Abstract
In the present study, acute stress responses of adult female Notopterus chitala were scrutinized by antioxidant status and inflammation reaction in the gill and liver at five different salinity exposures (0, 3, 6, 9, 12 ppt). Oxidative defense was assessed by determining superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase, and glutathione reductase activities, while malondialdehyde (MDA), glutathione, and xanthine oxidase levels were determined as indicators of oxidative load. Pro-inflammatory cytokines (IL-1β, IL-6, IL-10, and TNFα) and caspase 1 levels were also analyzed. Expression levels of transcription factors (NRF2 and NF-κB) and molecular chaperons (HSF, HSP70, and HSP90) were estimated to evaluate their relative contribution to overcome salinity stress. MDA showed a significant (P < 0.05) increase (gill, + 25.35–90.14%; liver, + 23.88–80.59%) with salinity; SOD (+ 13.72–45.09%) and CAT (+ 12.73–33.96%) exhibited a sharp increase until 9 ppt, followed by a decrease at the highest salinity (12 ppt) (gill, − 3.92%; liver, − 2.18%). Levels of cytokines were observed to increase (+ 52.8–127.42%) in a parallel pattern with increased salinity. HSP70 and HSP90 expressions were higher in gill tissues than those in liver tissues. NRF2 played pivotal role in reducing salinity-induced oxidative load in both the liver and gills. Serum cortisol and carbonic anhydrase were measured and noted to be significantly (P < 0.05) upregulated in salinity stressed groups. Gill Na+-K+-ATPase activity decreased significantly (P < 0.05) in fish exposed to 6, 9, and 12 ppt compared to control. Present study suggests that a hyperosmotic environment induces acute oxidative stress and inflammation, which in turn causes cellular death and impairs tissue functions in freshwater fish species such as Notopterus chitala.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity gradient has gradually been altered in the euryhaline freshwater ecosystems since last few decades (Sinha et al. 2015). Such salinity fluctuations are crucial, affecting fish physiology, reproductive ability, and abundance of different euryhaline freshwater fishes (Schlaff et al. 2014). Several studies have discussed different adaptive measures by the fish body system to adapt themselves with the fluctuating salinities at biochemical and molecular levels (Roessig et al. 2004; Sinha et al. 2015).
Non-enzymatic antioxidants such as malondialdehyde (MDA) and glutathione (GSH), and enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GRd), and glutathione S-tranferase (GST) have often been implicated as effective biomarkers of stress-induced physiological alterations in fish (Moniruzzaman et al. 2018). Studies on teleosts have indicated that stress caused by salinity fluctuations may be a reason for reduced antioxidant defense system (Choi et al. 2008; Yin et al. 2011). Due to the compromised defense system the fish body can no longer neutralise the increased reactive oxygen species (ROS) levels (Lushchak 2011).
Regulation and maintenance of ionic system in aquatic animals depend on numerous proteins such as Na+/K+-ATPase and carbonic anhydrase (CA), which regulate osmoregulation (Perry et al. 2003; Gilmour and Perry 2009; Pham and Van Phan. 2016). These enzymes are responsible for effective ion transport during osmoregulation (Hwang and Lee 2007; Blondeau-Bidet et al. 2019). CA is a Zn-metalloenzyme which is noted to catalyse the inter-conversion of carbon dioxide and bicarbonate through the reversible reaction, CO2 + H2O ← → H+ + HCO3− (Gilmour et al. 2012). CA plays a pivotal role in maintaining pH balance, transporting ions, and gas exchange. Na+/K+-ATPase is involved in regulation of ions in freshwater and marine fishes.
Salinity-induced stress also influences immune response; it affects some immune parameters depending on exposure to fluctuating salinities (Gonçalves et al. 2007; Dhabhar 2008; Tort 2011; Schmitz et al. 2017). Along with innate immunity, cytokines and interleukins are also directly affected by salinity fluctuations (Abo-Al-Ela et al. 2017). Heat shock protein (HSP) expression is considered a vital indicator of salinity stress as well (Deane and Woo 2011). HSP expression occurs continuously in the cells, however, when exposed to stress, normal processes get disrupted, and the cells can increase the expression of HSPs for maintaining cellular homeostasis (Puntila-Dodd et al. 2021). Nuclear factor kappa B (NF-κB), nuclear factor E2-related factor 2 (NRF2) are also considered to be crucial factors that mediate mRNA expression of several proteins related to stress physiology including antioxidant proteins in the stressed cells (Kumar et al. 2021; Mukherjee et al. 2019). A few previous reports suggested that many of the antioxidant and inflammatory genes under influence of stress were upregulated by NRF2 to safeguard the cells from ROS-induced apoptosis (Zheng et al. 2016). NRF2, after entering into the nucleus, regulates different antioxidant gene expression (Kumar et al. 2021; Cuadrado et al. 2018). NF-κB plays an important role to regulate the pro-inflammatory and anti-inflammatory cytokines. Caspases play an elementary role in cell immunity as an inflammatory reaction architect. Caspase activation occurs when the cellular architecture is distorted during osmotic stress, carrying out cell death (Rathore et al. 2015). Cortisol mediates a series of physiological changes against different types of abiotic stress (Moniruzzaman et al. 2018).
Gills represent the most important osmoregulatory organ in fish ensuring osmotic homeostasis (Wilson and Laurent, 2002), while the liver is the main site where turnover of glucose occurs. Osmotic adaptation requires increased supply of metabolic fuel to major osmoregulatory organ like the gills for efficient maintenance of internal salt and water balance (Peragón et al. 1998). Thus, the gills and liver have been chosen as the organs of interest in the present study.
Notopterus chitala (Hamilton 1822) (Clown knifefish or featherback fish or commonly known as Chitala) is an economically important fish species having a wide market value as food fish. Besides, the fish is considered “Near Threatened” in the IUCN red list category (Prakash and Singh 2020). Notopterus is generally considered euryhaline freshwater species (salinity range varies between 0.01 and 3 ppt for growth and normal physiological activity). However, our earlier observation has indicated that the fish may frequently enter into stagnant brackish water, and survive and maintain its natural activity even in high saline condition (up to 10 ppt) (unpublished data). Thus, it is important to gain information regarding adaptive ability of the fish at fluctuating salinities both in freshwater and estuaries. The present work was designed to determine the effects of salinity on fish physiology involving modulation of antioxidants, gill marker proteins for osmoregulation, molecular chaperones, interleukins, transcription factors, and cortisol. An attempt had also been made to postulate the maximum tolerant salinity level for Notopterus chitala.
