Abstract
We compared the levels of arsenic, cadmium, chromium, lead, manganese, mercury, and selenium in the blood, kidney, liver, muscle, and skin of northern water snakes (Nerodia sipedon) collected from the upper reach of East Fork Poplar Creek (EFPC) within the United States Department of Energy’s (USDOE’s) Y-12 National Security Complex with concentrations in tissues of northern water snakes from a reference reach of the Little River downstream from the Great Smoky Mountains National Park in East Tennessee. Our objectives were to determine whether concentrations of these metals were higher in tissues of water snakes collected from EFPC compared with the reference site and if northern water snakes were suitable bioindicators of metal contamination. Except for chromium, metal levels were significantly higher in tissues (kidney, liver, muscle, and skin) of EFPC northern water snakes compared with those in tissues of snakes from the reference site. Although female northern water snakes were significantly larger than male snakes, their tissues did not contain significantly higher metal concentrations compared with those from male snakes, possibly because of maternal transfer of metals to eggs. This study was the first to examine the accumulation of contaminants resulting from the operations of the USDOE’s Oak Ridge Reservation in snakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Reptiles are rarely included in studies of environmental contamination and ecological risk assessments. Gibbons et al. (2000) called attention to the ongoing global decrease of reptiles and listed six significant threats to reptiles, one of which is environmental pollution. Compared with the other classes of vertebrates, much less is known about the accumulation and effects of environmental contaminants in reptiles, specifically squamates (lizards and snakes) (Campbell and Campbell 2000, 2001, 2002; Hopkins 2000). Because water snakes are mid- to top-level predators, they are susceptible to the accumulation of persistent pollutants and may be useful environmental indicators of contamination (Bauerle et al. 1975; Stafford et al. 1976; Burger 1992). In the limited number of environmental contamination studies available for snakes, aquatic snakes are the most studied group (Campbell and Campbell 2001). Because they are an important trophic link to terrestrial and aquatic carnivores, aquatic snakes can provide vital insight as to how contaminants move through and affect communities (Hopkins et al. 1999).
The United States Department of Energy’s (USDOE’s) approximately 14,000-ha (approximately 35,000-acre) Oak Ridge Reservation (ORR) in East Tennessee was placed on the National Priorities List as a Superfund site in 1989 and contains three facilities: (1) Oak Ridge National Laboratory (ORNL); (2) East Tennessee Technology Park, formerly the K-25 Site; and (3) the Y-12 National Security Complex (Y-12), formerly the Y-12 Plant (Campbell et al. 1998). An estimated 75 to 150 tons of elemental mercury were released into East Fork Poplar Creek (EFPC) as a result of regular discharges, spills, and accidents associated with the lithium-isotope separation process used in the production of thermonuclear fusion weapons at Y-12 during the mid-1950s and early 1960s (Campbell et al. 1998). Although the primary mercury releases from Y-12 stopped in 1963, mercury continues to be released into EFPC from secondary sources such as contaminated buildings, equipment, and soils (USDOE 1995). The current release of mercury into EFPC averages approximately 20 g/d, which is down from 100 g/d in 1985 (USDOE 1995). Mercury releases continue to be decreased as a result of decontamination and decommissioning activities, the decrease of mercury in plant effluent, and the remediation of areas where mercury was used (USDOE 1995).
Because the accumulation of contaminants resulting from the operation and spills from the ORR have not been studied in squamate reptiles, we compared levels of metals (arsenic, cadmium, chromium, lead, manganese, mercury, and selenium) in northern water snakes (Nerodia sipedon) living in EFPC with levels in northern water snakes from a reference site. We tested the following hypotheses: (1) no intersite differences would exist in metal concentrations, and (2) metal levels would not be related to the sex or size of the snakes. Based on the literature and previous studies on northern water snakes in East Tennessee (Andreadis 1998), we expected female to be much larger than male snakes.
