Abstract
Salinity stress is one of the most important environmental factors that substantially affects the yield of plants and changes their secondary metabolites worldwide. Biochar is a vital eco-friendly amendment widely used to improve soil health and promote plant productivity under stress conditions. In the present study, the effect of biochar, a carbon-rich organic substance (0, 1, 2, and 3% of the total mass of the pot), on agro-morphological and physiological traits, essential oil and carvacrol percentage, and antioxidant activity of Satureja khuzistanica under salt stress conditions (0, 2, 4, and 8 ds m−1 NaCl). The plant agro-morphological traits and yield, including plant height, number of main and secondary branches, length and width of leaf, fresh and dry weight of aerial parts, and dry weight of leaves and flowers were decreased with increasing salinity level, but these traits were improved with the application of biochar. The highest yield was observed in the 3% biochar treatment in normal conditions. The highest percentage of essential oil (3.55%) and carvacrol (97.66%) were obtained from the plants under salinity stress (8 ds m−1) treated without and with 3% biochar. With increasing levels of salinity stress, the amount of SPAD decreased, and electrolyte leakage (EL) and the activities of peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) enzymes increased. However, biochar treatments effectively reduced the damage caused by salinity stress, so that the addition of 3% biochar treatment will decrease the destructive effects of salinity stress in the S. khuzistanica, so that decreased EL content and the activity of POD, SOD, and CAT enzymes. According to the positive effects of biochar on functional traits, essential oil content, carvacrol percentage, and SPAD index, its application can be suggested as a sustainable strategy to increase the yield of S. khuzistanica under salinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Satureja khuzistanica Jamzad (Lamiaceae) is a perennial medicinal plant that is widely distributed in the southwestern regions of Iran (Jamzad 2011; Khani et al. 2019; Ghorbanpour et al. 2016). The plant is locally utilized as a spice, herbal tea, analgesic, and antiseptic in traditional medicine and pharmaceutical industries (Shariat and Sefidkon 2021) and exhibits antifungal, antimicrobial, antibacterial, analgesic, antidiabetic, antioxidant, anti-inflammatory, and antilipemic properties (Hadian et al. 2011). Based on the results of the previous studies, several drugs such as dentol, saturex, and orthodentol are produced from this plant, and new formulations, like zagrol are prepared for the veterinary industry. Further, plant pomace, after extraction, is used as livestock forage (Hadian et al. 2011; Nooshkam et al. 2017).
Aerial parts of S. khuzistanica have been collected from its natural habitats for medicinal uses during the past two decades. Considering the growing importance of this plant and the need of the pharmaceutical and food industries for plant raw materials, it is necessary to domesticate and comprehensively cultivate these plants. The plant has unique qualities by growing on low-input lime soil and in a dry environment, making it a promising candidate to be cultivated in marginal lands and low-input farming systems (Hadian et al. 2016). Salinity is one of the main limiting factors in marginal lands and low-input farming systems. Salinity is rapidly rising, occupies approximately 20% of water bodies worldwide, and affects 50% of the area under cultivation in the most fertile lands of the world by 2050 (FAO 2017). Irrigation with saline water, improper drainage structures in agriculture, and Earth’s global warming are expected to increase the level of saline soil in various regions worldwide (Munns & Gilliham 2015). Salt stress negatively influences plant growth, normal physical-biochemical processes, and nutrient uptake. Furthermore, it disturbs photosystem II (PSII) reactions and indirectly causes molecular damage through reactive oxygen species (ROS) (Farouk et al. 2020; Jiang et al. 2020; Ren et al. 2020; Sheng et al. 2020; El-Banna et al. 2022; Farouk & AL-Huqail 2022). The application of organic amendments, which enhance soil fertility along with modifying soil, is known as one of the methods to remove salt from the soil profile. Given the expansion of saline lands in the world, biological approaches such as various organic materials (e.g., manure, plant residues, and biochar) can be adopted as one of the essential solutions to reduce the adverse effects of salt stress on plants (Xu et al. 2016).
