Abstract
While previous studies have addressed the desirable effects of biochar (BC) or magnesium nanoparticles (Mg NPs) on salinity stress individually, there is a research gap regarding their simultaneous application. Additionally, the specific mechanisms underlying the effects of BC and Mg NPs on salinity in Physalis alkekengi L. remain unclear. This study aimed to investigate the synergistic effects of BC and Mg NPs on P. alkekengi L. under salinity stress conditions. A pot experiment was conducted with salinity at 100 and 200 mM sodium chloride (NaCl), as well as soil applied BC (4% v/v) and foliar applied Mg NPs (500 mg L−1) on physiological and biochemical properties of P. alkekengi L. The results represented that salinity, particularly 200 mM NaCl, significantly reduced plant yield (58%) and total chlorophyll (Chl, 36%), but increased superoxide dismutase (SOD, 82%) and catalase (CAT, 159%) activity relative to non-saline conditions. However, the co-application of BC and Mg NPs mitigated these negative effects and improved fruit yield, Chl, anthocyanin, and ascorbic acid. It also decreased the activity of antioxidant enzymes. Salinity also altered the fatty acid composition, increasing saturated fatty acids (SFAs) and polyunsaturated fatty acids (PUFAs), while decreasing monounsaturated fatty acids (MUFAs). The heat map analysis showed that fruit yield, anthocyanin, Chl, and CAT were sensitive to salinity. The findings can provide insights into the possibility of these amendments as sustainable strategies to mitigate salt stress and enhance plant productivity in affected areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity can cause changes to soil composition, water quality, vegetation patterns, wetland degradation, agricultural productivity losses, and coastal erosion. These effects impact the overall health and functioning of ecosystems and can have economic consequences (Munir et al. 2022). Salinity stress has various effects on plants, including disruptions in water balance, ion toxicity, oxidative stress, altered metabolic processes, and changes in hormone signaling (Etesami et al. 2021; Yuan et al. 2023). These effects can result in wilting, stunted growth, nutrient deficiencies, damage to cellular structures, and impaired physiological processes. Understanding these effects is important for developing strategies to minimize the negative impact of salinity stress on plant growth and productivity. Managing salinity is essential for preserving biodiversity, ensuring water and food security, and maintaining the integrity of agricultural areas (Abdi et al. 2023; Yuan et al. 2023).
Biochar (BC) is a form of charcoal that is produced through the process of pyrolysis, which involves heating biomass (such as agricultural waste, wood chips, or crop residues) in the absence of oxygen (Zhang et al. 2020). This process converts the biomass into a stable carbon-rich material that can be used as a soil amendment. BC can help alleviate the negative effects of salinity stress on plants by improving nutrient availability, water holding capacity, and salt leaching. It also enhances plant defense mechanisms and regulates hormone levels, resulting in improved growth and productivity (Khosropour et al. 2022). Overall, BC offers a sustainable solution to modulate salinity stress and adjust yield and biochemical attributes in plants (Liu et al. 2023).
Magnesium (Mg) is an essential nutrient for plants that is involved in numerous physiological processes such as chlorophyll (Chl) production, enzyme activation, nutrient uptake and transport, stomatal regulation, and protein synthesis (Mirrani et al. 2024). Mg NPs can help plants cope with salinity stress by maintaining ion balance, providing antioxidant activity, facilitating osmotic adjustment, enhancing nutrient uptake, and promoting plant growth and development. These effects result in improved yield and biochemical attributes in plants under salinity stress conditions (Owusu Adjei et al. 2021; Mirrani et al. 2024).
Physalis alkekengi L., commonly known as Chinese lantern or strawberry groundcherry, is a perennial herbaceous plant that produces lantern-shaped orange or red fruits. It is native to both Europe and Asia and is highly valued for its exquisite ornamentation (Abdi et al. 2023). The plant belongs to the Solanaceae family and may reach a height of one meter. It features wide leaves and little white or pale yellow blooms. The fruit grows in a papery husk and has been employed in traditional medicine due to its possible therapeutic characteristics. Various parts of the plant, including the fruits and leaves, have been used in traditional medicine for their potential anti-inflammatory, antiviral, and antioxidant properties. Overall, P. alkekengi is a fascinating plant with both esthetic and medicinal values (Liang et al. 2022; Abdi et al. 2023).
Salinity stress poses a significant barrier to agricultural production globally, resulting in lower yields and quality. Finding effective and long-term ways to minimize the detrimental impacts of salinity stress is critical for global food security. This work focuses on the usage of BC and Mg NPs as viable solutions to reduce salinity stress in P. alkekengi L. Previous studies demonstrated that separate BC and Mg may modify salinity stress. For example, the positive role of soil-applied BC has been addressed on soybean (Zhang et al. 2020), satsuma mandarin (Wu et al. 2020), tomato (Malik et al. 2023), Chinese lantern (Abdi et al. 2023). In addition, the beneficial function of Mg NPs in modulating salinity stress has been explored in rice (Song et al. 2023) and Daucus carota (Faiz et al. 2022). These studies have demonstrated that BC and Mg NPs can help mitigate the negative effects of salinity on plant growth and improve overall plant health. However, the co-application of BC and Mg NPs on salinity stress is currently unknown. Investigating the effects of these materials on biochemical attributes and fatty acid profile can provide valuable insights into their potential as innovative approaches to enhance plant growth under saline conditions. While BC and Mg NPs have been separately studied for their potential effects on plant growth and stress tolerance, their co-application and impact on P. alkekengi L. under salinity stress is relatively unexplored. The hypothesis is that the synergic effect of BC and Mg NPs is more effective than their separate application on improving plant quality under salinity stress. The study aims to evaluate the potential of these materials in adjusting yield, biochemical attributes, and fatty acid profile of the plant under salinity stress conditions. By conducting this research, the study aims to contribute to the development of sustainable and effective strategies for improving plant growth and stress tolerance in salinity-affected environments.