Materials and methods
Model species and acclimation
Adult female Notopterus chitala of the same cohort (weight: 93.4 ± 2.3 gm, length: 21.3 ± 2.4 cm) were collected from a hatchery located at Diamond Harbour, West Bengal, India (22° 11′30″ North, 88° 11′ 5″ East) (salinity: 0.07 ± 0.01; pH: 7.2 ± 0.2). Physiological alterations due to stress often depend on biological factors such as age, sex, and growth, and females of some fish species have been reported in few earlier studies to be more sensitive towards adverse effect of toxicity (Biales et al. 2011; Hansen et al. 2007). Hence, female fish of same age and size was taken for the present study as well. Female N. chitala is larger than male, and the abdomen is bulged externally and can be seen prominently disposed as compared to male fish (Chondar 1999; Mitra et al. 2018). During breeding season, adult male N. chitala shows diffused but bright red colour at the base of paired and anal fins, while female fish does not show any such marked coloration. Collected fish were first acclimatized for 14 days in aerated cistern (5000 lit) in the laboratory. During acclimatization period, fish were fed commercially available blood worms (Evox freeze dried tubifex worms) twice everyday (at around 11:00 h in the morning and at around 18:00 h in the evening). Water in the cistern was maintained at 28.0 ± 0.5 °C, pH 7.0–7.5, and dissolved oxygen 6.5–7.0 mg/l. Salinity of the acclimatization tank was 0 ppt and levels of ammonia (< 1 ppm), nitrate (< 20 ppm), and nitrite (< 0.1 ppm) were measured daily using aquarium test kits (Ammonium test kit Quantofix®—37,212; Nitrate Test Kit, Aquasol; Nitrite test kit, Bioxen). If levels exceeded the normal limits, 50% water was changed or else 25% of the water was changed every day.
Experimental design
After 14 days of acclimation, 150 healthy fish from the stocking cistern were randomly divided into five experimental categories. Fish in each experimental category was equally distributed in three aquaria (120 × 60 × 50 cm; 10 fish/aquarium). Fish in one category (control) was maintained at 0 (± 0.01) ppt (only tap water), while fish in rest four experimental categories were directly exposed to four different doses of salinity (3, 6, 9, and 12 ppt) for 7 days.
The salinity range selected in this study was based on the environmental salinities. Salinity of the freshwater habitats of N. chitala at South and South Western part of Sundarban estuary, West Bengal, may change abruptly due to saltwater intrusion from the Bay of Bengal, and it has recently been observed to increase up to 3–6 ppt in the monsoon and around 9–12 ppt in the dry season (Unpublished data).
The experiment was conducted in semi static water system (Banaee et al. 2015) and water was renewed daily. A stock saline solution of 12 ppt was prepared by dissolving artificial sea salt (SEA SALT, AQUAFOREST, India) in tap water and desired experimental salinity levels (± 0.1 PSU) were obtained by diluting the stock solution with tap water in respective aquaria. Salinity in each treatment aquarium was periodically checked with a digital salinity meter (EUTECH Salt 6 + , Thermo Fisher Scientific, India). During the entire experimental duration, both control and experimental fish were fed with commercially available blood worms (Evox freeze dried tubifex worms) twice everyday (at around 11:00 h in the morning and at around 18:00 h in the evening), and water in each aquarium was maintained at 28.0 ± 0.5 °C, pH 7.0–7.5, dissolved oxygen 6.5–7.0 mg/l, ammonia level < 1 ppm, nitrate level < 20 ppm, and nitrite level < 0.1 ppm. Fish in each aquarium was observed for swimming activity, and mortality was also recorded during the course of the experiment.
Preparation of sample for enzyme assay, immunoassay, and immunoblot analysis
After 7 days of salinity exposure, 18 fish from each treatment category (6 fish/aquarium) were first anesthetized with phenoxy-ethanol (1: 20,000, v/v) and the blood was collected from the caudal vein. Fish were then rapidly decapitated and quick dissection was followed for the collection of gill and liver tissues from each fish. Collected blood was allowed to clot, and the clot was removed by centrifugation at 5000 g for 10 min allowing for blood serum to be isolated. Serum and tissue samples were stored at − 20 °C for further analysis.
Tissue samples were processed for separation of cytosolic and nuclear protein fractions (Kirici et al. 2019). Tissues were first homogenized in lysis buffer [25 mM tris–HCl (pH 7.4), 0.15 M NaCl, 2.0 mM ethylenediaminetetraacetic acid, 1 mM ethyleneglycoltetraacetic acid, 23 μM leupeptin, 6.5 μM aprotinin, 1.5 μM pepstatin A, 5 μg/ml soybean trypsin inhibitor, 1 mM phenylmethylsulfonyl fluoride, 1 μM sodium orthovanadate] at a ratio of tissue: buffer = 1: 5. The homogenates were centrifuged at 20,000 g for 30 min at 4 °C. The supernatants were collected as the cytoplasmic fraction, while the pellets were resuspended in the same lysis buffer [with additional 0.5% sodium dodecylsulphate] followed by incubation for 45 min at 4 °C. The samples were then centrifuged at 20,000 g for 30 min at 4 °C, and the supernatants were collected as the nuclear fraction. Both cytoplasmic and nuclear fractions were then stored at − 20 °C for further analysis (Moniruzzaman et al. 2018; Kumar et al. 2021).
Measurement of antioxidants (non-enzymatic and enzymatic)
Non-enzymatic antioxidants
Cytoplasmic fraction of gill and liver tissue homogenates of fish (n = 18) from each treatment category were used to quantify the level of malondialdehyde (MDA) equivalent, which was derived as a product of lipid peroxidation by TBARS (thiobarbituric acid reactive substances) assay (Draper and Hadley 1990) with slight modifications. Levels of reduced glutathione (GSH) were measured in gill and liver by using Ellman’s reagent at 412 nm.
Enzymatic antioxidants
Level of superoxide dismutase (SOD) was determined following a spectrophotometric method (Ewing and Janero 1995) based on the O2.− mediated NBT (nitro blue tetrazolium) reduction by a chemical mixture of PMS (phenazine methosulfate) and NADH. Superoxide radicals were generated in Tris–HCl buffer (16 mM, pH 8.0) containing NBT (50 μM) and NADH (78 μM) solution after sample extracts were mixed. PMS solution was added in order to start the reaction followed by incubation at 25 °C for 5 min. The absorbance was measured at 560 nm against the blank. Catalase (CAT) level was monitored at 240 nm up to 90 s at 15-s intervals (Aebi 1984). This assay was validated by treating the gill and liver tissue supernatants with sodium azide, an inhibitor of CAT activity (Aksoy et al. 2004). Glutathione reductase (GRd) activity was measured by assessing the oxidation of NADPH at 340 nm. The reaction mixture contained NADPH (0.15 mM), glutathione (0.5 mM), MgCl2 (3 mM), Tris (pH 7.5), and tissue extract (Pinto and Bartley 1969). Corrections were made for NADPH oxidation in the absence of glutathione. Glutathione S-transferase (GST) activity was assessed spectrophotometrically (Habig et al. 1974) by using GSH (2.4 mM/l) and 1-chloro-2, 4-dinitrobenzene (CDNB, 1 mM/l) as substrate. One hundred-microliter sample was added into 900 μl of assay cocktail (980 µl of PBS, pH 6.5, 100 mM CDNB, and 100 mM GSH). Phosphate buffer solution (pH 6.5) was used as a control and the absorbance was measured at 340 nm at a regular interval of 60 s for 5 min. Bradford assay kit (abcam, ab102535) was used for the purpose of protein concentration measurement in gill and liver tissues from all treatment groups. The assay protocol used an improved Coomassie blue G reagent which formed a blue complex in the presence of protein. The intensity of the blue complex is proportional to the amount of protein in the sample and it was measured by spectrophotometer at 595 nm.