Biochar, called black gold in agriculture, is a carbon-rich organic substance, which is obtained by pyrolyzing biomass or plant residues in the presence or absence of oxygen at 300–1000 °C (Akhtar et al. 2014). The material can affect soil physic-chemical characteristics, leading to a change in its biological yield and better fertility, and consequently, more plant yield (Farrell et al. 2014; El-Gamal et al. 2021; Farouk & AL-Huqail 2022). In addition, biochar results in absorbing and retaining nutrients, raising water holding capacity, increasing cation exchange capacity, improving soil structure, and subsequently providing mineral nutrients (e.g., P, Mg2+, Ca2+, and K+) for plants. The high specific surface area of biochar structure and the presence of many pores lead to decreased nutrients (Clough et al. 2013; Ghezzehei et al. 2014). Accordingly, biochar can enhance plant productivity rate and growth (Jeffery et al. 2011; Kim et al. 2016; El-Gamal et al. 2021; Farouk & AL-Huqail 2022; Farouk et al. 2023). Further, the percentage of biochar effect on plant growth and development depends on several items, including the type of biochar, the availability of nutrients following the use of biochar, plant species, and the texture of soil (Xu et al. 2016). The utilization of biochar retains macro- (P, N+, K+, Ca2+) and micronutrients (Zn2+, Mg2+) for plants. Several researchers demonstrated that biochar decreases sodium ion (Na+) uptake and improves the amount of potassium ion (K+) under salt stress, although the substance fails to influence N and Zn+ content (Brantley et al. 2016; Chaganti & Crohn 2015). According to previous studies, biochar enhances soil physicochemical properties (Lv et al. 2023; Ge et al. 2023). It diminishes the effects of salt stress during the growth of Brassica chinensis L. (Tang et al. 2020), Sorghum bicolor L. (Ibrahim et al. 2013), Glycyrrhiza uralensis Fisch. (Egamberdieva et al. 2021), and Borago officinalis L. (Farouk et al., 2020).
Little is known about the effects of wood-derived biochar amendments on agro-morphological and physiological traits, essential oil production (content and yield), and the major essential oil constituents (carvacrol) percentage, as well as their antioxidant activity of S. khuzistanica under salt stress conditions in a controlled pot experiment. We hypothesized that increasing biochar amendments would increasingly promote beneficial effects on S. khuzistanica growth, plant physiological properties, agro-morphological traits, as well as on essential oil percentage/yield and carvacrol content. Thus, the results of the present study can increase the knowledge about the effect of biochar in enhancing growth parameters, physiological, and phytochemical traits as well as essential oil compounds under salinity stress.
2 Materials and Method
2.1 Biochar and Plant Materials
To prepare biochar, the required amount of pomegranate wood (Punica granatum) was collected from the pomegranate orchards in the Saveh region, dried, packed in aluminum sheets, and placed in a furnace at 450 °C for 4 h to perform the pyrolysis process. Then, biochar was removed from the furnace and subjected to chemical analysis, the results of which are provided in Table 1. The plant seeds were obtained from Pakan Bazar Company (Isfahan, Iran) and planted in pots with 20 cm diameter and 25 cm depth, which were filled with sandy loam soil (71.43% sand, 20.81% silt, and 7.76% clay). Table 1 summarizes the physicochemical properties of the applied soil.
2.2 Experimental Design
This study was performed based on a factorial, completely randomized design with three replicates at the greenhouse of Medicinal Plants and Drugs Research Institute, Shahid Beheshti University (Tehran, Iran). The experimental treatments included four percentages of biochar (0, 1, 2, and 3% biochar in potting soil) and four concentrations of NaCl (0, 2, 4, and 8 ds/m). Before filling the pots, biochar was powdered, passed through a sieve with 1–2 mm diameter, and mixed with the soil in desired ratios. The mixtures were incubated for 1 week, followed by transferring S. khuzistanica transplants at the four-leaf stage to the pots containing biochar in the spring (10th of May) of 2022. One plant was placed in each pot, to which four salinity levels were applied at the six-leaf stage by adding NaCl to irrigation water. In order to prevent the accumulation of salt in the pots, two holes with a diameter of 1 cm were built at the bottom of them as drainage, and sand was poured at the bottom of each pot to a height of 5 cm. The pots were placed under greenhouse conditions at 17/26 °C (night/day) and 65–80% relative humidity. The soil moisture content was maintained at field capacity, and irrigation time was based on the soil moisture measurement using a time-domain reflectometer (TDR) (Model Sabta Barbara 6050X, USA).
