Abstract
Nanoparticles (NPs) are an emerging tool for mitigating environmental stresses. Although beneficial roles of NPs have been reported in some plants, there is little data on magnesium (Mg)-NPs in alleviating drought stress. Therefore, the field experiment was conducted to study changes in biochemical attributes and essential oil (EO) compositions of yarrow (Achillea millefolium L.) plants under drought stress and Mg-NPs in 2016 and 2017. Irrigation regimes were used in two levels as well-watered (irrigation intervals of 7 days) and drought stress (irrigation intervals of 14 days) conditions, and Mg-NPs were sprayed on leaves in four levels (0, 0.1, 0.3, and 0.5 g L−1). The results showed drought stress led to increased electrolyte leakage (EL), proline, carotenoid, anthocyanin, and total flavonoid content (TFC). However, flowers yield and EO yield were lower in plants exposed to drought stress as compared to well-watered conditions. The 0.3 and 0.5 g L−1 Mg-NPs were more effective in alleviating drought stress by enhancing these traits. Heat map results showed that EL and TSS represented the high variability upon different treatments. The GC and GC/MS results represented that α-pinene (8.60–12.20%), 1,8-cineol (9.03–14.02%), camphor (6.84–9.80%), α-bisabolol (8.54–18.81%), chamazulene (14.23–22.50%), and caryophyllene oxide (7.20–9.80%) were the min EO constitutes of yarrow plants. Totally, drought decreased monopertens but increased sesquiterpenes of EO. To sum up, foliar applied Mg-NPs in a range of 0.3–0.5 g L−1 can be recommended as effective tool to improve plant yield through changes in biochemical attributes of yarrow plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is an enhancing interest to replace synthetic medicines with herbal products; therefore, the medicinal plants are in a pressure of over harvesting in the world)Van Wyk and Prinsloo 2018). Asteraceae is a one of the largest family of medicinal and ornamental of plants, which includes major genera such as Cichorium, Stevia, and Achillea (Rolnik et al. 2021). Yarrow (Achillea millefolium) is widely grown due to great importance in the cosmetics, health, and pharmaceutical industries. This species is used in the treatment of intestinal, gastric, liver, and bile disorders (Applequist and Moerman 2011; Strzępek-Gomółka et al. 2021). In addition, it is used as an appetite-enhancing drug due to its bitter taste, wound healing and also against skin inflammations (Chou et al. 2013). In fact, the many properties and benefits of yarrow are due to the various compounds in its essential oils (EOs) (Rakmai et al 2017). Yarrow EOs contain sesquiterpene, monoterpene, phenolic compounds, and terpenoids such as cineole, camphor, and caryophylline (Howyzeh et al. 2019; Farhadi et al. 2020). The presence and amount of EOs constituents of medicinal plants is related to genetic and environmental factors (Saki et al. 2019). On the other hand, it has been reported that secondary metabolites in medicinal plants are the main response of plants to cope with environmental stresses (Mirzaie et al. 2020).
Drought stress is the important environmental challenge that has significant impacts on plant growth and development. In addition, it can strongly affect the secondary metabolites (Gharibi et al. 2019) and lead to overproduction of reactive oxygen species (ROS). Plants also have different molecular and biochemical mechanisms to inhibit free radicals, including enzymatic antioxidant systems (catalase, peroxidase, etc.), and various non-enzymatic antioxidants (flavonoids, anthocyanins, phenylpropanoids, etc.) (Tohidi et al. 2017). Among the physiological strategies of the plant, the accumulation of osmolites is one of the most effective methods to help plants cope with the deleterious effects of water scarcity. Increased accumulation of osmolites such as total flavonoids, total phenol, proline, malondialdehyde, and hydrogen peroxide has been reported in various plants exposed to drought stress (Gharibi et al. 2015; Mirzaie et al. 2020; Afshari et al. 2021).
Foliar application of elements improves the mechanism of tolerance to water stress and thus increases plant yield (Aslam et al. 2018). One of the most important elements in the growth and development of plants is magnesium (Mg). The most important role of Mg in plants is to participate in the construction of chlorophyll, which contains about 15–30% of the total Mg in plants. Mg also activates more than 300 enzymes such as RNA polymerases, ATPases, protein kinases, phosphatases, glutathione synthase, and carboxylases, and regulates ion transport and cation balance in plants (Bose et al. 2011). It plays a key role in the biosynthesis of nucleic acids and proteins, the transport of photosynthetics in the phloem, the control of plant diseases, and finally in the growth, yield, and product quality of plants (Le et al. 2020). In general, foliar application of fertilizers is used to quickly supply the nutrients needed by the plant, and in these conditions, the use of nanoparticles (NPs) as foliar application is significantly more efficient for plant uptake (Afshari et al. 2021). Since NPs are less than 100 nm in size, it causes interesting and remarkable properties and behaviors of these materials such as high mobility, self-control, and intelligence properties in these particles (Ghasemian et al. 2021). The NPs are more effective than their usual particles due to their higher adsorption efficiency and higher specific surface area (Tripathi et al. 2017; Afshari et al. 2021).