Activity of gill enzymes
Na+-K+-ATPase activity was expressed as micromolars of Pi liberated per mg of protein per hour in the gill tissue of fish (n = 18) from each treatment category (Agrahari and Gopal 2008; Mukherjee et al. 2019). Carbonic anhydrase activity in gill tissues was measured using carbonic anhydrase activity assay kit (Biovision; Catalog # K472-100). Samples were mixed with acetazolamide (a CA inhibitor) and OD was obtained at 405 nm. Xanthine oxidase (XO) activity in gill and liver tissues was determined using the standard method of Westerfeld and Richert (1949). Spectrophotometric determination of XO activity was based on determining uric acid production from xanthine or hypoxanthine substrate at around 295 nm.
Immunoassay of cytokines (IL-1β, IL-6, TNFα, and IL-10)
Serum samples of fish (n = 18) from each treatment category were used for the measurement of cytokines (IL-1β, IL-6, TNFα, and IL-10) by using standard ELISA (enzyme-linked immunosorbent assay) kit (MyBioSource; Catalog#MBS700230, Catalog#MBS704369, Catalog#MBS175904, and Catalog#MBS282130, respectively). These are ready-to-use microwell, strip plate ELISA kit for analyzing the presence of the cytokines. The kit targets analytes in biological samples. The ELISA analytical biochemical technique of the kit is based on cytokine antibody-cytokine antigen interactions (immunosorbency) and an HRP colorimetric detection system to detect respective cytokine antigen targets in samples. The absorbance was measured by using Tecan-Spectra automatic Microplate reader.
Protein expression analysis
Expression of cytosolic HSF, HSP70, HSP90, and nuclear NF-κB and NRF2 in liver and gill tissues of fish (n = 18) from each treatment category were assessed through 12.5% Laemmli SDS-PAGE. A wet electro blotting system (BIO-RAD Trans-Blot) was followed for the immunoblot. Relevant primary Ab (MyBioSource) were used at a standard dilution of 1:1000 whereas secondary at 1:500. Immunoblots were quantified following densitometric method after comparing with the internal control (β-actin) (Moniruzzaman et al. 2018).
Immunoassay of cortisol (SH)
Serum cortisol level in fish (n = 18) from each treatment category was measured by standard ELISA method using kit (MyBiosource; Catalog#MBS704055). It is a ready-to-use microwell, strip plate ELISA kit for analyzing the presence of the Cortisol. The kit targets analytes in the biological samples. The concentration gradients of the kit standards or positive controls render a theoretical kit detection range in biological research samples containing cortisol. The ELISA analytical biochemical technique of the MBS704055 kit is based on cortisol antibody-cortisol antigen interactions (immunosorbency) and an HRP colorimetric detection system to detect cortisol antigen targets in samples. (Moniruzzaman et al. 2020).
Statistical analysis
Level of antioxidants, gill enzymes, cytokines, and cortisol and expression of each protein blot in fish were evaluated by univariate ANOVA using SPSS (version 20.0). Significance was calculated at 5% level of Pr > F values.
Results
Behaviour and mortality
Swimming activity was observed to be reduced in fish exposed to 12 ppt salinity. Exposure to 12 ppt salinity also resulted in mortality and a total of 8 fish were found dead after 7 days in this treatment category. No mortality was observed in other treatment categories.
Non-enzymatic and enzymatic antioxidants
MDA level in both gill and liver tissues increased significantly (P < 0.05) (+ 25.35–90.14% in gill and + 23.88–80.59% in liver) at all salinity doses than that in control. GSH level in gill and liver tissues increased significantly (P < 0.05) (+ 14.55–37.09% in gill and + 11.76–37.74% in liver) from the control with increased salinity doses until 9 ppt but decreased significantly (P < 0.05) from there (− 3.92% in gill and − 2.18% in liver) at 12 ppt. Similar pattern was observed for SOD (+ 13.72–45.09%) and CAT (+ 12.73–33.96%) activities in gill tissues. In liver tissues, however, SOD (+ 12.98–50.48%) and CAT (+ 15.11–48.44%) activities increased significantly (P < 0.05) until 9 ppt but no significant changes were noted at 12 ppt. In gill and liver tissues GRd activity increased significantly (P < 0.05) until 9 ppt but no significant changes were noted at 12 ppt. In both the tissues, GST activity increased significantly (P < 0.05) from 3–9 ppt while decreased significantly (P < 0.05) at 12 ppt (Fig. 1).
Bar diagram representing changes in non-enzymatic and enzymatic stress markers in the (i) gill and (ii) liver of Notopterus chitala at five different salinity exposures (data = mean ± standard error, n = 18). Different superscript alphabets denote significant difference (P < 0.05) in mean values between different treatment groups. Notes: MDA: malondialdehyde (nmol/mg protein), GSH: reduced glutathione (nmol/mg protein), SOD: superoxide dismutase (U/mg protein/min), CAT: catalase (U/mg protein/min), GRd: glutathione reductase (U/mg protein/min), GST: glutathione S-transferase (U/mg protein/min)
Protein expression analysis
HSP70 (gill, + 17.4–83.58%; liver, + 13.6–70.42%), HSP90 (gill, + 7.15–76.95%; liver, + 3.86–65.94%), and HSF (gill, + 8.96–83.68%; liver, + 7.11–72.13%) expression level increased significantly (P < 0.05) from those in control at all the salinities in both tissues. Nuclear NRF2 (gill, + 12.82–57.49%; liver, + 9.94–59.74%), nuclear NF-κB (gill, + 4.11–42.69%; liver, + 8.80–48.27%), and caspase 1 (gill, + 4.28–99.4%; liver, + 3.97–71.19%) expression level also increased significantly (P < 0.05) at all the salinities in both tissues (Fig. 2).
Bar diagram representing changes in HSP70, HSP90, HSF, NRF2, NF-κB, and caspase 1 expression levels in the (i) gill and (ii) liver of Notopterus chitala at five different salinity exposures (data = mean ± standard error, n = 18). Different superscript alphabets denote significant difference (P < 0.05) in mean values between different treatment groups. Individual band intensity of each immunoblot was quantified by densitometry using β-actin as control
Activity of gill enzymes
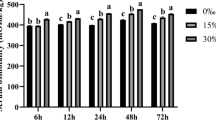
XO level increased significantly (P < 0.05) (+ 8.33–145.83%) at all salinities compared to control in gill tissues, while in liver tissues, XO level increased significantly (P < 0.05) from 3–9 ppt (+ 9.52–133.33%) but decreased significantly (P < 0.05) thereafter (− 11.36%) at 12 ppt. Na+K+-ATPase level decreased (− 7.41 to 62.07%) significantly (P < 0.05) at all salinities in a dose dependent manner. On the other hand, carbonic anhydrase level increased (+ 45.83–232.09%) significantly (P < 0.05) from the control at all salinities (Fig. 3).