Materials and methods
Plant materials and growth conditions
The seeds of P. alkekengi L. were purchased from Pakan Bazr company, Isfahan, Iran. The experimental soil was a sandy loam with pH: 7.09, and EC: 0.99 dS m−1. N: 0.23%, P: 12.1 mg kg−1; K: 255 mg kg−1.
This work was conducted as factorial based on a completely randomized block design (CRBD) with three replicates in 2022. The seeds were planted in 4-L pots with in a greenhouse in Tehran, Iran. The plants were normally irrigated up to up to the 4-leaf stage. After that, they were subjected to a salty solution (400 mL in each pot) at concentrations of 100, and 200 mM NaCl every three days for 40 days. When using BC in the soil, it is typically recommended to add it at a rate of 4% of the pot volume. In addition, plants were sprayed with Mg NPs at 500 mg L−1 three times in 15-day intervals from the 4-leaf stage. Mg NPs were provided in the powder form of MgO (Sigma Aldrich) with Chemical Abstracts Service (CAS) number: 1309–48-4, 99% purify, molecular weight of 40.3 g mol−1, size of ≤ 20 nm, and density of 3.58 g cm−2. When the fruits had ripened, samples of the plants were taken to determine their physiological and biochemical characteristics.
BC production
The process for creating BC involved washing grape tree residues, drying them outdoors, grinding, and then placing the samples in containers that were covered. The containers were then heated in a furnace for 4 h at a rate of 20 °C min−1. Finally, the temperature was increased to 500 °C, at which point the grape tree residues were transformed into BC (Yuan et al. 2011).
Fruit weight
For fruit weight, the mean of all ripe fruits in each plant was recorded and reported as g per plant.
Chl assay
Leaf Chl content was quantified using Arnon’s (1949) technique. Liquid nitrogen was used to grind the 0.1 g of fresh leaves. A centrifuge was then used for 10 min at 6000 rpm after 10 mL of acetone (80%) had been added. A spectrophotometer (Shimadzu UV-160) was used to test the absorbance of samples at wavelengths of 645 and 663 nm. The results were utilized in the following equations:
where A645 and A663 are the absorbance values read at 645 and 663 nm, respectively; V is the final volume of acetone consumed in mL; W is fresh tissue weight.
Enzyme essay
At a temperature of 0–4 °C, 0.5 g of freshly homogenized samples in potassium phosphate buffer with EDTA–Na2 and ascorbate were used for enzyme extraction. The decrease in optical density at 240 nm as the hydrogen peroxide vanished was used to calculate the catalase (CAT) activity (Dhindsa et al. 1981). The activity of superoxide dismutase (SOD) was determined by measuring the absorbance at 560 nm using a procedure including riboflavin, methionine, potassium cyanide (KCN), and nitroblue tetrazolium salt (Beyer and Fridovich 1987).
Preparation of fruit extraction
A solution of liquid nitrogen was used to freeze fruit tissue samples. Next, 5 g of fruit tissue was homogenized in 10 mL of 50 mmol L–1 of phosphate buffer at a pH of 7.8. According to Yaghubi et al. (2019), the homogenate was centrifuged at 15000 × g for 20 min at 4 °C. The fruit extract-containing supernatant was collected to guide next studies.
Anthocyanin measurement
The total anthocyanin content was then calculated using the method described by Giusti and Wrolstad (2001). It was determined using two buffer solutions: a 25 mM KCl buffer at pH 1.0 and a 0.4 M Na acetate buffer at pH 4.5. Firstly, the samples were diluted with the KCl buffer until the absorbance at 510 nm fell within the linear range of the spectrophotometer. The sample was then further diluted using the same dilution factor with the Na acetate buffer. After an incubation period of 15 min in the two buffers, the absorbance was measured at wavelengths of 510 nm and 700 nm. Finally, it was determined as:
where A: (A510 – A700); MW: molecular weight; DF: dilution factor; MA: molar absorptive coefficient of cyanidin-3-glucoside (C3G). Results were expressed as mg C3G 100 g–1 of juice.
Ascorbic acid measurement
The ascorbic acid content in fruit flesh was measured using a titration method with 2,6-dichlorophenolindophenol. The fruit flesh was digested with 3% metaphosphoric acid, then filtered and diluted with more metaphosphoric acid. A portion of the filtered solution was titrated with dichlorophenol-indophenol until a pale pink color appeared. The volume of pigment used in the titration was used to calculate the amount of ascorbic acid in mg per 100 g of fruit flesh extract (Yaghubi et al. in 2019).