Recently, NPs have been widely used to mitigate drought stress on plants. The mitigation of drought stress with the use of different NPS have been reported on coriander (Afshari et al. 2021), rice (Ahmed et al. 2021); maize (Van Nguyen et al. 2021), eggplant (Semida et al. 2021), pomegranates (Zahedi et al. 2021), cucumber (Ghani et al. 2022), and tomato (El-Zohri et al. 2121). However, there is little information for Mg-NPs on medicinal plants upon irrigation regimes. The aims of present study were (1) to assess the effect of Mg-NPs on electrolyte leakage, carbohydrate, proline, anthocyanin, carotenoids, and flavonoid under drought stress in yarrow plants and (2) to study the changes of essential oil (EO) yield and EO compositions of yarrow plants at two different irrigation regimes and foliar application of Mg-NPs.
Materials and methods
Nanoparticles properties
Mg-NPs were provided in the powder form of MgO (Sigma Aldrich) with CAS number: 1309–48-4, 99% purify, molecular weight of 40.3 g mol−1, size of ≤ 20 nm, and density of 3.58 g cm−2.
Experimental design and treatments
The field experiment was conducted to evaluate the effect of Mg-NPs on biochemical properties of yarrow (Achillea millefolium L.) under two irrigation grimes as a split design with three replicates on in Safadsht, Iran (1165 m asl, 35° 41′ 17″ N, 50° 50′ 25″ E), during 2016–2017. The experiment included irrigation regime as main plot at two levels including normal irrigation (irrigation intervals of 7 days) and water stress conditions (irrigation intervals of 14 days); the subplot was the concentration of foliar applied Mg-NPs at four levels (0, 0.1, 0.3, and 0.5 g L−1).
Growth conditions
The operations of land preparation were carried out according to the custom of the region by plowing and disking. The seeds were cultured in 3.5 × 5 m plots with 1-m distance between the plots and 30 cm distance between plants on the rows. Before culturing, soil samples from two depths (0–20 and 20–40 cm) were transferred to laboratory to determine physiochemical properties (Table 1), and according to soil properties and fertilizer recommendation, the NPK fertilizers were added to soil. The plants were sprayed by the solutions of Mg-NPs at three times including leaf development, stem elongation, and budding. Irrigation regimes were applied in two levels based on evaporation from class A pan with irrigation after 60 mm (irrigation intervals of 7 day( and 120 mm (irrigation intervals of 14 day( evaporation from class A pan. Irrigation was done using drip irrigation system. The weeds were manually harvested during the growth period.
Electrolyte leakage measurement
To determine EL, 1-cm disks of fresh leaves were prepared and moved to Erlenmeyer’s containing 10 mL of water twice distilled at 25 °C. After shaking for 24 h, the EC of samples was measured by a gage digital EC (EL1 μS/cm). Subsequently, the solution was placed in an autoclave for 1 h at 120 °C and then its EC was determined (EL2 μS/cm). Finally, electrolyte leakage (EL) was calculated as follow (Sheppard et al. 1995). EL = [(EL2 – EL1)/EL1)].
Determination of total soluble sugar
The 95% and 70% ethanol was used to determine total soluble sugar (TSS). The 0.5 g of frozen flowers was grinded liquid nitrogen and mixed with 5 mL of 95% ethanol. After that, 5 mL of 70% ethanol was added to the mixture in two times and centrifuged at 3500 rpm for 10 min and followed by keeping in the refrigerator for 7 days. Subsequently, 0.1 mL of the stored stock was mixed with 3-mL antron (150-mg antron and 100-mL 72% sulfuric acid). The solution was placed in the boiling water bath at 90 °C for 10 min. The absorbance was measured at 625 nm using a UV–Vis spectrophotometer (Shimadzu, Tokyo, Japan). For a standardized diagram, it was used the solutions with 0, 1, 2, 3, 4, 5, 7, and 10 ppm concentrations (Irigoyen et al. 1992).
Total flavonoid content
The aluminum chloride colorimetric method was used to determine total flavonoid content (TFC). According to this instruction, 0.5 mL of the extract solution with mixed with 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water. After keeping the samples at room temperature for 30 min, the adsorption of the mixture was read at 415 nm. The quercetin standard was used to draw the curve (Chlopicka et al. 2012).