Box plot representing changes in (i) XO, Na+-K+-ATPase and carbonic anhydrase in the gill and (ii) XO in liver of Notopterus chitala in four different salinity exposures (data = mean ± standard error, n = 18). Different superscript alphabets denote significant difference (P < 0.05) in mean values between different treatment groups. Notes: XO: xanthine oxidase (nmol/mg protein), carbonic anhydrase (nmol/mg protein), Na+-K.+-ATPase (U/mg protein/min)
Immunoassay of cytokines (IL-1β, IL-6, TNFα, and IL-10) and cortisol
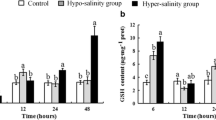
IL-1β, IL-10, and TNFα levels increased significantly (P < 0.05) from the control at all salinity exposures. However, IL-6 level increased significantly (P < 0.05) only at 9 (+ 52.8%) and 12 ppt (+ 55.63%) (Fig. 4). Serum cortisol level increased significantly (P < 0.05) at all salinities (+ 33.33–311.11%) (Fig. 5).
Bar diagram representing changes in IL-1β, IL-6, IL-10, and TNFα in the serum samples of Notopterus chitala at five different salinity exposures (data = mean ± standard error, n = 18). Different superscript alphabets denote significant difference (P < 0.05) in mean values between different treatment groups. Notes: IL-1β: Interlukin 1β (pg/ml), IL-6: Interlukin 6 (pg/ml), IL-10: Interlukin 10 (pg/ml), TNFα: Tissue Necrosis Factor α (pg/ml)
Discussion
Salinity is one of the most critical abiotic regulators to maintain physiological homeostasis in aquatic ecosystem. Several reports have stated the effects of natural and induced salinity stress on growth performance of fish (Boeuf and Payan 2001). Salinity alteration of 50% could induce a reduction in the growth of sablefish (Kim et al. 2017). When most of the fishes counter a higher osmotic pressure, a primary stress response is always initiated. Cortisol is primarily released which in turn activate the cellular signaling processes that assist in sustaining the homeostasis (Barton and Iwama 1991). In freshwater fishes, body fluid is hyperosmotic to the exterior surroundings. Thus, they should counter the water gain and further salt loss by excreting huge quantity of dilute urine and activate salt uptake within their gills. However, in saltwater environment, the body fluids are hyposmotic to the environment. Thus, fish must reduce the loss of water and gain of salt by up taking seawater and excreting little quantity of concentrated urine and secretion of extra salt through the gill epithelium (Evans 2008). However, loss of this osmotic homeostasis leads to reactive oxygen species generation that in turn hampers the prooxidant-antioxidant balances ultimately causing inflammation.
Increase in salinity has previously been shown to induce behavioral alterations and mortality in a number of euryhaline freshwater fish species (Edeline et al. 2005; Tietze and Gerald 2016; Nepal and Fabrizio 2019; Leite et al. 2022). We observed reduced swimming activity and mortality only in fish exposed to the highest salinity level (12 ppt) as well. However, stress caused by salinity up to 9 ppt was not enough to alter behaviour and induce mortality in N. chitala. The present results indicate the adaptive ability of N. chitala where it can thrive well up to 9 ppt but cannot withstand 12 ppt salinity exposure. However, experiments with longer exposure period will be required to fully comprehend the consequences of salinization on the fish species.
In aquatic animals, fluctuating salinities can induce oxidative impairment by generating ROS (Lushchak 2011). Li et al. (2007) have suggested that salinity stress is directly related to increased ROS production, which induces oxidative injury. We examined the level of enzymatic and non enzymatic antioxidants along with XO activity as biomarkers to assess effect of salinity. The induced salinity stress demonstrated that oxidative disbalance was generated when Notopterus was exposed to increased salinities. MDA, one of the major products of lipid peroxidation increased in response to salinity in the present study. Salinity stress was also reported previously to augment lipid peroxidation in sturgeons (Acipenser naccarii) (Martinez-Alvarez et al. 2002). Glutathione always plays a crucial function against oxidative impairment through removal of free radicals (Moniruzzaman et al. 2018). Therefore, discrepancy in GSH level due to salinity exposure signified surplus of free radical in gill and liver tissues. It consequently might decrease the reduced and non-reduced glutathione level ratio (Moniruzzaman et al. 2018). Present results indicated that ROS generation was induced as a result of salinity increase eventually creating a pro-oxidant condition. GST and GRd were found to increase during initial salinity stress up to 9 ppt. This indicated their role in the process of detoxification to eliminate the xenobiotic substances from the tissue (Moniruzzaman et al. 2016). However, significant (P < 0.05) drop in GST at high salinity indicated towards alteration in reduced and oxidized glutathione ratio due to accumulation of free radical. Increase in SOD and CAT activity stands as an indicator of surplus of ROS assembly (Wu et al. 2010; Oruc 2010). Significant (P < 0.05) increase in activity of SOD and CAT was noted in fish tissues exposed to salinity stress followed by a decrease at the highest salinity. The increase of SOD activity indicates enhancement in superoxide radical assembly and an ensuing elevation in antioxidant system to recompense the associated damage (Oruc 2012). Since these enzymes provide primary line of defense against oxidative impairment (Pandey et al. 2003), the level of SOD and CAT decreases after exposure to higher salinities. XO is also reported to generate ROS which disrupt the cellular architecture of the cell and leads to cell death (Ghosh et al. 2018). Increase in XO level during salinity stress indicates imbalance between ROS and levels of endogenous antioxidants (Murata et al. 2013). Decreasing trend of antioxidants at high salinity indicates promotion of lipid peroxidation and disruption of the membrane lipid bilayer arrangement that may inactivate membrane-bound receptors and enzymes. It may also cause fragmentation of the peptide chain, alteration of electrical charge of proteins, cross-linking of proteins, and oxidation of specific amino acids and therefore may lead to increased susceptibility to proteolysis by degradation. Therefore, it can be concluded that adaptive ability of N.chitala to the environment declines when salinity crosses 9 ppt (Yadav et al. 2015).