2.3 Evaluation of Agro-Morphological Traits and Yield
Following the treatment application, the plants were grown for 70 days, and four subshrubs were selected from each treatment to assess morphological and functional characteristics. Morphological properties, such as plant height, leaf length and width, as well as the number of main and side branches, were examined at the full flowering stage. After harvesting, the shoot’s fresh weight was determined, and then the plant materials were dried at room temperature. The flower and leaf were removed from the shoot, and the dry weight of the shoot, leaf, and flower was measured by using the scale.
2.4 Antioxidant Enzyme Activity Assay
A Powerwave XS Microplate spectrophotometer (Bio-Tek Instruments, Inc., USA) was utilized to evaluate the activity of enzymes. Regarding the antioxidant enzyme activity assay, 0.1 g of fresh leaf samples was mixed with 1 ml of cold 50 mM phosphate buffer (pH = 7.8) and centrifuged at 4 °C for 20 min at 12,000 × g. Catalase (CAT) enzyme activity measurement was performed based on H2O2 decomposition rate and reduction of absorbance rate at 240 nm during 3 min. The reaction mixture contained potassium phosphate buffer (50 mM), H2O2 (15 mM), and 100 µL of enzymatic extract. Enzyme activity was expressed as U g−1 FW unit (Aebi 1984). Peroxidase (POD) activity: 1 g of each leaf sample was separately milled in 5 mL of assay buffer. The homogenates were centrifuged at 12,000 × g for 30 min at 4 °C (Zhang 1992). Five milliliters of the assay buffer for the peroxidase activity contained the following: 125 µM of phosphate buffer, 50 µM of pyrogallol, 50 mM of H2O2, pH 6.8, and 1 mL of the 20 times diluted enzyme extract. This was incubated for 5 min at 25 °C, and subsequently, the reaction was stopped by adding 0.5 mL of 5% (v/v) H2SO4. The amount of purpurogallin was determined by measuring the absorbance at 420 nm. In order to evaluate the superoxidase dismutase (SOD) activity rate, 100 µL of enzymatic extract was added to the reaction mixture (contained potassium phosphate buffer 50 mM, methionine 0.013 M, EDTA 0.1 µM, and riboflavin 2 µM) and then, absorbance rates were recorded at 560 nm. Enzyme activity was expressed as U g−1 FW unit (Beauchamp & Fridovich 1971). The extraction and measurement of free proline in leaf tissues were performed according to the acid ninhydrin method described by Bates et al. (1973). Briefly, samples of 100 mg frozen leaves were homogenized with 2 mL of 3% sulphosalicylic acid in a pre-chilled mortar and pestle. The extracts were shaken for 30 min at 750 rpm. The residues were removed by centrifugation at 12,000 × g for 20 min. Supernatants (0.8 mL) were mixed with an equal volume of an acid-ninhydrin reagent (1.25 g ninhydrin, 30 mL of glacial acetic acid, and 20 mL of 2 M orthophosphoric acid) and incubated for 60 min in boiling water. After cooling, the reaction mixture was extracted with 2 mL of toluene, mixed vigorously, and left at room temperature for 20 min until separation of the two phases occurred. The absorbance of the toluene phase was measured at 520 nm using pure toluene as a blank.
2.5 Cell Membrane Damage
The cell membrane leakage was examined by determining electrolyte leakage (EL) according to Lutts et al. (2004). To this end, 0.30 g of fresh leaves were collected, washed three times with distilled water, cut into smaller pieces (length, 1 cm), and transferred to a test tube with distilled water (10 mL). After keeping in room condition for 24 h, primary EL (EL1) was obtained by using an electrical conductivity meter (Inobl, Japan). Then, the samples were incubated at 121 °C for 15 min. Afterward, the solution was cooled at the room condition, the electrical conductivity of which was assessed (EL2). Finally, the rate of cell membrane damage was evaluated by dividing EL1 by EL2 and expressed as EL%.
2.6 SPAD Measurements
The number of 10 leaves of each plant with different ages and colors was randomly chosen to determine leaf chlorophyll content under diffuse lighting (Xiong et al. 2015). Each leaf measurement averaged 5–10 readings using a chlorophyll meter SPAD-502 (Minolta, Japan) (Uddling et al. 2007).