Materials and Methods
Northern water snakes were collected from two locations in East Tennessee (Figure 1). Adult northern water snakes were collected by hand or in modified minnow traps (Casazza et al. 2000) between May 7 and June 24, 2002. Twenty northern water snakes (10 females and 10 males) were collected from the uppermost 2.5 km of EFPC inside Y-12 (Figure 1). The reference site was a reach of the Little River downstream from Great Smoky Mountains National Park between the Picnic Area and Perry’s Mill (Figure 1), where a large population of northern water snakes was known to occur (Andreadis 1998). Twenty-seven northern water snakes (11 females and 16 males) were collected from the Little River reference site.
In conjunction with the collection of northern water snakes, 20 Central stonerollers (Campostoma anomalum) were collected from each site by backpack electroshocking or nighttime dip netting. Central stonerollers were determined to be the main prey of northern water snakes inhabiting upper EFPC. Details regarding Central stoneroller collection, analyses, and results are presented in a previous publication (Burger et al. 2005a).
On collection, each snake was anesthetized by placing its head in a small ziplock bag containing a cotton ball saturated with isoflurane and then sexed, weighed, and measured. Between 0.5 and 3 ml of blood was collected from the heart using a 25-gauge heparinized syringe while the snakes were under anesthesia. Water snakes were then euthanized with Beuthanasia (Schering-Plough Animal Health). Snakes and blood samples were frozen and shipped to the Environmental and Occupational Health Sciences Institute (EOHSI), Rutgers University, for dissection and metals analysis, respectively.
At EOHSI, skin samples were cleaned using a triple rinse, first by gently swirling in a beaker of deionized water, then in a beaker of acetone, and finally in deionized water. Tissues were digested in ultrex ultrapure nitric acid in a microwave (MD 2000 CEM) using a digestion protocol of three stages of 10 minutes each under 50, 100, and 150 pounds/in.2 (3.5, 7.0, and 10.6 kg/cm2) at 70x power. Digested samples were subsequently diluted in deionized water. Mercury was analyzed by cold-vapor atomic absorption, and all other metals (arsenic, cadmium, chromium, lead, manganese, and selenium) were analyzed by graphite furnace atomic absorption. All tissue metal levels were expressed in parts per billion (ppb; ng/g on wet weight). Detection limits were 0.2 ppb for arsenic, 0.1 ppb for cadmium, 1.0 ppb for chromium, 2.0 ppb for lead, 1.0 ppb for manganese, 2.0 ppb for mercury, and 0.5 ppb for selenium.
All specimens were run in batches that included blanks, a standard calibration curve, and spiked specimens. The accepted recoveries for spikes ranged from 85% to 105%. The coefficient of variation on replicate samples ranged from 3% to 7%. Further quality control included periodic blind analysis of an aliquot from a large sample of known concentration and blind runs of duplicate samples during the analysis for each metal (acceptable criterion ± 15%).
The data were analyzed using SPSS software (Release 11.5.0; SPSS, Chicago, IL). To meet the assumptions of multiple analysis of variance (MANOVA) and analysis of variance (ANOVA), all metal levels data were log transformed. To determine the effects of organ (blood, kidney, liver, muscle, skin), sex (female, male), and site (EFPC, Little River) on metal concentrations, data were first cast into a three-way MANOVA using organ, sex, site, and their interaction as factors (p = 0.05). Significant effects and interactions were further explored with one-way ANOVAs and multiple comparisons tests (p = 0.05).
Results
Northern water snakes ranged in size from 44.5 to 80 mm snout-vent length and 53 to 417.5 g in weight (Table 1). As was expected, females were significantly larger and heavier than male snakes. There were no significant intersite differences in size for either females or male snakes.
The MANOVA indicated that site (F = 14.7, df = 206, p < 0.000), sex (F = 3.5, df = 206, p < 0.001), and organ (F = 18.6, df = 836, p < 0.000) were significant effects. In addition, the site–sex interaction (F = 3.1, df = 206, p < 0.004), the site–organ interaction (F = 3.4, df = 836, p < 0.000), and the sex–organ interaction (F = 1.8, df = 836, p < 0.007) were also significant effects. The interaction of site, sex, and organ was not a significant effect (F = 0.87, df = 836, p < 0.660).