Fatty acid profile
The content of fatty acid methyl esters (FAMEs) in fruit flesh was estimated using gas chromatography (GC) coupled with mass spectrometry. A Varian CP-3800 GC instrument and an Agilent 5973N mass spectrometer were used, along with a capillary column DB-5MS. Helium gas at a pressure of 25 bar was used as the carrier gas. The detector and injector temperatures were set at 255 °C and 270 °C, respectively. The temperature program consisted of three stages, starting at 125 °C for 0.5 min, followed by 150 °C for 2 min, and finally 200 °C for 90 min (Morrison and Smith 1964).
Data analysis
The data were subjected to SAS (version 9.3, SAS Institute, Cary, NC). The least significant difference (LSD) test was used to compare the mean values and the data were statistically evaluated at a 5% probability level (P ≤ 0.05). Multivariate analyses were accomplished by XLSTAT. To generate a heat map, the online tool CIMMiner was utilized. This tool, accessible at https://discover.nci.nih.gov/cimminer/oneMatrix.do, allows for the creation of heat maps based on the provided data.
Results
Fruit yield and Chl content
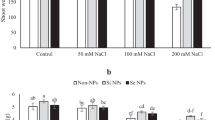
According to the findings, fruit yield was negatively impacted by salinity, with a larger yield decline occurring at higher salt levels. Nevertheless, there was a noticeable increase in fruit yield with the administration of both BC and Mg NPs. Salinity at 100 and 200 mM decreased fruit yield by 16 and 59%, respectively, compared to the control. Under severe salt stress (200 mM NaCl), the administration of BC, Mg NPs, and BC + Mg boosted fruit output by 25, 23, and 30%, respectively, when compared to non-fertilizer (Fig. 1a). Total Chl decreased as salinity increased; nevertheless, BC and Mg had a significant role in boosting Chl. Salinity stress at 100 and 200 mM caused substantial reductions in total Chl of 20 and 36%, respectively, compared to the control. However, the use of BC, Mg NPs, and BC + Mg significantly increased total Chl levels by 12, 14, and 21%, respectively (Fig. 1b).
SOD and CAT
Salinity led to increased SOD and CAT in P. alkekengi L. leaves. Without BC and Mg, salinity at 100 and 200 mM increased SOD by 51 and 82%, respectively, relative to the control. However, when BC and Mg NPs were co-applied, the SOD activity decreased by 21% in plants exposed to severe salinity, relative to plants that did not receive any BC and Mg (Fig. 2a). In addition, CAT increased by salinity and decreased by BC and Mg NPs in stressful plants. The use of BC, Mg, BC + Mg lowered CAT activity by 19, 25, and 24%, respectively, relative to the non-BC and Mg treatment in plants exposed to severe salinity (Fig. 2b).
Anthocyanin and ascorbic acid
Salinity had a meaningful impact on anthocyanin levels, with an increased quantity occurring in response to mild salinity stress (100 mM). Furthermore, using BC and Mg NPs resulted in an increase in anthocyanin content. In plants exposed to severe salinity stress, the application of BC, Mg NPs, and their co-application led to increased anthocyanin content. Accordingly, co-applied BC, Mg, and BC + Mg resulted in a 21%, 16, and 33% increases, respectively, in anthocyanin levels compared to plants that did not receive BC and Mg NPs (Fig. 3a). Ascorbic acid levels rose in response to moderate salinity, as did soil-applied BC and foliar-applied Mg NPs. The maximum ascorbic acid was observed in plants under the interaction of salinity at 100 mM and co-application of BC and Mg with a 15% increase relative to the control (Fig. 3b).
Fatty acid profile
The analysis of fatty acid profiles revealed that salinity, as well as the application of BC and Mg NPs, had an impact on the fatty acid profile in P. alkekengi L. fruit (Table 1). The primary changes in fatty acid composition were observed under salinity stress. Under salinity conditions, the levels of saturated fatty acids (SFAs) increased. Specifically, palmitic acid (C16:0) showed a significant increase of 16% in plants exposed to salinity at 200 mM compared to the control. Palmitoleic acid (C18:0) was found in a range of 1.23–2.66% of total fatty acid content. Stearic acid (C18:0) did not show a significantly trend under the treatments. Oleic acid (C18:1) decreased under salinity, while BC and Mg increased it. The maximum oleic acid was obtained in plants treated with co-applied BC and Mg NPs without salinity. In contrast, linoleic acid (C18:1) rose with salinity stress but reduced when BC and Mg NPs were applied. The lowest amounts of linoleic acid were observed in plants subjected to 200 mM salinity without BC and Mg, but the maximum levels were achieved in non-saline conditions with the co-application of BC and Mg NPs. Linolenic acid (C18:3) was obtained in a range of 1.83–4.33%. Overall, the salinity stress led to a decrease in monounsaturated fatty acids (MUFAs) and an increase in SFAs and polyunsaturated fatty acids (PUFAs) in the P. alkekengi L. plants. The application of BC and Mg NPs had varying effects on the fatty acid profile, with BC and Mg reducing SFAs and influencing the levels of oleic and linoleic acids (Table 1).