Cortisol is considered crucial indicator during stress (Barton 2002; Pankhurst and Munday 2011). Cortisol can enhance Na+-K+-ATPase activity in gill cells (Dang et al. 2000). Cortisol levels can regulate the plasma ion fluctuations and immune reaction (McCormick 2011). Similar to the results of the present study, Elarabany et al. (2017) noticed increase in cortisol level during salinity exposure in Nile tilapia. Carbonic anhydrase activity plays a putative role in modulating the systemic acid–base equilibrium in the gills (Georgalis et al. 2006). The enzyme catalyzes CO2 within the branchial cells to produce the acid–base equivalent (Evans et al. 2005; Hirose et al. 2003). Carbonic anhydrase was increased in fish groups exposed to salinity stress. In a previous study, rainbow trout (Oncorhynchus mykiss) exposed to hypercarbia showed increased activity of carbonic anhydrase (Georgalis et al. 2006). The homeostasis in fish transferred to a hyperosmotic medium is regulated by gill Na+-K+-ATPase activity. In general, gill Na+-K+-ATPase activity is positively correlated with salinity of the external environment in most telesosts. However, in Notopterus chitala, Na+-K+-ATPase activity in gills was observed to be negatively correlated with ambient salinity. Similar observations were noted in few other euryhaline freshwater fish species living in habitats with extreme salinity fluctuations (Tomy et al. 2009). Such change in the enzyme activity plays role in adaptation to fluctuating salinities (Stewart et al. 2016; Zhang et al. 2017) as the fish may take a strategy to save extra energy on ion-/osmoregulation and to channel it for other uses. This observation is consistent with a previous finding that suggests energy saving during osmoregulation enables fish to adapt well to salinity fluctuation (Nordlie 2009).
Molecular chaperones are considered cellular mechanisms to restore the distorted cellular machinery in situation of intense stress environment (Roberts et al. 2010). Expression of HSPs is often regulated by the heat shock factors. HSF1 is crucial for the transcriptional activation of most of the heat shock proteins (Padmini and Usha Rani 2009). HSP70, HSP90 along with HSF expression at salinity exposures were higher than its expression at the control. Deane and Woo (2005) found that HSP70 expression was increased in sea bream gills in response to salinity stress. Salinity stress enhances metabolic activity of osmoregulatory organs which subsequently leads to upregulation of HSP expression (Xu and Liu 2011).
Cytokines and chemokines are signal mediators generated by immune cells in the organism to promote inflammation at the infection site or during stressed environment. It consequently enhances the activity of phagocytic cells to kill the invading pathogenic agents (Secombes et al. 2001; Wang and Secombes 2013). IL-1β is a pro-inflammatory cytokine that performs crucial role during regulation of the inflammatory processes (Bird et al. 2002). IL-10 is an anti-inflammatory cytokine. It is involved with multiple pleiotropic effects in immunoregulation and inflammation. It can obstruct activity of NF-κB. It is also implicated in the regulation of the JAK-STAT signaling pathway. TNFα is a cell signaling protein (cytokine) involved in systemic inflammation and also makes up the acute phase reaction. It is produced by the cell of immune system in response to inflammation. In the present study, it was noted that during exposure to the salinity stress, there was upregulation of all interleukins.
Our data indicate that salinity stress induces caspase-dependent apoptosis in fish tissue. Caspase 1 plays a pivotal role during inflammatory reactions. Activation of caspase indicates that the cellular architecture is degraded by fluctuating salinities, which means while carrying out cell death there is minimal effect on surrounding tissues (Rathore et al. 2015). Current results suggest that during salinity stress there is activation of apoptotic pathways in the fish body. Once this pathway is activated, it initiates pro-inflammatory response and activation of pro-inflammatory cytokines. NF-κB modulates cell physiology and its response to external stimuli such as oxidative damage is studied previously (Mukherjee et al. 2019). Cellular responses such as survival, apoptosis, and other cell fates during oxidative damage are controlled by NF-κB (Morgan and Liu 2011). Nuclear NF-κB was significantly elevated during salinity stress indicating its translocation upon activation. This finding is similar to previous research, where significant upregulation in nuclear NF-κB has been recognized in the stressed cell (Sarada et al. 2008). Such elevated nuclear NF-κB expression might modulate the SOD-CAT system downstream.
Instigation of oxidative stress response is predominantly coordinated by NRF2, the most crucial and decisive transcription factor in the vertebrate system (Vargas-Mendoza et al. 2019). Under natural and undisturbed condition, NRF2 is remained in quiescent state in the cytosol by Keap1 protein. Under certain oxidative stress condition, intense free radical accumulation steers to the commencement of frequent transducers (kinases), which ultimately phosphorylate both Keap1 and NRF2. These radicals sometime may unswervingly alter the sulfhydryl rich Keap1 protein to finally bring about a conformational change in Keap1 protein. All these sequences of actions during the preliminary phase of the oxidative stress dislodge the Keap1-NRF2 complex and kindle the translocation of sturdy and stable NRF2 protein to the nucleus (Dinkova-Kostova 2012). In the nucleus, NRF2 associates with a number of small proteins and then binds directly to antioxidant response elements (AREs). Thus, NRF2 commences the transcription of most of the antioxidant genes. In the current result as well, a quick augmentation in nuclear NRF2 level was observed in both fish gill and liver exposed to high salinity.
Conclusion
Notopterus chitala is one of the most common euryhaline freshwater fish of tropical estuary and it has been observed to survive and colonize habitats with fluctuating salinity range. Consequently, the fish is expected to gain an advantage over its stenohaline relatives in the foreseeable future. Salinity stress can severely affect specific physiological traits like oxidative balance that may enhance pro-inflammatory cytokines and alter anti-inflammatory cytokines in fish. Therefore, inflammation may be induced by fluctuating salinity. Molecular chaperons and transcription factors were observed to be involved in salinity stress tolerance in both respiratory (gill) and metabolic (liver) organs of Notopterus chitala for developing secondary stress response mechanisms that facilitated ion balance by altering the concentration of transport and antioxidant proteins to regulate ion flux rate and metabolic status. However, significant protein function is associated with being highly osmotic stress-tolerant and these may cost high energy to resist osmotically unstable environments. Although a medium salinity range was noted to be nonlethal for Notopterus chitala, a higher osmotic challenge might antagonize retention of the physiological capacity for such euryhaline species. Most proteins required for the physiological adaptability appeared to be exhausted at high salinity (12 ppt) and severely affected the immune functions and cellular endurance of the fish. Therefore, high osmotic pressure hampered the biochemical and physiological mechanisms that enabled the fish to cope with limited salinity fluctuations. Finally, keeping in view of the mortality and antioxidant and inflammatory responses as observed in the present study, 9 ppt may be postulated as the maximum salinity tolerance level for survival of Notopterus chitala.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Abo-Al-Ela HG, El-Nahas AF, Mahmoud S, Ibrahim EM (2017) Vitamin C modulates the immunotoxic effect of 17α-methyltestosterone in Nile tilapia. Biochemistry 56:2042–2050. https://doi.org/10.1021/acs.biochem.6b01284