Proline concentration
To determine the proline content, 0.1 g of fresh leaf tissue was mixed with 10 mL of sulfosalicylic acid (3% w/v) and centrifuged at 4000 × g for 20 min. Then, the mixture was supplied with 2 mL of ninhydrin acid and 2 mL of glacial acetic acid. Simultaneously, 2 mL of standard 0, 4, 8, 12, 16, and 20 mg of proline were mixed with 2 mL of ninhydrinic acid and 2 mL of acetic acid. All samples were heated in a hot water bath for 60 min and then placed on ice to cool completely. The 4 mL of toluene was added to the solution. Using 0, 4, 8, 12, 16, and 20 mg proline, the regression equation of the standard curve was determined spectrophotometrically at 520 nm (Bates et al. 1973).
Flower carotenoids content
Arnon (1967) method was used to measure carotenoid content in flowers. The 0.5 g of fresh samples was grinded using liquid nitrogen and then 20 mL acetone 80% was added and centrifuged at 6000 rpm for 10 min. The supernatant was transferred to a glass balloon. The values were recorded by a spectrophotometer in the absorbance read at 470, 645, and 663 nm. The carotenoid content was calculated according to the following equations.
Chl a = (12.7 \(\times\) A663 − 2.69 \(\times\) A645) V/1000 W.
Chl b = (22.9 \(\times\) A645 − 4.68 \(\times\) A663) V/1000 W.
Carotenoid = 100(A470) − 1.82(mg Chl a) − 85.02(mg Chl b)/198.
Determination of anthocyanins
To measure the anthocyanin content of the petals, 0.3 g of fresh petals were first weighed and turned into small pieces and crushed in a fine mortar. To extract anthocyanins, the solution containing 1 cc methanol and 99 cc hydrochloric acid (1%) was added to each sample and then the sample was transferred into falcon and refrigerated for 24 h at 4 °C. After that, the samples were centrifuged for 24 h at 4000 rpm, and then the supernatant was used. Finally, the amount of anthocyanin after proper dilution was measured at 530 and 657 nm with a spectrophotometer (Shimadzu, Tokyo, Japan) (Sankhla et al. 2005). Anthocyanin content: = A530 − 1/4A657.
Essential oil distillation
In order to obtain the EO, the aerial parts of the plants were harvested at the flowering stage and dried at shade conditions. The 80–100 g of dried samples were hydro-distillated using Clevenger type apparatus for 3 h and then the essential oil yield of each population was measured based on dry plant weight (Sefidkon et al. 2006).
Essential oil profile
The EOs analysis was performed by gas chromatography (GC) and gas chromatography-mass spectrometry (GC–MS). Dry sodium sulfate was used to dehydrate the oil samples. After injecting the essential oils into the GC and finding the most suitable column temperature program, the EOs were diluted with dichloromethane and injected to the GC–MS, and the retention index, mass spectra, and related chromatograms, and finally, the quantitative and qualitative amounts of the active ingredients of EOs were identified. The EO composition were analyzed by Varian 3400 GC–MS system equipment with AOC-5000 auto injector and DB-5 fused silica capillary column (30 m × 0.25 mm i.d.; film thicknesses 0.25 μm). Helium was used as carrier gas.
Statistical analysis
The data were analyzed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA) and the means were compared using Duncan’s multiple range test at 0.05 probability level. Before analyzing the ANOVA, Bartlett’s test was formed to normality. Because the experiment was done in 2 years, the data were analyses as a combined split design.
Results
Electrolyte leakage and proline concentration
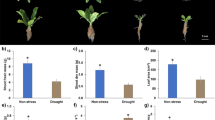
Electrolyte leakage and proline content were significantly changed upon irrigation regimes and foliar application of Mg-NPs (P ≤ 0.05). Electrolyte leakage increased under drought stress, while M-NPs decreased its amount. At non-foliar application of Mg-NPs, drought stress increased electrolyte leakage by 93% compared with control. All levels of Mg-NPs decreased electrolyte leakage relative to control in normal irrigation and drought stress conditions. In plants experiencing drought stress, 0.1, 0.3, and 0.5 g L−1 Mg-NPs decreased electrolyte leakage by 12, 23, and 27% as compared with non-foliar application of Mg-NPs (Fig. 1a). Like electrolyte leakage, proline concentration increased by drought stress, the maximum proline amount was observed in plants exposed to drought stress and 0.5 g L−1 Mg-NPs as 2.92 μg g−1. In contrast, the minimum proline content was found in plants under normal irrigation and 0.5 g L−1 Mg-NPs to be 0.98 μg g−1 (Fig. 1b).