PCA analysis
The changes in fruit yield, Chl, CAT activity, SOD activity, and the fatty acids palmitic, palmitoleic, oleic, linoleic, and linolenic acids could all be justified by the first component (F1) based on the PCA of salinity. This indicates a strong correlation between these variables and their contribution to the same underlying pattern in the data. In contrast, the changes in stearic acid, ascorbic acid, and anthocyanin concentration was justified by the second component (F2) in the PCA of salinity stress. This shows that these variables have additional correlations with one another and help to explain a unique pattern in the data that the first component is unable to explain (Fig. 4a). All of the traits, with the exception of stearic acid, were explained by F1, according to PCA of BC and Mg. This suggests that these variables are highly correlated with one another and work together to form a common overall pattern. But F2 was only connected to stearic acid (Fig. 4b). Overall, the PCA results demonstrate that various variables, or traits, are most impacted by either the first or the second component, pointing to different patterns of correlations between the traits.
Heat map
The heat map analysis showed that the control group and the salinity treatment at 100 mM were grouped together in a cluster, suggesting that their reactions are similar. In contrast to the control and 100 mM treatments, the salinity treatment at 200 mM was found in a different cluster, indicating that it has a different reaction. It was discovered that fruit yield, anthocyanin, Chl content, and CAT activity were more susceptible to salinity. It can be inferred from this that these characteristics varied more under the salinity treatment than they did under the control. These characteristics may be employed as markers or indicators for saline conditions (Fig. 5a). Based on to the examination of Mg NPs and BC, the control and the Mg NPs treatment were assigned to the same cluster, meaning that their responses were identical. However, the BC treatment was placed in a separate cluster, indicating a different reaction than the control and Mg NPs treatments. Palmitoleic and linolenic acids were shown to have higher sensitivity to BC and Mg than the other traits. This suggests that the BC and Mg treatments produced more variances or changes in these fatty acids than the control. These traits might serve as markers or indicators for the impact of BC and Mg on the investigated system (Fig. 5b).
Discussion
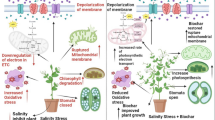
Salinity stress had a major effect on fruit production of P. alkekengi L. via a variety of biochemical processes. Salinity stress can disturb the ion balance within plant cells, notably the Na+ to K+ ratio. High quantities of Na+ can accumulate in plant tissues, causing toxicity and impeding normal physiological activities. (Zahedi et al. 2020). This ionic imbalance interferes with essential biochemical reactions, ultimately resulting in reduced fruit yield. Salinity stress can induce the generation of reactive oxygen species (ROS) within plant cells. These ROS, O2− and H2O2, can cause oxidative damage to cellular components such as lipids, proteins, and DNA (Costan et al. 2020). This oxidative stress negatively affects various biochemical pathways essential for optimal fruit development, leading to decreased yield. Salinity stress often disrupts the photosynthetic process, reducing the plant’s ability to produce energy through photosynthesis. High salt concentrations can inhibit the activity of enzymes involved in carbon fixation, such as Rubisco, and can also impair electron transport chains in chloroplasts. As a result, the production of energy-rich compounds, such as carbohydrates, is compromised, ultimately impacting fruit yield. Salinity stress imposes an osmotic challenge on plants by increasing the concentration of salts in the surrounding soil. Salinity stress can disrupt hormonal balance within the plant. For example, it can increase the levels of stress hormones like abscisic acid (ABA) and ethylene while decreasing the levels of growth-promoting hormones like auxins and cytokinins. These hormonal alterations can interfere with various biochemical pathways involved in cell division, elongation, and fruit development, negatively impacting yield (Dias et al. 2022). The combined application of BC and Mg can positively impact fruit yield under salinity stress. This is achieved through improved nutrient availability, enhanced water holding capacity, buffering of excessive sodium ions, alleviation of oxidative stress, and restoration of hormonal balance. The addition of BC and Mg helps mitigate the negative effects of salinity stress on fruit development, ultimately leading to increased yields in salt-affected environments (Wu et al. 2020). Although detrimental impacts on salinity have been documented in many research (Abdi et al. 2023; Li et al. 2023), beneficial effects of BC (Wu et al. 2022; Harhash et al. 2022) and Mg NPs (Owusu Adjei et al. 2021) have also been reported.
Salinity stress increased the activity of SOD and CAT P. alkekengi L. This is a critical component of the plant’s response to ROS buildup. Superoxide dismutase converts superoxide radicals into less toxic molecules, whereas CAT degrades hydrogen peroxide. These enzymes aid in the plant’s defense against oxidative damage induced by salt stress. Salinity stress can accumulate ROS in plants, causing oxidative stress and damage to numerous cellular components (Moniruzzaman et al. 2022). Superoxide dismutase and CAT are responsible for scavenging ROS and protecting the plant from oxidative damage (Dawood et al. 2022). The application of BC and Mg NPs in this study affected the biochemical pathways involved in this antioxidant defense system. BC is known to have high sorption capacity and can retain nutrients, reducing their availability to plants. This limitation of nutrient availability may have contributed to the decrease in SOD and CAT activity. Mg NPs, on the other hand, have been shown to interact with ROS and inhibit their formation, thereby reducing the need for antioxidant enzymes like SOD and CAT (Song et al. 2023). This direct interaction with ROS may have resulted in the downregulation of these enzymes. In salt-exposed plants, when BC and Mg NPs are applied, ROS can decrease, resulting in decreased activity of antioxidant enzymes. Similarly, Kul et al. (2021) reported the mitigation of salinity by applying BC through changes in ACT and SOD activity in tomato plants. Though the usage of Mg NPs to modulate salinity is unknown, Faiz et al. (2022) found that it has a good function in controlling other abiotic stress on Daucus carota.
