkins WA (2006) Use of tissue residues in reptile ecotoxicology: a call for integration and experimentalism. In: Gardner SC, Oberdörster (eds) Toxicology of reptiles. CRC Press, Boca Raton, FL, pp 35–62
Hopkins WA, Rowe CL, Congdon JD (1999) Elevated trace element concentrations and standard metabolic rate in banded water snakes (Nerodia fasciata) exposed to coal combustion wastes. Environ Toxicol Chem 18:1258–1263. doi: 10.1897/1551-5028(1999)018<1258:ETECAS>2.3.CO;2
Hopkins WA, Roe JH, Snodgrass JW, Jackson BP, Kling DE, Rowe CL, Congdon JD (2001) Nondestructive indices of trace element exposure in squamate reptiles. Environ Pollut 115:1–7. doi:10.1016/S0269-7491(01)00098-7
Hopkins WA, Roe JH, Snodgrass JW, Staub BP, Jackson BP, Congdon JD (2002) Effects of chronic dietary exposure to trace elements on banded water snakes (Nerodia fasciata). Environ Toxicol Chem 21:906–913. doi: 10.1897/1551-5028(2002)021<0906:EOCDET>2.0.CO;2
Hopkins WA, Snodgrass JW, Baionno JA, Roe JH, Staub BP, Jackson BP (2005) Functional relationships among selenium concentrations in the diet, target tissues, and nondestructive tissue samples of two species of snakes. Environ Toxicol Chem 24:344–351. doi:10.1897/03-601.1
Jayne BC, Bennett AF (1989) The effect of tail morphology on locomotor performance of snakes: a comparison of experimental and correlative methods. J Exp Zool 252:126–133. doi:10.1002/jez.1402520204
Ohlendorf HM, Hothem RL, Aldrich TW (1988) Bioaccumulation of selenium by snakes and frogs in the San Joaquin Valley, California. Copeia 1988:704–710. doi:10.2307/1445391
Rainwater TR, Reynolds KD, Cañas JE, Cobb GP, Anderson TA, McMurry ST, Smith PN (2005) Organochlorine pesticides and mercury in cottonmouths (Agkistrodon piscivorus) from northeastern Texas, USA. Environ Toxicol Chem 24:665–673. doi:10.1897/04-223R.1
Rytuba JJ (2000) Mercury mine drainage and processes that control its environmental impact. Sci Total Environ 260:57–71. doi:10.1016/S0048-9697(00)00541-6
Rytuba JJ (2003) Mercury from mineral deposits and potential environmental impact. Environ Geol 43:326–338
Shine R, Olsson MM, Moore IT, LeMaster MP, Mason RT (1999) Why do male snakes have longer tails than females? Proc R Soc Lond B 266:2147–2151. doi:10.1098/rspb.1999.0901
Sparling DW, Bishop CA, Linder G (2000) The current status of amphibian and reptile ecotoxicological research. In: Sparling DW, Linder G, Bishop CA (eds) Ecotoxicology of amphibians and reptiles. Society of Environmental Toxicology and Chemistry, Pensacola, FL, pp 1–13
U.S. Fish Wildlife Service (1993) Endangered and threatened wildlife and plants; determination of threatened status for the giant garter snake. Fed Register 58:54053–54066
U.S. Fish Wildlife Service (1999) Draft recovery plan for the giant garter snake (Thamnophis gigas). U.S. Fish and Wildlife Service, Portland, OR
Willis L, Threlkeld ST, Carpenter CC (1982) Tail loss patterns in Thamnophis (Reptilia: Colubridae) and the probable fate of injured individuals. Copeia 1982:98–101. doi:10.2307/1444273
Wylie GD, Casazza ML (2000) Investigations of giant garter snakes in the Natomas Basin: 1998–1999—Project Summary. USGS-BRD, Western Ecological Research Center. Dixon Field Station, Dixon, CA
Wylie GD, Casazza ML, Daugherty J (1997) 1996 progress report for the giant garter snake study. USGS-BRD, Western Ecological Research Center. Dixon Field Station, Dixon, CA
Wylie GD, Casazza ML, Carpenter M (2003a) Diet of bullfrogs in relation to predation on giant garter snakes at Colusa National Wildlife Refuge. Calif Fish Game 89:139–145
Wylie GD, Casazza ML, Martin LL, Carpenter NM (2003b) Monitoring giant garter snakes at Colusa National Wildlife Refuge: 2003 progress report. USGS-BRD, Western Ecological Research Center. Dixon Field Station, Dixon, CA
Acknowledgments
This study was conducted in cooperation with the U.S. Fish and Wildlife Service Sacramento National Wildlife Refuge and the Sacramento Office of Ecological Services. Funding was provided by the U.S. Geological Survey and the U.S. Fish and Wildlife Service. Specimens were collected in accordance with our U.S. Fish and Wildlife Service Recovery Permit TE-020548-5. Numerous U.S. Geological Survey biological technicians and biologists assisted with field collections. Kate DeClercq assisted with snake dissection and prepared snakes for shipment to the contract laboratory, and Melissa Farinha prepared the figure. Tom Suchanek, Julie Yee, Melissa Farinha, and an anonymous reviewer provided many helpful comments on the manuscript. Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wylie, G.D., Hothem, R.L., Bergen, D.R. et al. Metals and Trace Elements in Giant Garter Snakes (Thamnophis gigas) from the Sacramento Valley, California, USA. Arch Environ Contam Toxicol 56, 577–587 (2009). https://doi.org/10.1007/s00244-008-9265-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-008-9265-8