Total soluble sugar and total flavonoid continent
Drought stress and foliar application of Mg-NPs significantly affected TSS and TFC (P ≤ 0.05). Drought stress increased TSS, but Mg NPs decreased it. There was an interesting results of 0.5 g L−1 of Mg-NPs under irrigation regimes; where it decreased TSS at drought stress, but increased it upon normal irrigation. However, 0.1 and 0.3 g L−1 decreased TSS in bot normal and stress conditions. Under drought stress, 0.1, 0.3, and 0.5 g L−1 Mg-NPs decreased TSS by 7, 13, and 31% compared with non-sprayed plants (Fig. 1c). Drought stress and Mg-NPs led to increased TFC in yarrow leaves. Although 0.1 g L−1 Mg-NPs showed no significant difference with control, 0.3 and 0.5 g L−1 increased TFC by 14 and 18%, respectively, when compared with non-foliar application (Fig. 1d). Drought significantly increased TFC, so that in non-foliar application, 0.1, and 0.3 g L−1 of Mg-NPs, drought stress increased TFC by 55, 52, and 48% relative to normal irrigation. In addition, in 0.5 g L−1 of Mg-NPs, TFC in plants exposed to drought represented 2.4-fold increases compared with normal irrigation (Fig. 1d).
Anthocyanin and carotenoid content
Anthocyanin was significantly changed under drought stress and foliar-applied Mg-NPs (P ≤ 0.05). The highest anthocyanin content was obtained in plants exposed to drought stress and 0.5 g L−1 Mg-NPs. Upon nonfoliar application of Mg-NPs and also compared with normal irrigation, the 36% increase of anthocyanin was observed when plants exposed to drought stress (Fig. 2a). Although Mg-NPs showed no significant change of anthocyanin at normal irrigation, they increased this secondary metabolite in drought treatments (Fig. 2a). Carotenoid revealed a similar response to drought stress and leaf spraying of Mg-NPs. It ranged from 0.55 mg g−1 in normal irrigation and 0.5 g L−1 of Mg-NPs to 1.03 in drought stress and 0.5 g L−1 of Mg-NPs (Fig. 2b).
Flower weight yield and essential oil yield
The flower yield remarkably decreased when exposed to drought stress. At non-foliar application, 40% reduction of flower yield was obtained in plants under drought stress. On the contrary, 0.3 and 0.5 g L−1 Mg-NPs increased flower yield by 10 and 18%, respectively, compared with non-sprayed plants (Fig. 2c). Like flower yield, drought stress decreased EO yield, but Mg-NPs improved EO yield. The 0.3 and 0.5 g L−1 Mg-NPs were more effective in enhancing EO content under drought stress. The 0.1, 0.3, and 0.5 g L−1 of Mg-NPs increased EO yield by 10, 17, and 35%, respectively, in comparison with non-foliar application (Fig. 2d).
Essential oil profile
The GC and GC/MS results showed 21 compounds of yarrow EOs, which represented 98.16–99.97% of total EOs (Tables 2 and 3). The main EO compounds were α-pinene, 1,8-cineol, camphor, α-bisabolol, chamazulene, and caryophyllene oxide. These constitutes had different amounts under drought stress and foliage application of Mg-NPs. α-pinene ranged from 8.60% in non-application of Mg-NPs at drought stress to 12.20% in 0.3 g L−1 of Mg-NPs upon well-watered conditions. The amount of 1,8-cineol was obtained in a range of 9.03–14.02, which its highest was observed in 0.3 g L−1 of Mg-NPs under well-watered conditions. Camphor represented the different amounts under irrigation regimes and Mg-NPs, ranging from 14.23 to 17.25%. Caryophyllene oxide was obtained in a range of 6.84–9.80%. The amount of α-bisabolol was obtained in a range of 8.54% in 0.3 g L−1 of Mg-NPs at normal irrigation to 18.81% in non-application of Mg-NPs under drought stress. Chamazulene amount varied from 14.23 in 0.3 g L−1 of Mg-NPs under well-watered conditions to 22.50% in 0.1 g L−1 of Mg-NPs upon drought stress.
Heat map results
Based on heat map analysis, the traits distribute according to their value from 0 as blue to 1 as red. The TSS and EL represented the highest variability among the traits. However, flower and EO yield, anthocyanin, and carotenoid had the minimum change under Mg-NPs application at two irrigation regimes. Therefore, TSS and EL can be considered as biochemical markers of yarrow plants with high sensibility under drought and foliar application of Mg-NPs. Moreover, irrigation regime was more effective than Mg-NPs on creating two main clusters of biochemical traits of yarrow plants (Fig. 3).