Salinity stress reduced Chl content in P. alkekengi L. plants through biochemical pathways such as impaired chl synthesis, inhibition of chlorophyll degradation, increased chloroplast damage, disturbed nutrient uptake, and altered hormonal balance (Ababsa et al. 2023). These effects collectively lead to a decrease in Chl content, impacting photosynthesis and overall plant growth. Co-application of BC and Mg can potentially increase Chl content in plants under salinity stress. BC has a high cation exchange capacity, which helps retain and release essential nutrients, including Mg, in the root zone (Hafez et al. 2021). Mg is a key component of the Chl molecule, and its availability is crucial for Chl synthesis. The addition of BC and Mg can supply an ample amount of Mg to plants, promoting the biosynthesis of Chl and increasing its content. Salinity stress can disrupt photosynthetic processes, including Chl synthesis and function. BC has been shown to improve photosynthetic efficiency by enhancing the physiological and biochemical functions of chloroplasts. The presence of BC can protect the chloroplast membranes from salt-induced damage and maintain optimal photosynthetic activity, leading to increased Chl content. Salinity stress induces oxidative stress in plants, resulting in the accumulation of ROS. Oxidative stress can lead to chloroplast damage and reduction in Chl content. BC possesses antioxidant properties and can mitigate ROS accumulation by scavenging these harmful molecules. The combined application of BC and Mg can reduce oxidative stress levels, thus preserving chloroplast integrity and increasing Chl content (Mirrani et al. 2024).
The co-applied BC and Mg could potentially increase anthocyanin content in P. alkekengi L. plants under moderate salinity stress. Salinity stress can induce oxidative stress in plants, leading to the production of ROS. Anthocyanins are known to possess strong antioxidant properties and can help scavenge ROS, reducing oxidative damage (Mansour 2023). In a similar study, Abdi et al. (2023) found that salinity at 50–100 mM Nacl enhanced anthocyanin while 150 mM reduced it. BC has been shown to enhance the antioxidant capacity of plants by promoting the synthesis of antioxidants, including anthocyanins. The addition of BC and Mg can stimulate the production of anthocyanins, increasing their content in plant tissues. Salinity stress can affect hormone levels, particularly ABA, which is known to negatively regulate anthocyanin synthesis. It has been addressed that BC can modulate hormone levels, including reducing ABA accumulation and promoting the activity of other growth-promoting hormones, such as auxins and cytokinins (Abd El-Wahed et al. 2023). By regulating hormone balance, BC and Mg can enhance anthocyanin biosynthesis and increase their content under salinity stress. Mg is an essential micronutrient involved in various physiological processes, including anthocyanin production. Salinity stress can interfere with Mg uptake, leading to nutrient imbalances and potentially reducing anthocyanin synthesis. The application of Mg along with BC can provide an adequate supply of Mg to plants, ensuring optimal nutrient availability for anthocyanin biosynthesis and increasing anthocyanin content (Gautam et al. 2023). Salinity stress triggers stress signaling pathways in plants, which can activate genes involved in anthocyanin synthesis. BC has been shown to influence gene expression and signaling pathways related to stress responses (Ojagh and Moaveni 2022). The presence of BC and Mg can enhance the stress signaling pathways, promoting the expression of genes associated with anthocyanin biosynthesis and consequently increasing anthocyanin content. This increase in anthocyanin content can contribute to enhanced antioxidant activity, stress tolerance, and potentially improved plant growth under salinity conditions (Abdi et al. 2023). Similarly, Ghassemi-Golezani and Rahimzadeh (2023) observed that BC had a favorable effect on anthocyanin levels in dill.
The amount of ascorbic acid was significantly enhanced with co-application of BC and Mg in P. alkekengi L. plants under salinity stress. Salinity stress induces oxidative stress in plants, leading to the accumulation of ROS. Ascorbic acid acts as a potent antioxidant and plays a crucial role in scavenging ROS (Shahzad et al. 2023). It has been reported that BC can enhance antioxidant activity in plants by promoting the synthesis of antioxidants, including ascorbic acid. The application of BC and Mg can stimulate the production of ascorbic acid, increasing its content in plant tissues and effectively mitigating oxidative damage caused by salinity stress. Ascorbic acid is involved in the activation of various enzymes that play essential roles in plant metabolism and stress responses (Malik et al. 2023). Mg acts as a cofactor for enzymes involved in ascorbic acid biosynthesis. Salinity stress can interfere with Mg uptake, leading to a decrease in enzyme activity associated with ascorbic acid synthesis (Younis et al. 2021). The addition of Mg along with BC can provide sufficient Mg supply, ensuring optimal enzyme activity and promoting ascorbic acid production. Salinity stress triggers stress signaling pathways in plants, which can activate genes involved in ascorbic acid biosynthesis. BC can influence gene expression and signaling pathways related to stress responses. The presence of BC and Mg can enhance the stress signaling pathways, promoting the expression of genes associated with ascorbic acid synthesis and consequently increasing ascorbic acid content. Mg is an essential nutrient involved in various metabolic processes, including ascorbic acid biosynthesis (Hussien and Gad El-Kareem 2021). Salinity stress can disrupt nutrient uptake and alter nutrient balance, potentially affecting ascorbic acid synthesis. The application of Mg along with BC can provide an adequate supply of Mg to plants, ensuring optimal nutrient availability for ascorbic acid biosynthesis and increasing its content. Through these mechanisms, the combined application of BC and Mg can potentially increase ascorbic acid levels in plants subjected to salinity stress. This increase in ascorbic acid content can contribute to enhanced antioxidant activity, stress tolerance, and potentially improved plant growth under salinity conditions (Abdi et al. 2023).