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksoy Y, Balk M, ÖĞÜŞ H, Özer N (2004) The mechanism of inhibition of human erythrocyte catalase by azide. Turk J Biol 28:65–70
Agrahari S, Gopal K (2008) Inhibition of Na+–K+-ATPase in different tissues of freshwater fish Channa punctatus (Bloch) exposed to monocrotophos. Pestic Biochem Physiol 92:57–60. https://doi.org/10.1016/j.pestbp.2008.06.003
Banaee M, Mohammadipour S, Madhani S (2015) Effects of sublethal concentrations of permethrin on bioaccumulation of cadmium in zebra cichlid (Cichlasoma nigrofasciatum). Toxicol Environ Chem 97:200–207. https://doi.org/10.1080/02772248.2015.1031668
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann Rev Fish Dis 1:3–26. https://doi.org/10.1016/0959-8030(91)90019-G
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525. https://doi.org/10.1093/icb/42.3.517
Biales AD, Bencic DC, Flick RL, Blocksom KA, Lazorchak JM, Lattier DL (2011) Proteomic analysis of a model fish species exposed to individual pesticides and a binary mixture. Aquat Toxicol 101(1):196–206
Bird S, Wang T, Zou J, Cunningham C, Secombes CJ (2002) The first cytokine sequence within cartilaginous fish: IL-1β in the small spotted catshark (Scyliorhinus canicula). J Immunol 168:3329–3340. https://doi.org/10.4049/jimmunol.168.7.3329
Blondeau-Bidet E, Hiroi J, Lorin-Nebel C (2019) Ion uptake pathways in European sea bass Dicentrarchus labrax. Gene: 692:126-137 https://doi.org/10.1016/j.gene.2019.01.006
Bœuf G, Payan P (2001) How should salinity influence fish growth? Comp Biochem Physiol C Toxicol Pharmacol 130:411–423. https://doi.org/10.1016/S1532-0456(01)00268-X
Bray WA, Lawrence AL, Leung-Trujillo JR (1994) The effect of salinity on growth and survival of Penaeus vannamei, with observations on the interaction of IHHN virus and salinity. Aquac 122:133–146. https://doi.org/10.1016/0044-8486(94)90505-3
Choi K, Kim Y, Park J, Park CK, Kim M, Kim HS, Kim P (2008) Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Sci Total Environ 405:120–128. https://doi.org/10.1016/j.scitotenv.2008.06.038
Chondar SL (1999) Biology of finfish and shellfish. SCSC Publishers, Howrah, West Bengal, India. xvi, 514 p
Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, Ghezzi P, León R, López MG, Oliva B, Pajares M, Rojo AI, Robledinos-Antón N, Valverde AM, Guney E, Schmidt HHHW (2018) Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmac Rev 70:348–383. https://doi.org/10.1124/pr.117.014753
Dang ZC, Flik G, Ducouret B, Hogstrand C, Bonga SEW, Lock RA (2000) Effects of copper on cortisol receptor and metallothionein expression in gills of Oncorhynchus mykiss. Aquat Toxicol 51:45–54. https://doi.org/10.1016/s0166-445x(00)00102-8
Deane EE, Woo NYS (2005) Cloning and characterization of the hsp70 multigene family from silver sea bream: modulated gene expression between warm and cold temperature acclimation. Biochem Biophys Res Commun 330:776–783. https://doi.org/10.1016/j.bbrc.2005.03.039
Deane EE, Woo N (2011) Advances and perspectives on the regulation and expression of piscine heat shock proteins. Rev Fish Biol Fisher 21:153–185. https://doi.org/10.1007/s11160-010-9164-8
Dinkova-Kostova AT (2012) The role of sulfhydryl reactivity of small molecules for the activation of the KEAP1/NRF2 pathway and the heat shock response. Scientifica 19 p. https://doi.org/10.6064/2012/606104
Dhabhar FS (2008) Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection versus immunopathology. All Asth Clin Immun 4:2–11. https://doi.org/10.1186/1710-1492-4-1-2
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol 186:421–431
Edeline E, Dufour S, Elie P (2005) Role of glass eel salinity preference in the control of habitat selection and growth plasticity in Anguilla anguilla. Mar Ecol Prog Ser 304:191–199
Elarabany N, Bahnasawy M, Edrees G, Alkazagli R (2017) Effects of salinity on some haematological and biochemical parameters in Nile tilapia Oreochromus niloticus. Agric For Fish 6:200–205. https://doi.org/10.11648/j.aff.20170606.13
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177. https://doi.org/10.1152/physrev.00050.2003
Evans DH (2008) Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am J Physiol Regul Integr Comp Physiol 295:R704–R713. https://doi.org/10.1152/ajpregu.90337.2008
Evans TG, Kültz D (2020) The cellular stress response in fish exposed to salinity fluctuations. J Exp Zool A Ecol Integr Physiol 333:421–435. https://doi.org/10.1002/jez.2350
Ewing JF, Janero DR (1995) Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem 232:243–248. https://doi.org/10.1006/abio.1995.0014
Georgalis T, Perry SF, Gilmour KM (2006) The role of branchial carbonic anhydrase in acid-base regulation in rainbow trout (Oncorhynchus mykiss). J Exp Biol 209:518–530. https://doi.org/10.1242/jeb.02018
Ghosh M, Ta S, Banerjee M, Mahiuddin M, Das D (2018) Exploring the scope of photo-induced electron transfer–chelation-enhanced fluorescence–fluorescence resonance energy transfer processes for recognition and discrimination of Zn2+, Cd2+, Hg2+, and Al3+ in a ratiometric manner: application to sea fish analysis. ACS Omega 3:4262–4275. https://doi.org/10.1021/acsomega.8b00266
Gilmour KM, Perry SF (2009) Carbonic anhydrase and acid–base regulation in fish. J Exp Biol 212:1647–1661. https://doi.org/10.1242/jeb.029181
Gilmour KM, Perry SF, Esbaugh AJ, Genz J, Taylor JR, Grosell M (2012) Compensatory regulation of acid–base balance during salinity transfer in rainbow trout (Oncorhynchus mykiss). J Comp Physiol B 182:259–274. https://doi.org/10.1007/s00360-011-0617-8
Gonçalves AMM, Castro BB, Pardal MA, Gonçalves F (2007) Salinity effects on survival and life history of two freshwater cladocerans (Daphnia magna and Daphnia longispina). Ann Limnol-Int J Limnol 43:13–20. https://doi.org/10.1051/limn/2007022
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hansen AC, Rosenlund G, Karlsen Ø, Koppe W, Hemre GI (2007) Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) I—Effects on growth and protein retention. Aquac 272(1–4): 599–611.