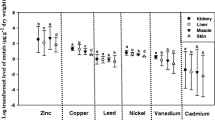
Overall, mean arsenic, cadmium, and selenium concentrations were highest in liver and kidney tissues than all other water snake tissues (Table 2 and Figure 2). Compared with other tissues, levels of chromium and lead were highest in water snake skin. Average manganese levels were highest in muscle, followed by skin, of northern water snakes from both sites and of both sexes (Table 2 and Figure 2). Concentrations of mercury were highest in livers of northern water snakes from both study sites followed by kidney tissue for EFPC snakes and muscle tissue for Little River snakes (Table 2). Mercury levels were also highest in liver tissue for all males and females (Figure 2).
Mean metal concentrations (ppb, wet weight) by sex in tissues of northern water snakes (N. sipedon) collected from East Tennessee (two sites combined: female snakes N = 21 and male snakes N = 26) from May through June 2002. Error bars represent the SE, and an asterisk indicates significant differences in female and male tissue levels.
Arsenic levels in the liver and skin were significantly higher in water snakes from EFPC compared with those from the Little River reference site (Table 2). For cadmium, levels in kidney and liver from EFPC snakes were significantly higher than those from Little River snakes. Chromium concentrations in kidney and muscle of snakes from the Little River were significantly higher compared with levels in tissues of water snakes from EFPC (Table 2). For lead, levels in kidney, liver, and skin from EFPC water snakes were significantly higher than concentrations in tissues of snakes from the Little River reference site. Manganese levels in liver and skin from water snakes collected from EFPC were significantly higher than concentrations in liver and skin from the Little River snakes. Mercury concentrations in kidney and liver from EFPC snakes were significantly higher compared with levels in tissues of Little River water snakes (Table 2). However, muscle mercury levels were significantly higher in northern water snakes from the Little River compared with snakes from EFPC. Levels of selenium in kidney, liver, and muscle tissue of water snakes from EFPC were significantly higher than those in tissues of snakes collected from the Little River.
Despite the significant sex-related differences in size, overall there were few sex-related differences in tissue metal levels (Figure 2). Blood from male northern water snakes collected from two sites in East Tennessee had significantly higher levels of cadmium compared with blood from female snakes. Mercury concentrations in muscle tissue of male snakes from both sites were significantly higher than levels in female northern water snake muscle.
Discussion
This study examined the accumulation of metals in northern water snakes from a Superfund site and a reference site in East Tennessee. Recent controlled laboratory studies have demonstrated that prey items can be an important source of metal exposure for aquatic snakes (Hopkins et al. 2001, 2002, 2004). A comparison of average metal levels in liver tissue from water snakes from both sites (Table 2) with average metal concentrations in whole Central stonerollers collected from both sites (Table 3) indicated that cadmium, mercury, and selenium levels in water snake livers were up to six times higher than concentrations found in Central stonerollers. Mean arsenic and lead levels in water snake livers and Central stonerollers were similar, whereas manganese concentrations in snake livers were much lower than levels found in Central stonerollers. Northern water snakes appear to be accumulating cadmium, mercury, and selenium as a result of consuming Central stonerollers.
Although Central stonerollers are not the exclusive food source for northern water snakes in the Little River (Andreadis 1998), they are the main, if not only, food source for water snakes in uppermost EFPC. Central stonerollers are the only fish species found in upper EFPC and they occur there in unusually large numbers. Because concentrations of six of seven metals (except for arsenic) in Central stonerollers collected from upper EFPC were significantly higher than those found in stonerollers from the Little River (Burger et al. 2005a), and levels of six of seven metals (except for chromium) in livers of northern water snakes from EFPC were significantly higher than concentrations in livers of Little River snakes, we can assume that the stonerollers in EFPC were a significant source of metal exposure for northern water snakes. However, controlled laboratory studies, similar to those conducted by Hopkins et al. (2001, 2002, 2004), are needed to confirm these relationships. The ORNL’s Biological Monitoring and Abatement Program has been monitoring mercury levels in fish and water collected from EFPC since 1985 (www.esd.ornl.gov/BMAP/efpc.htm). Although levels of mercury have decreased in Central stonerollers and water from upper EFPC in recent years, concentrations in fish remain high compared with uncontaminated reference streams, similar to what was observed in our investigation (Burger et al. 2005a).