In salt-exposed plants, the levels of SFAs are generally increased, while the levels of PUFAs are also increased. However, the levels of MUFAs are typically decreased. The increase in SFAs is thought to be a response to salt stress, as these fatty acids provide stability to cell membranes and protect them from damage caused by high salinity levels. The increase in PUFAs is believed to be a protective mechanism against salinity stress, as these fatty acids are more fluid and can maintain the fluidity and functionality of cell membranes under salt exposure. On the other hand, the decrease in MUFAs is likely due to their susceptibility to oxidative damage caused by ROS accumulation in salt-stressed plants (Hojjati et al. 2023). Similarly, the increased in PUFAs and SFAs and a decreased in MUFAs has been reported in P. alkekengi L. plants by Abdi et al. (2021). However, the use of BC and Mg NPs enhanced MUFAs but decreased SFAs. Application of BC and Mg NPs can help alleviate the negative effects of salt stress on fatty acid composition. These additives can improve the soil environment and nutrient availability, leading to enhanced plant growth and development. The increase in MUFAs can be beneficial as they have been associated with various health benefits and are considered healthier fats compared to SFAs. MUFAs are known for their positive effects on cardiovascular health and can reduce the risk of chronic diseases. Additionally, the decrease in SFAs is favorable as high levels of SFAs have been linked to negative health outcomes, such as increased cholesterol levels and cardiovascular risks (Prasad et al. 2021). Nasirzadeh et al. (2022), Ghasemzadehet al. (2022), and Hojjati et al. (2023) previously discovered increased PUFAs, SFAs, and decreased MUFAs in response to different abiotic stressors. Interestingly, Abdi et al. (2023) discovered a similar fatty acid pattern in P. alkekengi L. under salinity and silicon treatments.
Conclusions
The use of biochar and magnesium nanoparticles has been shown to successfully reduce salt stress in Physalis alkekengi L. plants. This modification has positively altered various critical aspects, including yield, biochemical characteristics, and fatty acid profile. The usage of biochar and magnesium nanoparticles has shown encouraging results in increasing plant yield in salty environments. Moreover, the use of charcoal and magnesium nanoparticles increased the plants’ biochemical characteristics. Salinity stress can alter a number of physiological processes, including oxidative stress and the accumulation of reactive oxygen species. However, biochar and magnesium nanoparticles has been demonstrated to reduce ROS levels while increasing antioxidant enzyme activity, hence lowering oxidative damage and enhancing plant health. Furthermore, biochar and magnesium nanoparticles have been shown to increase the levels of monounsaturated fatty acids while decreasing the levels of saturated fatty acids, creating a more favorable and healthier fatty acid composition of Physalis alkekengi. fruits. Overall, soil-applied biochar and foliar-applied magnesium nanoparticles appears to be a potential technique for reducing the deleterious effects of salt stress on Physalis alkekengi L. plants. These chemicals not only increase yield but also improve biochemical properties and fatty acid profiles, eventually adding to the overall health and quality of the plants.
Data availability
The data are available on request.
References
Ababsa N, Boudjabi S, Chenchouni H (2023) Biochar amendments changed soil properties and improved cereal crop growth under salt stress. J Soil Sci Plant Nutr 1–14. https://doi.org/10.1007/s42729-023-01453-7
Abd El-Wahed MH, Eissa MA, Almasoudi NM, Abo-Elyousr KA (2023) Macronutrient-rich biochar induces boron nanoparticles in improving the salt tolerance of pomegranate (Punica granatum L.) in arid degraded soils. Sci Hort 313:111908. https://doi.org/10.1016/j.scienta.2023.111908
Abdi MJ, Ghanbari Jahromi M, Mortazavi SN, Kalateh Jari S, Nazarideljou MJ (2023) Foliar-applied silicon and selenium nanoparticles modulated salinity stress through modifying yield, biochemical attribute, and fatty acid profile of Physalis alkekengi L. Environ Sci Pollut Res 30:100513–100525. https://doi.org/10.1007/s11356-023-29450-4
Beyer JWF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Costan A, Stamatakis A, Chrysargyris A, Petropoulos SA, Tzortzakis N (2020) Interactive effects of salinity and silicon application on Solanum lycopersicum growth, physiology and shelf-life of fruit produced hydroponically. J Sci Food Agric 100:732–743. https://doi.org/10.1002/jsfa.10076
Dawood MA, Alkafafy M, Sewilam H (2022) The antioxidant responses of gills, intestines and livers and blood immunity of common carp (Cyprinus carpio) exposed to salinity and temperature stressors. Fish Physiol Biochem 48:397–408. https://doi.org/10.1007/s10695-022-01052-w