Discussion
In this experiment, drought stress increased electrolyte leakage (Fig. 1a). Water deficit can damage cell membrane by increasing reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radical. It leads to rapid oxidation of lipids and fatty acids due to high sensitivity to oxygen (Xie et al. 2019). Since the cell membrane is a type of phospholipid membrane, oxygen can stimulate it and eventually leaks electrolytes to extracellular environment (Kong et al. 2020). Drought stress causes the stomatal closure in leaves, which decreases the rate of CO2 stabilization. The increased IL under drought stress have been reported on maize (Bijanzadeh et al. 2019) and coffee (Silva et al. 2018), and tomato plants (Cornejo-Ríos et al. 2021). Water deficit reduces the availability, transport, and metabolism of nutrients in plants, so that the lack of water by affecting the active transfer and permeability of the membrane, reduces the root access of plants to absorb cations such as Mg (Hussainet et al. 2018). Therefore, in this study, to compensate for Mg deficiency, its NPs were sprayed on yarrow plants. Mg-NPs in all levels reduced ion leakage. Regarding the special properties of NPs like high specific surface area, small size, and low weight of these particles, they are can move easily through the cell wall of plants leaves and results in changes in physiological and biochemical attributes (Afshari et al. 2021). The concentration of Mg-NPs showed different response under normal irrigation and drought stress. High concentration of Mg (0.5 g L−1) was more effective at drought stress. The use of Mg-NPs can reduce oxidative stress by accumulating antioxidants and osmolytic compounds, thereby reducing the amount of ROS. These NPs increased the stability of the cell membrane and reduce the leakage of electrolytes in yarrow plants. Similarly, Siddiqui et al. (2018) showed that Mg by increasing antioxidant enzymes and accumulating osmolites such as glycine betaine and proline can reduce ROS under drought stress.
Proline concentration increased under drought stress as well as Mg-NPs (Fig. 1b). The increased proline upon drought stress is a stagey of most plants to cope the undesirable conditions of environmental stress (Furlan et al. 2020). Plants increase proline contents to reduce the water potential in their tissues and to maintain the turpentine pressure. Increasing the activity of delta-1-proline-5-carboxylase reductase enzymes in the production cycle of this substance and preventing the activity of proline oxidizing enzymes, including proline dehydrogenase, increases the proline content in plant cells (Furlan et al. 2020). The yarrow plants enhanced their praline content to improve the antioxidant capacity under drought tress, and it was in accordance with the results of Hammad and Ali (2012) and Zegaoui et al. (2017) on cowpea plants. In addition, 0.3 and 0.5 g L−1 of Mg-NPs enhanced proline content in plants exposed to drought stress. Similarly, Rehman et al. (2018) represented the accumulation of proline under foliar application of Mg in sunflower plants. Mg participates in many biochemical pathways in plants, and it affects the production of proline when plants exposed to drought. Totally, increased proline in yarrow plants is a stagey to alleviate draft stress by foliar application Mg-NPs.
TSS increased under drought stress but had different response to foliar applied Mg-NPs. During drought stress, plants convert larger molecules such as starch to small molecules like sucrose and then to glucose and fructose molecules to escape the process of plasmolysis and to reduce cell turgescence (Afshari et al. 2021). The increased TSS in drought-exposed plants have been reported by Moloi and Merwe (2021) on wheat and Sadaghiani et al. (2019) on German chamomile, which support the results of present study. The levels of 0.1 and 0.3 g L−1 Mg-NPs decreased TSS, but 0.3 g L−1 increased it compared with non-foliar application under normal irrigation. Mg is required to the function of the H+-ATPase, which pumps protons from the plasma membrane to the apoplast. Screening elements — the cell is loaded, and this leads to the accumulation of sucrose in the apoplast, which in turn affects the rate of proton/sucrose transcription. Therefore, poor performance of the ATPase-H + proton pump has a negative effect on sucrose transfer (Su et al. 2020). Under drought stress, all levels of Mg-NPs decreased TSS (Fig. 1c). In line with the results, Barbosa et al. (2019) also reported decreased TSS in potato plants with foliar applied Mg. Finally, drought stress increased TSS, but foliar application of Mg-NPs decreased it in plants exposed to drought stress.
The amount of non-enzymatic antioxidants like TFC, anthocyanin, and carotenoid increased under drought stress. Although carotenoids have a significance role in chlorophyll, the main function of these pigments is correlated to elimination of ROS toxicity (Kang et al. 2018). Due to the participation of Mg in the synthesis of chlorophyll and carotenoids (Pourranjbari Saghaiesh et al. 2019), increasing the concentration of Mg-NPs in yarrow plants led to enhanced carotenoids. In addition, phenolic compounds such as flavonoids and anthocyanins are produced through the phenylpropanoid pathway (Laura et al. 2019). These compounds act as a defense barrier, protecting the plant against biotic and abiotic stresses (Sharma et al. 2019a, b). The present study showed yarrow plants that received the desirable amount of Mg-NPs prevented further damages imposed by drought stress by increasing phenolic compounds. The increased carotenoids (Pourranjbari Saghaiesh et al. 2019) and anthocyanins (Faiz et al. 2021) have been reported. The significant effects on Si NPs like significantly improved TFC under different irrigation regimes (Afshari et al. 2021).