2.7 Essential Oil Extraction and Analysis
To extract the essential oil, about 30 g of dried flowers and leaves were finely chopped and heated in a Clevenger apparatus for 3.5 h, according to the British Pharmacopoeia (1993). The obtained essential oil was dehydrated with dry sodium sulfate, weighed carefully, and stored in a refrigerator (4 °C) until analysis. The amount of essential oil was calculated based on the weight obtained from 100 g of the plant samples (w/w). Then, gas chromatography coupled with mass spectrometry (GC–MS) was applied to identify the essential oil constituents quantitatively and qualitatively. The essential oil was analyzed using an Agilent 7890A gas chromatograph (Agilent Technology, USA) coupled to a mass spectrometer equipped with an HP-5MS column (length, 30 m; internal diameter, 0.25 mm; and thin layer thickness, 0.25 µm). Further, oven temperature increased from 50 to 250 °C at a rate of 4 °C/min and was kept at 250 °C for 10 min. Helium carrier gas with a flow rate of 1 mm/min was utilized, and the ionization energy and ionization source temperature were 70 eV and 270 °C, respectively. Finally, the GC-FID and GC–MS analyses were performed based on the equipment and approach of Hadian et al. (2014) to quantify the carvacrol content of the essential oils.
2.8 Statistical Analysis
Regarding the experimental data analysis, the normality of the data was first checked in Minitab 16 software. Then, the data were subjected to the ANOVA using R 4.0.4 software (https://www.r-project.org), followed by utilizing the least significant difference (LSD) test to compare the means. All diagrams were drawn by using Origin 2021 software.
3 Results
3.1 Agro-Morphological Characteristics
The results suggested decreased morphological and functional properties after salt stress. An increase in the stress level led to a further reduction in the traits so that their minimum was observed in the salinity of 8 ds/m (Table 2). In addition, the use of biochar, especially 3%, significantly improved the morphological and functional characteristics (Table 2). The results of ANOVA revealed the significant interaction effect of biochar amendment and salt stress on the plant height, shoot fresh weight, leaf length and width, as well as the dry weight of the flower, leaf, and shoot, and the number of main and side branches (P < 0.01) As shown in Table 2, the plant height is maximized (63.66 cm) following the B3S0 treatment (3% biochar + 0 ds/m salinity). In comparison, the lowest height (34.00 cm) is related to the exposure to the salinity of 8 ds/m without biochar (B0S3). The plant height increased sharply with increasing levels of biochar 3% treatments, which significantly increased by 18.70% compared with the control. In addition, applying salt reduced the plant height by 41.98% compared to the control.
Applying biochar under the various levels of salinity promoted the number of main and side branches, as well as leaf length and width compared to the control (B0S0). The treatment could moderate the effect of salinity and lead to a lower decrease in the properties compared to the control. The highest number of main (36) and side branches (4.66), as well as the largest leaf length (17 cm) and width (11.6 mm), was found after applying the B3S0 (Table 2). Biochar treatments (B3S0) increased the number of main branches, number of secondary branches, leaf length, and leaf width by 46.38%, 18.75%, 79.45%, and 60.11%, respectively, compared to the control. Further, biochar amendment in salinity conditions was effective on shoot fresh weight, as well as the wet weight of the shoot, flower, and leaf so that the traits were maximized following the B3S0 (176.66, 84. 33, and 33 g/plant in order) (Table 2). The application of biochar improved the fresh weight, dry weight, and leaf and flower dry weight by 221.2%, 237.32%, and 205.55%, respectively, compared to the conditions of salinity stress (B0S3).
3.2 Essential Oil and Carvacrol Content
The content of essential oil was determined at the full flowering stage. The main and interaction effects of biochar amendment and salt stress on essential oil and carvacrol percentages were significant (P < 0.01). Biochar utilization under various salinity levels led to a rise in the two parameters compared to the control. The mean comparison results introduced B0S3 (0% biochar + 8 ds/m salinity) and B3S3 (3% biochar + 8 ds/m salinity) as the treatments leading to the highest essential oil (3.55%) and carvacrol percentage (97.66%), respectively. However, the lowest amounts of the two variables equaled 2.18 and 83.00%, respectively, which were related to the control. At the highest salinity level, the biochar application increased the essential oil and carvacrol percentage by about 56.42% and 17.66%, respectively, compared with control. The results indicated higher levels of essential oil and carvacrol by elevating the concentrations of biochar and salt (Table 2, Fig. 1).