Dhindsa RS, Plumb-Dhindsa PAMELA, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. https://doi.org/10.1093/jxb/32.1.93
Dias AS, Lima GSD, Gheyi HR, Melo ASD, Silva PCC, Soares LADA, Silva SSD (2022) Effect of combined potassium-phosphorus fertilization on gas exchange, antioxidant activity and fruit production of West Indian cherry under salt stress. Arid Land Res Manag 36:163–180. https://doi.org/10.1080/15324982.2021.1959464
Etesami H, Fatemi H, Rizwan M (2021) Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: a review. Ecotox Environ Saf 225:112769. https://doi.org/10.1016/j.ecoenv.2021.112769
Faiz S, Yasin NA, Khan WU, Shah AA, Akram W, Ahmad A, Riaz L (2022) Role of magnesium oxide nanoparticles in the mitigation of lead-induced stress in Daucus carota: modulation in polyamines and antioxidant enzymes. Int J Phytorem 24:364–372. https://doi.org/10.1080/15226514.2021.1949263
Gautam A, Sharma P, Ashokhan S, Yaacob JS, Kumar V, Guleria P (2023) Magnesium oxide nanoparticles improved vegetative growth and enhanced productivity, biochemical potency and storage stability of harvested mustard seeds. Environ Res 229:116023. https://doi.org/10.1016/j.envres.2023.116023
Ghasemzadeh N, Iranbakhsh A, Oraghi-Ardebili Z, Saadatmand S, Jahanbakhsh-Godehkahriz S (2022) Cold plasma can alleviate cadmium stress by optimizing growth and yield of wheat (Triticum aestivum L.) through changes in physio-biochemical properties and fatty acid profile. Environ Sci Pollut Res 29:35897–35907. https://doi.org/10.1007/s11356-022-18630-3
Ghassemi-Golezani K, Rahimzadeh S (2023) Biochar-based nutritional nanocomposites: a superior treatment for alleviating salt toxicity and improving physiological performance of dill (Anethum graveolens). Environ Geochem Health 45:3089–3111. https://doi.org/10.1007/s10653-022-01397-4
Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr Porotoc Food Anal Chem 1:1–2. https://doi.org/10.1002/0471142913.faf0102s00
Hafez EM, Omara AED, Alhumaydhi FA, El-Esawi MA (2021) Minimizing hazard impacts of soil salinity and water stress on wheat plants by soil application of vermicompost and biochar. Physiol Plant 172:587–602. https://doi.org/10.1111/ppl.13261
Harhash MM, Ahamed MM, Mosa WF (2022) Mango performance as affected by the soil application of zeolite and biochar under water salinity stresses. Environ Sci Pollut Res 29:87144–87156. https://doi.org/10.1007/s11356-022-21503-4
Hussien M, Gad El-Kareem M (2021) Response of valencia orange trees to foliar application of some antioxidants (ascorbic and salicylic acids), magnesium and micronutrients. Alex Sci Exch 42:883–891. https://doi.org/10.21608/asejaiqjsae.2021.205326
Hojjati M, Jahromi MG, Abdossi V, Torkashvand AM (2023) exogenous melatonin modulated drought stress by regulating physio-biochemical attributes and fatty acid profile of sweet cherry (Prunus avium L.). J Plant Growth Regul 43:299–313. https://doi.org/10.1007/s00344-023-11085-xa
Khosropour E, Weisany W, Tahir NAR, Hakimi L (2022) Vermicompost and biochar can alleviate cadmium stress through minimizing its uptake and optimizing biochemical properties in Berberis integerrima bunge. Environ Sci Pollut Re 29:17476–17486. https://doi.org/10.1007/s11356-021-17073-6
Kul R, Arjumend T, Ekinci M, Yildirim E, Turan M, Argin S (2021) Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Soil Sci Plant Nutr 67:693–706. https://doi.org/10.1080/00380768.2021.1998924
Li H, Hou X, Bertin N, Ding R, Du T (2023) Quantitative responses of tomato yield, fruit quality and water use efficiency to soil salinity under different water regimes in Northwest China. Agric Water Manag 277:108134. https://doi.org/10.1016/j.agwat.2022.108134
Liang L, Li, C, Wang Y, Yue Y, Zhang H, Yang M, Shu Z (2022) Physalis alkekengi L. var. franchetii (Mast.) Makino: a review of the pharmacognosy, chemical constituents, pharmacological effects, quality control, and applications. Phytomedicine 154328. https://doi.org/10.1016/j.phymed.2022.154328
Liu Q, Meki K, Zheng H, Yuan Y, Shao M, Luo X, Xing B (2023) Biochar application in remediating salt-affected soil to achieve carbon neutrality and abate climate change. Biochar 5:45. https://doi.org/10.1007/s42773-023-00244-8
Malik Z, Malik N, Noor I, Kamran M, Parveen A, Ali M, Fahad S (2023) Combined effect of rice-straw biochar and humic acid on growth, antioxidative capacity, and ion uptake in maize (Zea mays L.) grown under saline soil conditions. J Plant Growth Regul 42:3211–3228. https://doi.org/10.1007/s00344-022-10786-z
Mansour MMF (2023) Anthocyanins: biotechnological targets for enhancing crop tolerance to salinity stress. Sci Hort 319:112182. https://doi.org/10.1016/j.scienta.2023.112182
Mirrani HM, Noreen Z, Usman S, Shah AA, Mahmoud EA, Elansary HO, Javed T (2024) Magnesium nanoparticles extirpate salt stress in carrots (Daucus carota L.) through metabolomics regulations. Plant Physiol Biochem 108383. https://doi.org/10.1016/j.plaphy.2024.108383