Flower yield and EO yield decreased under drought stress, but increased by Mg-NPs. Under stress conditions, plants use their energy to survival and thereby they decrease cell deviation (Khosropour et al. 2021). Drought can reduce photosynthesis materials to flowers and results in decline of flower weight (Sehgal et al. 2017). Plants have different responses of their EO content to drought stress and foliar application of metal NPs. The increased EO content under stress conditions is a strategy in medicinal plants to protect plants against the harmful effects of drought stress (Afshari et al. 2021). EO yield is affected by EO percentage and plants weight (Saki et al. 2019). Therefore, the main factor in reducing the EO content in this study is the decreased flower yield under drought stress. In fact, foliar application of Mg-NPs improves the retransfer of photosynthetic materials and carbohydrates, promotes photosynthesis pigments, and prevents leaf aging. Similarly, Aslam et al. (2018) reported that foliar application of Mg increased plant yield.
The EO profile of yarrow plants showed different responses to drought and foliar application of Mg-NPs. Totally, 21 compounds were identified in the present study, and their main compounds were α -pinene, 1,8-cineol, camphor as monoterpenes and also α-bisabolol, chamazulene, and caryophyllene oxide as sesquiterpenes. Farhadi et al. (2020) identified 28 EO components of yarrow plants, which the main constituent was 1,8-cineole (21.28–34.51%), camphor (7.27–14.07%), chamazulene (4.18–11.34%), and α-eudesmol (2.09–9.63%). Howyzeh et al. (2019) identified 32 EO components with main compounds as caryophyllene oxide, camphor, γ-muurolene, γ-eudesmol, borneol, β-eudesmol, spatulenol, germacrene D, and γ-gurjunene. The changes of EO profile under drought stress have been reported different plants (Afshari et al. 2021; Babaei et al. 2021). Drought stress increased sesquiterpenes and decreased monoterpenes. Babaei et al. (2021) showed increased sesquiterpenes in Mexican marigold plants under drought stress. Drought can change the quality and quantity of EOs in medicinal plants through altering the expression of genes or the activity of enzymes involved in the biosynthesis of monoterpenes and sesquiterpenes in EOs (Caser et al. 2019). In addition, the EO profile of yarrow plants was altered under different Mg-NPs. According to previous studies, there is a strong correlation between EO compounds and photosynthesis. Hence, since Mg has a significant role on photosynthesis, it can affect the EO composition. Similar to the results, Afshari et al. (2021) showed changes in EO profile of coriander plants under Si-NPs.
Conclusion
According to the results of the present study, it can be concluded that foliar application of Mg-NPs is effective in mitigating drought stress through changes in biochemical attributes. Mg-NPs in a range of 0.3–0.5 g L−1 is effective in alleviating drought stress. Among the biochemical traits, EL and TSS showed the high variability under drought stress; hence, they can be considered as smart traits to study the biochemical changes. Irrigation regime was more effective in aviation of EO profile so that we can manage the amount of the EO compounds according to changes in irrigation water.
Data availability
Not applicable.
References
Afshari M, Pazoki A, Sadeghipour O (2021) Foliar-applied silicon and its nanoparticles stimulates physio-chemical changes to improve growth, yield and active constituents of coriander (Coriandrum Sativum L.) essential oil under different irrigation regimes. Silicon 3(11):4177–4188
Ahmed T, Noman M, Manzoor N, Shahid M, Abdullah M, Ali L, Li B (2021) Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol Environ Saf 209:111829
Applequist WL Moerman DE (2011) Yarrow (Achillea millefolium L.): A neglected panacea? A review of ethnobotany, bioactivity, and biomedical research 1. Econ Bot 65(2):209–225
Arnon AN (1967) Method of extraction of chlorophyll in the plants. Agron J 23(1):112–121
Aslam MR, Maqsood M, Ahmad Z, Akhtar S, Rizwan M, Hameed MU (2018) Effect of foliar applied magnesium sulphate and irrigation scheduling on quality and yield of maize hybrid. Pak J Agric Res 31(2):173–179
Babaei K, Moghaddam M, Farhadi N Pirbalouti AG (2021) Morphological, physiological and phytochemical responses of Mexican marigold (Tagetes minuta L.) to drought stress. Sci Hort 284: 110116
Barbosa RP, Cairo PAR, Lacerda JDJ, Botelho VV (2019) The effects of magnesium deficiency on sugar partitioning do not restrict the root growth in Eucalyptus young plants. Ciência Florestal 29:622–631
Bates LS, Waldran RP, Tear ID (1973) Rapid determination of free proline for water studies. Plant Soil 39:205–208
Bijanzadeh E, Naderi R, Egan TP (2019) Exogenous application of humic acid and salicylic acid to alleviate seedling drought stress in two corn (Zea mays L.) hybrids. J Plant Nutr 42(13): 1483–1495.