3.3 Physiological Parameters
3.3.1 Leaf Photosynthetic Pigment Content
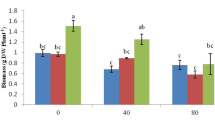
According to variance analysis, the interaction effect of the various levels of biochar and salinity on the SPAD index was significant (P < 0.01). SPAD readings varied from 13.23 to 31.20. Furthermore, SPAD diminished by increasing salinity, while it improved after amending with biochar. The greatest SPAD was 31.20, which was observed following the application of 3% biochar without salt stress, an increase of 18.18% compared to the control (Fig. 2A).
Effect of salinity levels and biochar application on leaf photosynthetic pigment (A) and cell membrane injury (B) of S. khuzistanica. S, salt; S0, without salt; S1, 2 dS m−1; S2, 4 dS m−1; S3, 8 dS m−1 NaCl/; B, biochar; B0, non-biochar; B1, 1% of total pot mass; B2, 2% total pot mass; B3, 3% total pot mass. Bars with the same letter(s) are not significantly different at P < 0.05 according to the LSD test
3.3.2 Determination of Membrane Damage
The membrane damage in the plant was significantly affected by the main and interaction effects of the various levels of biochar and salinity (P < 0.01). The use of biochar reduced the EL, while a significant rise was detected in the variable by exposing it to more salinity (Fig. 2B). The highest salinity treatment of 8 ds/m significantly increased the EL by 88.12% compared with the control. So that, the B0S3 (0% biochar + 8 ds/m salinity) and B3S0 (3% biochar + 0 ds/m salinity) treatments led to the maximum (74.12%) and minimum (30.42%) membrane damage, respectively (Fig. 2B).
3.3.3 Antioxidant Enzyme Activities and Leaf Proline Content
The exposure of the plant to the various concentrations of salt in the absence of biochar improved the activity of POD, SOD, and CAT, although the enzymes exhibited lower activities when biochar was added. Further, the enzyme activities increased by raising salinity, while biochar decreased the stress regulation and enzyme activities. The highest salinity level (8 ds/m) SOD and POD increased about 302.22% and 226.07%, respectively. So, the highest activity of SOD and POD was related to the B0S3 treatment (Fig. 3A, B), although the B3S8 and control ones resulted in maximizing (with a 135% increase compared to the control) and minimizing CAT activity, respectively (Fig. 3C). Furthermore, proline concentration elevated under salt stress, and the salinity of 8 ds/m significantly increased (345.45%) accumulation in the vegetative tissue compared to the control. However, using biochar at various salinity levels diminished proline content (Fig. 3D).
Effect of salinity levels and biochar application on superoxide dismutase (SOD) (A), catalase (CAT) (B), peroxidase (POD) (C), and proline (D) of S. khuzistanica. S, salt; S0, without salt; S1, 2 dS m−1; S2, 4 dS m−1; S3, 8 dS m−1 NaCl/; B, biochar; B0, non-biochar; B1, 1% of total pot mass; B2, 2% total pot mass; B3, 3% total pot mass. Bars with the same letter(s) are not significantly different at P < 0.05 according to the LSD test
4 Discussion
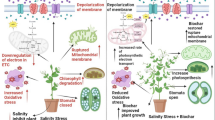
Salt stress is one of the main abiotic risks, which limits plant growth and biomass production (Sheng et al. 2020; Yang et al. 2020; Zhang et al. 2021). The results of the present study represented that salt stress decreased morphological and functional properties. An elevation in the stress level led to more reduction in the characteristics so that their lowest amount was related to the salinity 8 ds/m, which is consistent with the results of Taarit et al. (2011) on the growth traits of Salvia sclarea under salt stress and those of Mehdizadeh et al. (2020) on S. hortensis under stress. Salinity reduces plant growth rate by affecting metabolic pathways and molecular responses associated with the present results. Furthermore, salt stress can disrupt plant ion homeostasis leading to lower photosynthesis activity and membrane stability. Ion toxicity, lower cell water, less osmotic potential, osmotic stress stimulation, and lower dry weight are the other dominant adverse effects of salinity (Ren et al. 2020; Yang et al. 2020; El-Banna et al. 2022). Salinity disrupts chloroplast function and reduces cell water potential, resulting in closing stomata and absorbing a limited level of CO2 after a short time, consequently stopping cell division (Bukhat et al. 2019; Farouk & AL-Huqail 2022). Following the salt stress, ROS accumulation increases, which accelerates oxidative damage, leads to constant oxidative damage by weakening essential cellular compounds, inactivates antioxidant capacity, interrupts plant-water relations, and diminishes nutrient uptake (Ahmad et al. 2021; Bukhat et al. 2019; Farouk et al. 2020; Yang et al. 2020).