Hirose S, Kaneko T, Naito N, Takei Y (2003) Molecular biology of major components of chloride cells. Comp Biochem Physiol B 136:593–620. https://doi.org/10.1016/s1096-4959(03)00287-2
Hwang PP, Lee TH (2007) New insights into fish ion regulation and mitochondrion-rich cells. Comp Biochem Physiol A Mol Integr Physiol 148:479–497. https://doi.org/10.1016/j.cbpa.2007.06.416
Imsland AK, Foss A, Gunnarsson S, Berntssen MH, FitzGerald R, Bonga SW, Ham E, Naevdal G, Stefansson SO (2001) The interaction of temperature and salinity on growth and food conversion in juvenile turbot (Scophthalmus maximus). Aquac 198:353–367. https://doi.org/10.1016/S0044-8486(01)507-5
Kim JH, Park HJ, Kim KW, Hwang IK, Kim DH, Oh CW, Lee JS, Kang JC (2017) Growth performance, oxidative stress, and non-specific immune responses in juvenile sablefish, Anoplopoma fimbria, by changes of water temperature and salinity. Fish Physiol Biochem 43:1421–1431. https://doi.org/10.1007/s10695-017-0382-z
Kirici M, Nedzvetsky VS, Agca CA, Gasso VY (2019) Sublethal doses of copper sulphate initiate deregulation of glial cytoskeleton, NF-kB and PARP expression in Capoeta umbla brain tissue. Regul Mech Biosyst 10: 103–110. https://doi.org/10.15421/021916
Kumar S, Moniruzzaman M, Chakraborty A, Sarbajna A, Chakraborty SB (2021) Crosstalk between heat shock proteins, NRF2, NF-κB and different endogenous antioxidants during lead-induced hepatotoxicity in Puntius ticto. Aquat Toxicol 233:105771. https://doi.org/10.1016/j.aquatox.2021.105771
Leite T, Branco P, Ferreira MT, Santos JM (2022) Activity, boldness and schooling in freshwater fish are affected by river salinization. Sci Total Environ 819:153046. https://doi.org/10.1016/j.scitotenv.2022.153046
Li E, Chen L, Zeng C, Chen X, Yu N, Lai Q, Qin JG (2007) Growth, body composition, respiration and ambient ammonia nitrogen tolerance of the juvenile white shrimp, Litopenaeus vannamei, at different salinities. Aquac 265:385–390. https://doi.org/10.1016/j.aquaculture.2007.02.018
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Martinez-Alvarez RM, Hidalgo MC, Domezain A, Morales AE, García-Gallego M, Sanz A (2002) Physiological changes of sturgeon Acipenser naccarii caused by increasing environmental salinity. J Exp Biol 205:3699–3706
McCormick SD (2011) The hormonal control of osmoregulation in teleost fish. Life Sci 1:1466–1473. https://doi.org/10.1016/B978-0-12-374553-8.00212-4
McCormick SD, Moyes CD, Ballantyne JS (1989) Influence of salinity on the energetics of gill and kidney of Atlantic salmon (Salmo salar). Fish Physiol Biochem 6:243–254. https://doi.org/10.1007/BF01875027
Mitra A, Mukhopadhyay PK, Homechaudhuri S (2018) An overview of biology and culture potentials of Humped featherback Chitala chitala (Hamilton, 1822)–a new candidate for aquaculture diversification. Rev Fish Sci Aquac 26(3):371–380
Moniruzzaman M, Hasan KN, Maitra SK (2016) Melatonin actions on ovaprim (synthetic GnRH and domperidone)-induced oocyte maturation in carp. Reproduction 151:285–296. https://doi.org/10.1530/REP-15-0391
Moniruzzaman M, Mukherjee J, Jacquin L, Mukherjee D, Mitra P, Ray S, Chakraborty SB (2018) Physiological and behavioural responses to acid and osmotic stress and effects of Mucuna extract in Guppies. Ecotox Environ Safe 163:37–46. https://doi.org/10.1016/j.ecoenv.2018.07.053
Moniruzzaman M, Mukherjee M, Das D, Chakraborty SB (2020) Effectiveness of melatonin to restore fish brain activity in face of permethrin induced toxicity. Environ Pollut 266:115230. https://doi.org/10.1016/j.envpol.2020.115230
Morgan MJ, Liu Z-g (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21:103–115. https://doi.org/10.1038/cr.2010.178
Mukherjee J, Moniruzzaman M, Chakraborty SB, Lek S, Ray S (2017) Towards a physiological response of fishes under variable environmental conditions: an approach through neural network. Ecol Indic 78:381–394. https://doi.org/10.1016/j.ecolind.2017.03.038
Mukherjee A, Bhowmick AR, Mukherjee J, Moniruzzaman M (2019) Physiological response of fish under variable acidic conditions: a molecular approach through the assessment of an eco-physiological marker in the brain. Environ Sci Pollut Res 26:23442–23452. https://doi.org/10.1007/s11356-019-05602-3
Murata M, Fukushima K, Takao T, Seki H, Takeda S, Wake N (2013) Oxidative stress produced by xanthine oxidase induces apoptosis in human extravillous trophoblast cells. J Reprod Dev 59:7–13. https://doi.org/10.1262/jrd.2012-053
Nepal V, Fabrizio MC (2019) High salinity tolerance of invasive blue catfish suggests potential for further range expansion in the Chesapeake Bay region. PLoS ONE 14(11):e0224770. https://doi.org/10.1371/journal.pone.0224770
Nordlie FG (2009) Environmental influences on regulation of blood plasma/serum components in teleost fishes: a review. Rev Fish Biol Fish 19:481–564. https://doi.org/10.1007/s11160-009-9131-4
Oruc EÖ (2010) Oxidative stress, steroid hormone concentrations and acetylcholinesterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pest Biochem Physiol 96:160–166. https://doi.org/10.1016/j.pestbp.2009.11.005
Oruc E (2012) Oxidative stress responses and recovery patterns in the liver of Oreochromis niloticus exposed to chlorpyrifos-ethyl. Bull Environ Contam Toxicol 88:678–84. https://doi.org/10.1007/s00128-012-0548-4
Oruc EO, Sevgiler Y, Uner N (2004) Tissue-specific oxidative stress responses in fish exposed to 2, 4-D and azinphosmethyl. Comp Biochem Physiol C Toxicol Pharmacol 137:43–51. https://doi.org/10.1016/j.cca.2003.11.006
Padmini E, Rani MU (2009) Evaluation of oxidative stress biomarkers in hepatocytes of grey mullet inhabiting natural and polluted estuaries. Sci Total Environ 407:4533–4541. https://doi.org/10.1016/j.scitotenv.2009.04.005
Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 309:105–115. https://doi.org/10.1016/S0048-9697(03)00006-8
Pankhurst NW, Munday PL (2011) Effects of climate change on fish reproduction and early life history stages. Mar Freshw Res 62:1015–1026. https://doi.org/10.1071/MF10269
Peragón J, Barroso JB, de la Higuera M, Lupiáñez JA (1998) Relationship between growth and protein turnover rates andnucleic acids in the liver of rainbow trout (Oncorhynchus mykiss) during development. Can J Fish Aquat 55:649–657. https://doi.org/10.1139/cjfas-55-3-649
Perry SF, Shahsavarani A, Georgalis T, Bayaa M, Furimsky M, Thomas SLY (2003) Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid-base regulation. J Exp Zool A Comp Exp Biol 300(1):53–62. https://doi.org/10.1002/jez.a.10309