Consistent with results found in other investigations in reptiles (Linder and Grillitsch 2000; Rainwater et al. 2005), mercury levels were highest in northern water snake liver tissue (Table 2). In a previous publication, we demonstrated that blood and skin were suitable nondestructive indicators of mercury levels in aquatic snakes because mercury levels in blood and skin of northern water snakes collected from two locations in East Tennessee were significantly correlated with liver, kidney, and muscle tissue levels (Burger et al. 2005b). Arsenic and cadmium concentrations were highest in livers of water snakes from EFPC compared with the other tissues examined (Table 2), similar to what was observed in a controlled laboratory study on banded water snakes (N. fasciata) (Hopkins et al. 2002). Although selenium levels in our field investigation were highest in water snake livers (Table 2), concentrations of selenium were highest in kidneys in banded water and brown house snakes (Lamprophis fuliginosus) in controlled laboratory studies (Hopkins et al. 2002, 2004). As indicated by our results, northern water snakes can sequester chromium, lead, and manganese in their skin; depuration of these metals through frequent shedding of skin may significantly decrease body burdens of these contaminants (Burger 1992). The source of chromium contributing to the high chromium levels in kidney and muscle tissue of Little River snakes, compared with concentrations in EFPC water snake kidney and muscle tissue, is unknown. Few studies have examined the effects of metal accumulation on snakes (Campbell and Campbell 2001), and toxicity thresholds currently do not exist for reptiles (Hopkins et al. 2005). Mercury concentrations ranging from 1,000 to 13,700 ppb in livers of water birds (ducks and herons) have been associated with decreases in reproductive fitness, effects on growth and behavior, and mortality (Zillioux et al. 1993; Wolfe et al. 1998); levels of mercury in livers of northern water snakes from EFPC were within this range (Table 2). Concentrations of arsenic, cadmium, and selenium in liver tissue of water snakes from EFPC were many times lower than those found in livers of banded water snakes that were associated with increased standard metabolic rates and increased allocations of energy to maintenance (less energy available for growth, reproduction, and storage) (Hopkins et al. 1999). Selenium concentrations in livers of northern water snakes collected from EFPC were within the range of levels known to be reproductively toxic to both fish and birds (Lemly 1996; Heinz 1996).
Although female northern water snakes were significantly larger than male snakes, significantly higher metal concentrations were not found in tissues of female snakes compared with those of male snakes (Figure 2). Ohlendorf et al. (1988) did not find a significant correlation between selenium concentration and size of gopher snakes (Pituophis melanoleucus). Mercury levels in all tissues examined increased with body size in both female and male cottonmouths (Agkistrodon piscivorous) in a recent study conducted by Rainwater et al. (2005). Male cottonmouths, which were significantly larger than female snakes, exhibited higher mercury concentrations than female snakes in both liver and kidney. Sex-specific differences in metal accumulation occurred in a controlled laboratory study on juvenile banded water snakes (Hopkins et al. 2002). Differences in metal accumulation in female and male snakes could occur because of sex-based differences in food preference, body size, or elimination during reproduction (e.g., female snakes transferring the metals to their eggs) (Burger 1992; Hopkins et al. 2002, 2004).
The results of our investigation indicate that EFPC is contaminated by metals other than mercury. Northern water snakes and Central stonerollers inhabiting upper EFPC may have adapted with time to living in the metal-contaminated environment and may be unaffected by the chronic exposure. During our investigation, the water snake population appeared to be extremely dense, healthy, and thriving, possibly because of the large amount of artificial rock habitat along the stream banks and the boundless supply of Central stonerollers. Many juvenile snakes of all size classes were trapped in EFPC (because we were interested in only adult snakes, all juveniles were released). However, we did observe unusual feeding behavior of northern snakes in EFPC. Instead of feeding in the open at night, which is typical for northern water snakes and what we observed for northern water snakes in the Little River, water snakes in EFPC fed openly during the mid-morning (which meant snakes had to be trapped instead of hand-captured at night). This could be related to the extremely dense population of stonerollers (being seen was not an issue) or the fact that the upper reach of the creek is fairly isolated (the presence of humans along the creek banks of upper EFPC is minimal because it is inside the secured boundary of Y-12).