Further, a significant improvement was observed in the morphological and functional properties after utilizing biochar, especially 3%. Biochar enhances plant ability to resist environmental stress factors and elevates agricultural productivity (Ran et al. 2019; Farouk & AL-Huqail 2022; Farouk et al. 2023). The substance results promote the growth of S. hortensis (Mehdizadeh et al. 2020) and Solanum tuberosum (Akhtar et al. 2015) under salt stress, which are in line with the results of the present study. Furthermore, the application of biochar increases shoot weight in maize (Abiven et al. 2015), as well as the biomass of aerial parts and the length of roots in potato crops (Akhtar et al. 2015) under salt stress. Limited information is available concerning the exact mechanism of biochar on plant growth and the interaction between biochar and salinity.
Biochar may stabilize salt ions in saline soils or form non-saline microsites to enhance nutrient uptake (Ghezzehei et al. 2014). However, the enhanced growth rate could be related to increased stomatal conductivity and plant water consumption (Akhtar et al. 2015; Zhang et al. 2013). It seems that a rise in water-holding capacity and ionic solute uptake because of utilizing biochar in saline conditions is associated with lower salt concentration in the soil, leading to less adverse effects of NaCl on plant growth parameters. Additionally, biochar stimulates leaf photosynthetic performance and decreases oxidative stress, causing more biomass and plant yield under salinity conditions (Yang et al. 2020). Some of the most important characteristics of soil, such as pH, enzyme activity, microbial biomass, and nutrient and water holding capacity, elevate after treatment with biochar (Jeffery et al. 2011). In the biochar-amended soil, root sensitivity to osmotic stress diminishes by increasing soil moisture and improving soil properties, as well as Na binding to the biochar structure (Akhtar et al. 2015; Mehdizadeh et al. 2020).
The quantity and quality of the essential oil of medicinal plants can be influenced by environmental stresses and nutrient availability. Despite the reduced plant productivity, different results have been reported regarding the changes in the quantity and quality of medicinal plant essential oil in response to salinity levels. Some researchers have found a rise in the essential oil content of Coriandrum sativum (Nefati et al. 2011), Ocimum basilicum (Farasaraei et al. 2020), Mentha spicata (El-Danasoury et al. 2010), and Lallemantia iberica (Heydari & Pirzad 2020) at high salinity level. In some plant species, essential oil accumulation can be ascribed to an increased density of essential oil glands along with the production of more glands during stress (Ghassemi-Golezani & Farhadi 2022), as well as net assimilation or assimilate distribution during growth and differentiation processes (Chrysargyris et al. 2018). Further, a reduction in plant primary metabolism during stress can result in the accumulating of specific products (intermediates), which can be shifted toward the synthesis of secondary metabolites such as essential oils (Ghassemi-Golezani & Farhadi 2022). In the present study, the essential oil percentage was significantly affected by biochar. The treatments could enhance plant yield and essential oil content under salt stress because of having specific physicochemical traits, minimizing sodium uptake, and maximizing the amounts of available nutrients (Ghassemi-Golezani & Farhadi 2022). The results of several studies have demonstrated different carvacrol biosynthesis and accumulation in response to various environmental factors (Majdi et al. 2017; Mohammadi et al. 2019). Some plant species like mint (Aziz et al. 2008) and basil (Ashraf & Orroj 2006) have less essential oils, especially monoterpenes, following salt stress. However, the stress is associated with the elevated concentrations of essential oil components in some other plant species, such as chamomile (Baghalian et al. 2008). The results of the present study represented a more significant carvacrol percentage in salinity conditions, which is in line with those of Neffati and Marzouk (2008).
Furthermore, SPAD significantly diminished under salt stress, which is in agreement with the results of Taïbi et al. (2016) on Phaseolus vulgaris L., Taffouo et al. (2010) on Vigna subterranean L., and Farouk et al. (2020) on Ocimum basilicum L. The lower photosynthetic pigment level of plants under salt stress is considered a typical sign of oxidative stress and is attributed to the inhibition of chlorophyll synthesis and activation of its degradation by chlorophyllase (Santos 2004; Smirnoff 1996; Taïbi et al. 2016). The declined chlorophyll content caused by slow synthesis or rapid degradation suggests the presence of a light protection mechanism through decreasing light absorption by reducing chlorophyll amount (Elsheery & Cao 2008). Based on the results of the previous studies, a rise in salinity level diminishes the chlorophyll content of basil due to light inhibition, increased chlorophyllase activity, and enhanced ROS production, as well as the instability of the protein structure of pigments (Heidari 2012). In the present study, a higher SPAD was found by applying biochar under salinity conditions. The results of other studies indicated that biochar treatment elevates chlorophyll amount, improves PSII activity, and facilitates electron transfer (Lyu et al. 2016), as well as maximizing the production of chlorophyll, carotenoid, amino acids, and protein in plants (Younis et al. 2015).