Pham HQ, Van Phan U (2016) Embryonic and larval development and effect of salinity levels on egg and ovary performance in rabbit fish (Siganus guttatus). Isr J Aquac 68:1301. https://doi.org/10.46989/001c.20807
Prakash SA, Singh DH (2020) Conservation status of Ichthyofauna of Baghel Taal, a wetland of district Bahraich (UP), India. IRE Journals 3:52–56
Pinto RE, Bartley W (1969) The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J 112:109–115. https://doi.org/10.1042/bj1120109
Puntila-Dodd R, Bekkevold D, Behrens JW (2021) Estimating salinity stress via hsp70 expression in the invasive round goby (Neogobius melanostomus): implications for further range expansion. Hydrobiologia 848:421–429. https://doi.org/10.1007/s10750-020-04449-x
Rathore S, Datta G, Kaur I, Malhotra P, Mohmmed A (2015) Disruption of cellular homeostasis induces organelle stress and triggers apoptosis like cell-death pathways in malaria parasite. Cell Death Dis 6:e1803. https://doi.org/10.1038/cddis.2015.142
Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY (2010) Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis 33:789–801. https://doi.org/10.1111/j.1365-2761.2010.01183.x
Roessig JM, Woodley CM, Cech JJ, Hansen LJ (2004) Effects of global climate change on marine and euryhaline freshwater fishes and fisheries. Rev Fish Biol Fisheries 14:251–275. https://doi.org/10.1007/s11160-004-6749-0
Sarada S, Himadri P, Mishra C, Geetali P, Ram MS, Ilavazhagan G (2008) Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema. Exp Biol Med (Maywood) 233:1088–1098. https://doi.org/10.3181/0712-RM-337
Sinha AK, Dasan AF, Rasoloniriana R, Pipralia N, Blust R, De Boeck G (2015) Hypo-osmotic stress-induced physiological and ion-osmoregulatory responses in European sea bass (Dicentrarchus labrax) are modulated differentially by nutritional status. Comp Biochem Physiol A 181:87–99. https://doi.org/10.1016/j.cbpa.2014.11.024
Schlaff AM, Heupel MR, Simpfendorfer CA (2014) Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev Fish Biol Fisheries 24:1089–1103. https://doi.org/10.1007/s11160-014-9364-8
Schmitz M, Ziv T, Admon A, Baekelandt S, Mandiki SN, L’Hoir M, Kestemont P (2017) Salinity stress, enhancing basal and induced immune responses in striped catfish Pangasianodon hypophthalmus (Sauvage). J Proteomics 167:12–24. https://doi.org/10.1016/j.jprot.2017.08.005
Secombes CJ, Wang T, Hong S, Peddie S, Crampe M, Laing KJ, Cunningham C, Zou J (2001) Cytokines and innate immunity of fish. Dev Comp Immunol 25:713–723. https://doi.org/10.1016/s0145-305x(01)00032-5
Stewart HA, Noakes DL, Cogliati KM, Peterson JT, Iversen MH, Schreck CB (2016) Salinity effects on plasma ion levels, cortisol, and osmolality in Chinook salmon following lethal sampling. Comp Biochem Physiol A Mol Integr Physiol 192:38–43. https://doi.org/10.1016/j.cbpa.2015.11.011
Tietze SM, Gerald GW (2016) Trade-offs between salinity preference and antipredator behaviour in the euryhaline sailfin molly Poecilia latipinna. J Fish Biol 88:1918–1931
Tomy S, Chang YM, Chen YH, Cao JC, Wang TP, Chang CF (2009) Salinity effects on the expression of osmoregulatory genes in the euryhaline black porgy Acanthopagrus schlegeli. Gen Comp Endocrinol 161:123–132. https://doi.org/10.1016/j.ygcen.2008.12.003
Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35:1366–1375. https://doi.org/10.1016/j.dci.2011.07.002
Vargas-Mendoza N, Morales-Gonz´alez ´A, Madrigal-Santill´an EO, Madrigal- Bujaidar E, ´Alvarez-Gonz´alez I, García-Melo LF, Anguiano-Robledo L, Fregoso- Aguilar T, Morales-Gonzalez JA (2019) Antioxidant and adaptative response mediated by NRF2 during physical exercise. Antioxidants 8:196. https://doi.org/10.3390/antiox8060196
Wang T, Secombes CJ (2013) The cytokine networks of adaptive immunity in fish. Fish Shellfish Immunol 35:1703–1718. https://doi.org/10.1016/j.fsi.2013.08.030
Westerfeld WW, Richert DA (1949) The xanthine oxidase activity of rat tissues. Proc Soc Exp Biol 71:181–184. https://doi.org/10.3181/00379727-71-17125
Wilson JM, Laurent P (2002) Fish gill morphology: inside out. J Exp Zool 293:192–213. https://doi.org/10.1002/jez.10124
Wu CC, Liu CH, Chang YP, Hsieh SL (2010) Effects of hot-water extract of Toona sinensis on immune response and resistance to Aeromonas hydrophila in Oreochromis mossambicus. Fish Shellfish Immunol 29:258–263. https://doi.org/10.1016/j.fsi.2010.04.021
Xu Q, Liu Y (2011) Gene expression profiles of the swimming crab Portunus trituberculatus exposed to salinity stress. Mar Biol 158(10):2161–2172. https://doi.org/10.1007/s00227-011-1721-8
Yadav SS, Kumar R, Khare P, Tripathi M (2015) Oxidative stress biomarkers in the freshwater fish, Heteropneustes fossilis (Bloch) exposed to sodium fluoride: antioxidant defense and role of ascorbic acid. Toxicol Int 22:71
Yin H, Gao Y, Fan C (2011) Distribution, sources and ecological risk assessment of heavy metals in surface sediments from Lake Taihu. China Environ Res Lett 64:044012. https://doi.org/10.1088/1748-9326/6/4/044012
Zhang YT, Huang S, Qiu HT, Li Z, Mao Y, Hong WS, Chen SX (2017) Optimal salinity for rearing Chinese black sleeper (Bostrychus sinensis) fry. Aquac 476:37–43. https://doi.org/10.1016/j.aquaculture.2017.04.004
Zheng J-L, Zeng L, Shen B, Xu M-Y, Zhu A-Y, Wu C-W (2016) Antioxidant defenses at transcriptional and enzymatic levels and gene expression of Nrf2-Keap1 signaling molecules in response to acute zinc exposure in the spleen of the large yellow croaker Pseudosciaena crocea. Fish Shellfish Immunol 52:1–8. https://doi.org/10.1016/j.fsi.2016.02.031
Funding
MM1 received financial support from DST NPDF (PDF/2017/001308), Govt. of India, India.
Author information
Authors and Affiliations
Contributions
MM1: conceptualization, visualization, investigation, analysis, writing—original-draft, fund acquisition. MM2: methodology. SK: methodology. SBC: conceptualization, supervision, resources, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
The investigation was permitted by Institutional Animal Ethics Committee, University of Calcutta (Registration #885/ac/05/CPCSEA).
Consent to participate
Manuscript does not contain data from any individual person.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moniruzzaman, M., Mukherjee, M., Kumar, S. et al. Effects of salinity stress on antioxidant status and inflammatory responses in females of a “Near Threatened” economically important fish species Notopterus chitala: a mechanistic approach. Environ Sci Pollut Res 29, 75031–75042 (2022). https://doi.org/10.1007/s11356-022-21142-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21142-9