Tissue metal levels found in northern water snakes in this investigation were compared with levels found in aquatic snakes from other locations in the United States to determine the utility of using aquatic snakes as indicators of environmental contamination (Table 4). One advantage of using water snakes as indicators of environmental contamination is that they have been studied elsewhere (Campbell and Campbell 2001). However, in most cases a suite of metals was not examined, and different tissues were not analyzed. Aquatic snakes have a relatively small home range, making comparisons of conditions in multiple habitats within a relatively small geographic area possible (Hopkins et al. 1999).
Arsenic concentrations in muscle tissue of northern water snakes in this study were lower than those found in whole-body composite samples of water snakes (Nerodia spp.) collected from the upper and lower reaches of the Apalachicola River (FL) in 1978 (Table 4; Winger et al. 1984). The levels of arsenic found in livers of northern water snakes from EFPC were much lower than those found in banded water snakes inhabiting a contaminated coal–ash settling basin and nearby swamp associated with a power plant at the USDOE’s Savannah River Site (SRS) in South Carolina (Table 4; Hopkins et al. 1999).
Concentrations of cadmium in muscle tissue of northern water snakes from both EFPC and the Little River reference site were similar to those found in water snakes from the Apalachicola River and the Big and the Black Rivers in Missouri (Table 4; Winger et al. 1984; Niethammer et al. 1985). Cadmium levels in EFPC northern water snake liver tissue were similar to levels found in livers from banded water snakes from the contaminated site associated with the SRS power plant (Table 4; Hopkins et al. 1999). Chromium concentrations in livers of banded water snakes in the investigation conducted on the SRS were approximately 10 times higher than levels found in northern water snake liver tissue in this study (Table 4; Hopkins et al. 1999).
Lead levels in carcasses (minus head, skin, and gastrointestinal tract) of northern water snakes collected in 1981 to 1982 from the Big and the Black River drainages in two lead-mining districts of southeastern Missouri were much higher than snake muscle concentrations found in this study (Table 4; Niethammer et al. 1985). Concentrations of lead found in water snakes from the Apalachicola River were similar to levels found in muscle of northern water snakes from both EFPC and the reference site (Table 4; Winger et al. 1984).
Mercury concentrations in northern water snake blood and muscle tissue in this study were much higher than those found in a northern water snake from Pilot Island (Lake Michigan), water snakes from the Apalachicola River, and water snakes from two locations in Texas (Table 4; Heinz et al. 1980; Winger et al. 1984; Clark et al. 2000). Liver mercury levels in snakes from EFPC were similar to those found in livers of cottonmouths collected from Goose Prairie Creek, located within the Longhorn Army Ammunitions Plant, TX, whereas liver concentrations in Little River water snakes were similar to those in Harrison Bayou cottonmouths (Table 4; Rainwater et al. 2005). However, one cottonmouth collected from Central Creek, within the Longhorn Army Ammunitions Plant, had a liver mercury level of 8,613 ppb, almost three times higher than the highest level found in liver tissue of water snakes from EFPC (Table 4; Rainwater et al. 2005). Average concentrations of mercury found in kidney tissue from Little River water snakes in this study were similar to those found in cottonmouths collected from three locations on the Longhorn Army Ammunitions Plant; however, mercury levels in kidneys of EFPC water snakes were up to two times higher than those found in cottonmouths from the ammunitions plant (Table 4; Rainwater et al. 2005).
Levels of selenium in northern water snake muscle and blood in this investigation were higher than those found in whole bodies of water snakes from the Apalachicola River (FL) and blood from water snakes from two locations in Texas (Table 4; Winger et al. 1984; Clark et al. 2000). However, selenium concentrations found in livers of banded water snakes from the contaminated power plant site on the SRS were many times higher than those in liver tissue from EFPC northern water snakes (Table 4; Hopkins et al. 1999).
Aquatic snakes (e.g., genus Nerodia) are among the most common reptiles found in the southeastern United States and inhabit virtually all types of freshwater habitats (Mushinsky et al. 1982). The trophic status and site fidelity of water snakes makes them particularly useful in evaluating sites contaminated with compounds that are transferred by way of trophic mechanisms (Hopkins et al. 1999, 2001). The results of this study, those from similar studies conducted at another USDOE facility, the SRS (Hopkins et al. 1999, 2001), as well as additional investigations (Table 4) suggest that water snakes are well-suited for environmental toxicology studies.