Moniruzzaman M, Mukherjee M, Kumar S, Chakraborty SB (2022) Effects of salinity stress on antioxidant status and inflammatory responses in females of a “Near Threatened” economically important fish species Notopterus chitala: a mechanistic approach. Environ Sci Pollut Res 29:75031–75042. https://doi.org/10.1007/s11356-022-21142-9
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 5:600–608. https://doi.org/10.1016/S0022-2275(20)40190-7
Munir N, Hasnain M, Roessner U, Abideen Z (2022) Strategies in improving plant salinity resistance and use of salinity resistant plants for economic sustainability. Environ Sci Technol 52:2150–2196. https://doi.org/10.1080/10643389.2021.1877033
Nasirzadeh L, Kvarnheden A, Sorkhilaleloo B, Hervan EM, Fatehi F (2022) Foliar-applied selenium nanoparticles can alleviate soil-cadmium stress through physio-chemical and stomatal changes to optimize yield, antioxidant capacity, and fatty acid profile of wheat (Triticum aestivum L.). J Soil Sci Plant Nutr 22:2469–2480. https://doi.org/10.1007/s42729-022-00821-z
Ojagh SE, Moaveni P (2022) Foliar-applied magnesium nanoparticles modulate drought stress through changes in physio-biochemical attributes and essential oil profile of yarrow (Achillea millefolium L.). Environm Sci Pollut Res 29:59374–59384. https://doi.org/10.1007/s11356-022-19559-3
Owusu Adjei M, Zhou X, Mao M, Xue Y, Liu J, Hu H, Ma J (2021) Magnesium oxide nanoparticle effect on the growth, development, and microRNAs expression of Ananas comosus var. bracteatus. J Plant Interact 16:247–257. https://doi.org/10.1080/17429145.2021.1931720
Prasad P, Anjali P, Sreedhar RV (2021) Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Food Sci Nutr 61:1725–1737. https://doi.org/10.1080/10408398.2020.1765137
Shahzad AS, Younis U, Naz N, Danish S, Syed A, Elgorban AM, Battaglia ML (2023) Acidified biochar improves lead tolerance and enhances morphological and biochemical attributes of mint in saline soil. Sci Rep 13:8720. https://doi.org/10.1038/s41598-023-36018-2
Song Y, Zheng C, Li S, Chen J, Jiang M (2023) Chitosan-magnesium oxide nanoparticles improve salinity tolerance in rice (Oryza sativa L.). ACS Appl Mat Interf 15:20649–20660. https://doi.org/10.1021/acsami.3c00043
Wu S, Zhang Y, Tan Q, Sun X, Wei W, Hu C (2020) Biochar is superior to lime in improving acidic soil properties and fruit quality of Satsuma mandarin. Sci Total Environ 714:136722. https://doi.org/10.1016/j.scitotenv.2020.136722
Wu Z, Fan Y, Qiu Y, Hao X, Li S, Kang S (2022) Response of yield and quality of greenhouse tomatoes to water and salt stresses and biochar addition in Northwest China. Agric Water Manag 270:107736. https://doi.org/10.1016/j.agwat.2022.107736
Yaghubi K, Vafaee Y, Ghaderi N, Javadi T (2019) Potassium silicate improves salinity resistant and affects fruit quality in two strawberry cultivars grown under salt stress. Commun Soil Sci Plant Anal 50:1439–1451. https://doi.org/10.1080/00103624.2019.1621333
Younis IY, El-Hawary SS, Eldahshan OA, Abdel-Aziz MM, Ali ZY (2021) Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci Rep 11:16868. https://doi.org/10.1038/s41598-021-96377-6
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102(3):3488–3497. https://doi.org/10.1016/j.biortech.2010.11.018
Yuan Y, Liu Q, Zheng H, Li M, Liu Y, Wang X, Xing B (2023) Biochar as a sustainable tool for improving the health of salt-affected soil. Soil Environ Health 100033. https://doi.org/10.1016/j.seh.2023.100033
Zahedi SM, Hosseini MS, Abadía J, Marjani M (2020) Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria× ananassa Duch.). Plant Physiol Biochemi 149:313–323. https://doi.org/10.1016/j.plaphy.2020.02.021
Zhang Y, Ding J, Wang H, Su L, Zhao C (2020) Biochar addition alleviate the negative effects of drought and salinity stress on soybean productivity and water use efficiency. BMC Plant Biol 20:1–11. https://doi.org/10.1186/s12870-020-02493-2
Author information
Authors and Affiliations
Contributions
The data were provided by Zahra Amirfakhrian, Vahid Abdossi, Ali Mohammadi Torkashvand, Weria Weisany, and Marzieh Ghanbari Jahromi; The initial draft was prepared by Zahra Amirfakhrian and revised by the others.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval was not required for this work.
Consent to participate
The author agreed to submit the manuscript to Environmental Science and Pollution Research.
Consent for publication
The author approved the final manuscript to publish in Environmental Science and Pollution Research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amirfakhrian, Z., Abdossi, V., Mohammadi Torkashvand, A. et al. Co-applied magnesium nanoparticles and biochar modulate salinity stress via regulating yield, biochemical attribute, and fatty acid profile of Physalis alkekengi L. Environ Sci Pollut Res 31, 31806–31817 (2024). https://doi.org/10.1007/s11356-024-33329-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33329-3