Bose J, Babourina O, Zed Rengel Z (2011) Role of magnesium in alleviation of aluminum toxicity in plants. J Exp Bot 62(7):2251–2264
Caser M, Chitarro W, Angiolillo F, Perrone I, Demasio S, Lovisolo C, Pistelli L, Scariot P (2019) Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind Crop Prod 129:85–96
Chlopicka J, Pasko P, Gorinstein S, Jedryas A, Zagrodzki P (2012) Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT-Food Sci Technol 46(2):548–555
Chou ST, Peng HY, Hsu JC, Lin CC, Shih Y (2013) Achillea millefolium L. essential oil inhibits LPS-induced oxidative stress and nitric oxide production in RAW 264.7 macrophages. Int J Molecul Sci 14(7):12978–12993
Cornejo-Ríos K, Osorno-Suárez MDP, Hernández-León S, Reyes-Santamaría MI, Juárez-Díaz JA, Pérez-España VH, Saucedo-García M (2021) Impact of Trichoderma asperellum on chilling and drought stress in tomato (Solanum lycopersicum). Horticulturae 7(10):385
El-Zohri M, Al-Wadaani NA, Bafeel SO (2021) Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants 10(11):2400
Faiz S, Yasin NA, Khan WU, Shah AA, Akram W, Ahmad A, Riaz, L (2021) Role of magnesium oxide nanoparticles in the mitigation of lead-induced stress in Daucus carota: modulation in polyamines and antioxidant enzymes. Int J Phytoremediation 1–9
Farhadi N, Babaei K, Farsaraei S, Moghaddam M, Pirbalouti AG (2020) Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind Crop Prod 152:112570
Furlan AL, Bianucci E, Giordano W, Castro S, Becker DF (2020) Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol Biochem 151:566–578
Ghani MI, Saleem S, Rather SA, Rehmani MS, Alamri S, Rajput VD, Liu M (2022) Foliar application of zinc oxide nanoparticles: an effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 289:1–12
Gharibi Sh, Tabatabaei BES, Saeidi G-A, Goli SAH (2015) Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of achillea species. Appl Biochem Biotechnol 178(4):796–809
Gharibi Sh, Sayed Tabatabaei BE, Saeidi G-A, Majid Talebi M, Matkowski A (2019) The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 162:90–98
Ghasemian S, Masoudian N, Saeid Nematpour F, Safipour Afshar A (2021) Selenium nanoparticles stimulate growth, physiology, and gene expression to alleviate salt stress in Melissa officinalis. Biologia 76(10):2879–2888
Hammad SA, Ali OA (2014) Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Anna Agric Sci 59(1):133–145
Howyzeh MS, Aslani S, Pooraskari O (2019) Essential oil profile of an Iranian yarrow (Achillea millefolium). J Essent Oil Bear Plant 22(1):295–300
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018) Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci 9:1–21
Irigoyen JJ, Emerrich DW, Sanchez-Diaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa plant. Plant Physiol 84:55–60
Kang C, He S, Zhai H, Li R, Zhao N, Liu Q (2018) A sweet potato auxin response factor gene (IbARF5) is involved in carotenoid biosynthesis and salt and drought tolerance in transgenic Arabidopsis. Front Plant Sci 9:1307
Khosropour E, Weisany W, Tahir NAR, Hakimi L (2021) Vermicompost and biochar can alleviate cadmium stress through minimizing its uptake and optimizing biochemical properties in Berberis integerrima bunge. Environ Sci Pollut Res 1–11
Kong XM, Ge, WY, Wei BD, Zhou Q, Zhou X, Zhao YB, Ji SJ (2020) Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol Technol 170:111315
Laura A, Moreno-Escamilla JO, Rodrigo-García J, Alvarez-Parrilla E (2019) Phenolic compounds. In Postharvest physiology and biochemistry of fruits and vegetables (pp. 253–271). Woodhead Publishing
Mirzaie M, Ladanmoghadam AR, Hakimi L, Danaee E (2020) Water stress modifies essential oil yield and composition, glandular trichomes and stomatal features of lemongrass (Cymbopogon citratus) inoculated with arbuscular mycorrhizal fungi. J Agric Sci Technol 22(6):1575–1585
Moloi MJ, Merwe R (2021) Drought tolerance responses in vegetable-type soybean involve a network of biochemical mechanisms at flowering and pod-filling stages. Plants 10(8):1502
Van Nguyen D, Nguyen HM, Le NT, Nguyen K., Nguyen HT, Le HM, Van Ha, C (2021) Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J Plant Growth Regul 1–12
Pourranjbari Saghaiesh S, Souri MK, Moghaddam M (2019) Effects of different magnesium levels on some morphophysiological characteristics and nutrient elements uptake in Khatouni melons (cucumis melo var. inodorus). J Plant Nutri, 42(1):27–39