The direction of environmental toxicology studies on snakes during the past five years is encouraging. Research has gone from reporting tissue levels of contaminants in field-captured snakes to studying the mechanisms and effects of contaminant accumulation in snakes in both the field and controlled laboratory studies to developing nondestructive sampling methods. Functional relationships have recently been developed among dietary selenium levels, target tissue selenium concentrations, and selenium levels in nondestructive tissue samples for two species of snakes (Hopkins et al. 2005). The future of snake ecotoxicology is promising.
References
Andreadis PT (1998) Control of food intake and expression of hunger in the northern water snake, Nerodia sipedon (L.). Doctoral dissertation, University of Tennessee, Knoxville, TN
Bauerle B, Spencer DL, Wheeler W (1975) The use of snakes as a pollution indicator species. Copeia 1975:366–368
Burger J (1992) Trace element levels in pine snake hatchlings: Tissue and temporal differences. Arch Environ Contam Toxicol 22:209–213
Burger J, Campbell KR, Campbell TS, Shukla T, Dixon C, Gochfeld M (2005a) Use of Central stonerollers (Cyprinidae: Campostoma anomalum) from East Fork Poplar Creek, Tennessee as a bioindicator of metal contamination. Environ Monit Assess (in press)
Burger J, Campbell KR, Campbell TS, Shukla T, Jeitner C, Dixon C, et al. (2005b) The use of blood and skin as nondestructive indicators of heavy metal contamination in northern water snakes (Nerodia sipedon). Arch Environ Contam Toxicol (in press)
Campbell KR, Campbell TS (2000) Lizard contaminant data for ecological risk assessment. Rev Environ Contam Toxicol 165:39–116
Campbell KR, Campbell TS (2001) The accumulation and effects of environmental contaminants on snakes: A review. Environ Monit Assess 70:253–301
Campbell KR, Campbell TS (2002) A logical starting point for developing priorities for lizard and snake ecotoxicology: A review of available data. Environ Toxicol Chem 21:894–898
Campbell KR, Ford CJ, Levine DA (1998) Mercury distribution in Poplar Creek, Oak Ridge, Tennessee, USA. Environ Toxicol Chem 17:1191–1198
Casazza ML, Wylie GD, Gregory CJ (2000) A funnel trap modification for surface collection of aquatic amphibians and reptiles. Herp Rev 31:91–92
Clark DR Jr, Bickham JW, Baker DL, Cowman DF (2000) Environmental contaminants in Texas, USA, wetland reptiles: Evaluation using blood samples. Environ Toxicol Chem 19:2259–2265
Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville TD, Metts BS, et al. (2000) The global decline of reptiles, déjà vu amphibians. BioScience 50:653–666
Heinz GH (1996) Selenium in birds. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds). Environmental contaminants in wildlife: Interpreting tissue concentrations. Lewis, Boca Raton, FL, p 427
Heinz GH, Haseltine SD, Hall RJ, Krynitsky AJ (1980) Organochlorine and mercury residues in snakes from Pilot and Spider Islands, Lake Michigan-1978. Bull Environ Contam Toxicol 25:738–743
Hopkins WA (2000) Reptile toxicology: Challenges and opportunities on the last frontier in vertebrate ecotoxicology. Environ Toxicol Chem 19:2391–2393
Hopkins WA, Rowe CL, Congdon JD (1999) Elevated trace element concentrations and standard metabolic rate in banded water snake (Nerodia fasciata) exposed to coal combustion wastes. Environ Toxicol Chem 18:1258–1263
Hopkins WA, Roe JH, Snodgrass JW, Jackson BP, Kling DE, Rowe CL, et al. (2001) Nondestructive indices of trace elements exposure in squamate reptiles. Environ Pollut 115:1–7
Hopkins WA, Roe JH, Snodgrass JW, Staub BP, Jackson BP, Congdon JD (2002) Effects of chronic dietary exposure to trace elements on banded water snakes (Nerodia fasciata). Environ Toxicol Chem 21:906–913
Hopkins WA, Staub BP, Baionno JA, Jackson BP, Roe JH, Ford NB (2004) Trophic and maternal transfer of selenium in brown house snakes (Lamprophis fuliginosus). Ecotox Environ Saf 58:285–293
Hopkins WA, Snodgrass JW, Baionno JA, Roe JH, Staub BP, Jackson BP (2005) Functional relationships among selenium concentrations in the diet, target tissues, and nondestructive tissue samples of two species of snakes. Environ Toxicol Chem 24:344–351
Lemly AD (1996) Selenium poisoning in fish. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds). Environmental contaminants in wildlife: Interpreting tissue concentrations. Lewis, Boca Raton, FL, p 446
Linder G, Grillitsch B (2000) Ecotoxicology of metals. In: Sparling DW, Linder G, Bishop CA (eds). Ecotoxicology of amphibians and reptiles. Society of Environmental Toxicology and Chemistry, Pensacola, FL, pp 495
Mushinsky HR, Hebrard JJ, Vodopich DS (1982) Ontogeny of water snake foraging ecology. Ecology 63:1624–1629
Niethammer KR, Atkinson RD, Baskett TS, Samson FB (1985) Metals in riparian wildlife of the lead mining district of southeastern Missouri. Arch Environ Contam Toxicol 14:213–223
Ohlendorf HM, Hothem RL, Aldrich TW (1988) Bioaccumulation of selenium by snakes and frogs in the San Joaquin Valley, California. Copeia 1988:704–710
Rainwater TR, Reynolds KD, Cañas JE, Cobb GP, Anderson TA, McMurry ST, et al. (2005) Organochlorine pesticides and mercury in cottonmouths (Agkistrodon piscivorus) from northeastern Texas. Environ Toxicol Chem 24:665–673
Stafford DP, Plapp FW Jr, Fleet RR (1976) Snakes as indicators of environmental contamination: Relation of detoxifying enzymes and pesticide residues to species of occurrence in three aquatic ecosystems. Arch Environ Contam Toxicol 5:15–27
United States Department of Energy (1995) Record of decision for Lower East Fork Poplar Creek. DOE/OR/02-1370&D1. Office of Environmental Restoration and Waste Management, Oak Ridge, TN
Winger PV, Sieckman C, May TS, Johnson WW (1984) Residues of organochlorine insecticides, polychlorinated biphenyls, and heavy metals in biota from Apalachicola River, Florida, 1978. J Assoc Off Anal Chem 67:325–333
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife. Environ Toxicol Chem 17:146–160
Zillioux EJ, Porcella DB, Benoit JM (1993) Mercury cycling and effects in freshwater wetland ecosystems. Environ Toxicol Chem 12:2245–2264
Acknowledgments
Bill Hopkins and Thomas Rainwater provided insightful comments that improved an earlier version of this manuscript. This study was conducted under Oak Ridge National Laboratory Animal Care and Use Protocol No. 0287 and Rutgers University Animal Protocol No. 97-017 and funded by the Consortium for Risk Evaluation with Stakeholder Participation (CRESP) through the USDOE (AI Nos. DE-FC01-95EW55084 and DE-FG26-00NT-40938). Northern water snakes were collected under Tennessee Wildlife Resources Agency Scientific Collection Permits 1688-01 and 1688-02. We thank Paul Andreadis, R. Jason Dickey, Pat Parr, Mark Peterson, Mike Ryon, Greg Sievert, and Warren Webb for logistical support or help with collecting water snakes, and Carline Dixon, Chris Jeitner, and Tara Shukla for chemical analysis. Special thanks to Mick Wiest for coordination at the Y-12 National Security Complex and assistance with collecting snakes and to Dorcas O’Rourke for extensive assistance with animal care issues.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campbell, K.R., Campbell, T.S. & Burger, J. Heavy Metal Concentrations in Northern Water Snakes (Nerodia sipedon) from East Fork Poplar Creek and the Little River, East Tennessee, USA. Arch Environ Contam Toxicol 49, 239–248 (2005). https://doi.org/10.1007/s00244-004-0200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0200-3