Rakmai J, Cheirsilp B, Torrado-Agrasar A, Simal-Gándara J, Mejuto JC (2017) Encapsulation of yarrow essential oil in hydroxypropyl-beta-cyclodextrin: physiochemical characterization and evaluation of bio-efficacies. CyTA-Journal of Food 15(3):409–417
Rehman H, Alharby HF, Alzahrani Y, Rady MM (2018) Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol Biochem 126:97–105
Rolnik A, Soluch A, Kowalska I, Olas B (2021) Antioxidant and hemostatic properties of preparations from Asteraceae family and their chemical composition–comparative studies. Biomed Pharmacother 142:111982
Sadaghiani FM, Dehaghi MA, Pirzad A, Fotokian MH (2019) Variation in yield and biochemical factors of German chamomile (Matricaria recutita L.) under foliar application of osmolytes and drought stress conditions. J Herbm Pharmacol 8(2):90–100
Saki A, Mozafari H, Asl KK, Mirza SB, M, (2019) Plant yield, antioxidant capacity and essential oil quality of Satureja mutica supplied with cattle manure and wheat straw in different plant densities. Commun Soil Sci Plant Anal 50(21):2683–2693
Sankhla N, Mackay WA, Davis TD (2003) Corolla abscission and petal color in cut phlox flower heads: effects of sucrose and thidiazuron. In VIII International Symposium on Postharvest Physiology of Ornamental Plants. 669:389–394
Sefidkon F, Abbasi K, Khaniki GB (2006) Influence of drying and extraction methods on yield and chemical composition of the essential oil of Satureja hortensis. Food Chem 99(1):19–23
Sehgal A, Sita K, Kumar J, Kumar S, Singh S, Siddique KH, Nayyar H (2017) Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front Plant Sci 8:1776
Semida, WM. Abdelkhalik A, Mohamed G, El-Mageed A, Taia A, El-Mageed A, Ali EF (2021) Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 10(2): 421
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B (2019a) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9:285
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Zheng LM, B, (2019b) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24(13):2452
Sheppard LJ, Franssen, I, Cape JN (1995) Frost hardiness of Norway spruce treated with acid mist. Evaluation of the electrolyte leakage rate technique. Environ Exp Bot 35(2): 139–149
Siddiqui MH, Alamri SA, Al-Khaishany MYY, Al-Qutami MA, Ali HM, Mohamed H, Al-Wahibi A-W, Alharby MS, HF, (2018) Mitigation of adverse effects of heat stress on Vicia faba by exogenous application of magnesium. Saudi J Biolog Sci 25:1393–1401
Silva VA, Prado FM, Antunes WC, Paiva RMC, Ferrão MAG, Andrade AC, Almeida AM (2018) Reciprocal grafting between clones with contrasting drought tolerance suggests a key role of abscisic acid in coffee acclimation to drought stress. Plant Growth Regul 85(2):221–229
Strzępek-Gomółka, M., Gaweł-Bęben, K., Kukula-Koch, W (2021) Achillea species as sources of active phytochemicals for dermatological and cosmetic applications. Oxid Med Cell Long 2021
Su L, Lv A, Wen W, Zhou P, An Y (2020) Auxin is involved in magnesium-mediated photoprotection in photosystems of alfalfa seedlings under aluminum stress. Front Plant Sci 11:746
Tohidi B, Rahimmalek M, Arzani A (2017) Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem 220:153–161
Tripathi DK, Singh S, Singh VP, Prasad SM, Chauhan DNK, DK, (2017) Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110:70–81
Van Wyk AS, Prinsloo G (2018) Medicinal plant harvesting, sustainability and cultivation in South Africa. Biolog Conserv 227:335–342
Xie X, He Z, Chen N, Tang Z, Wang Q Cai Y (2019) The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res Int 2019
Zahedi SM, Hosseini MS, Meybodi DH, Peijnenburg N, W, (2021) Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J Sci Food Agric 101(12):5202–5213
Zegaoui Z, Planchais S, Cabassa C, Djebbar R, Belbachir OA, Carol P (2017) Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J Plant Physiol 218:26–34
Author information
Authors and Affiliations
Contributions
Seyyed Ebrahim Ojagh: collecting data and writing the initial draft; Payam Moaveni: conceptualization and methodology as well as editing the draft.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ojagh, S.E., Moaveni, P. Foliar-applied magnesium nanoparticles modulate drought stress through changes in physio-biochemical attributes and essential oil profile of yarrow (Achillea millefolium L.). Environ Sci Pollut Res 29, 59374–59384 (2022). https://doi.org/10.1007/s11356-022-19559-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19559-3