Some defense systems, like cell membrane stability, protect plants against the adverse effects of stress. Cell membrane stability and EL refer to the cell damage rate induced by environmental stress in plants, respectively. As for the basil, the EL increases significantly under salt stress because of enhancing membrane permeability, and consequently increasing solute leakage (Hu et al. 2012). Salinity conditions are accompanied by higher membrane damage and lipid peroxidation due to the greater concentrations of Na+ in plants (Banu et al. 2009). After exposure to salt stress, EL improves in O. basilicum (Farasaraei et al. 2020), S. hortensis (Mehdizadeh et al. 2020), and Thymus daenensis and T. vulgaris (Bistagni et al. 2019), which is consistent with the results of the present study. The stress causes nutritional imbalance in plant tissue by elevating the accumulation of specific ions like Na+, leading to toxic effects on membrane stability and permeability, as well as more EL (Tavakkoli et al. 2010). Further, it increases membrane damage by promoting the levels of cell free radicals in many plants (Farasaraei et al. 2020). Regarding the results of the present study, cell membrane damage was reduced by utilizing biochar, which aligns with the results of Mehdizadeh et al. (2020) on S. hortensis. Biochar enhances water-holding capacity in soil, improving soil moisture and lowering salt concentration in soil solution (Akhtar et al. 2015).
Like to other abiotic stresses, salt stress causes excessive ROS production in plants (An et al. 2016). Furthermore, SOD, POD, and CAT are the main components of the antioxidant enzyme system and play a crucial role in removing ROSs. Plant antioxidants are a natural defense system against various stresses (Zulfiqar et al. 2021). The results of the present study revealed that the activities of CAT, SOD, and POD elevated by increasing salinity, while they were modulated after amending with biochar. Some researchers have reported using biochar promotes the systemic resistance of antioxidant enzymes in plants (Quartacci et al. 2017; Rehman et al. 2019; Abideen et al. 2020; Rasool et al. 2021).
Proline, as one of the compatible soluble substances, plays an important role in maintaining cell osmotic balance in plants (Shtereva et al. 2015), the accumulation of which enhances salt resistance (Kishor et al. 2005). In the present study, a rise in salinity level led to a greater proline content in the leaves of S. khuzistanica, which is in agreement with those of Mehdizadeh et al. (2020) on summer savory and Huang et al. (2019) on sweet corn. Generally, proline concentration elevates through biosynthesis of protein, or its hydrolysis and oxidative degradation to protect against salinity. It serves as an osmotic protectant, ROS scavenger, and protein stabilizer, and plants can increase the accumulation of the material to deal with osmotic stress (Trovato et al. 2008). Based on the results of the previous studies, biochar differently influences the amount of proline in various plants. Biochar decreases proline content in bean (Farhangi-Abriz & Torabian 2017) and corn seedlings (Lashari et al. 2014), which is inconsistent with the results of the present study. However, some researchers found a greater proline concentration in summer savory after the treatment (Mehdizadeh et al. 2020), which is in line with the results of the present study.
5 Conclusion
The results of the present study revealed that salt stress reduced the growth of S. khuzistanica, and biochar amendment could moderate the adverse effects of salinity. Biochar amendment may effectively reduce salinity stress on plants by increasing the soil cation exchange capacity, total porosity, soluble and exchangeable K+, and fertilities, decreasing soil electrical conductivity, soluble Na+, and Cl− contents, and water evaporation, reducing the shoot Na+ accumulation, and relative electrical leakage; increasing the shoot K+ accumulation, improving leaf water status, increasing chlorophyll content, and increasing N use efficiency. The current study suggests that biochar advances salinity tolerance and may be considered a potential regulator of agricultural production under salt stress. This makes a significant contribution to the production of S. khuzistanica in salty and low-yielding lands. Thus, we conclude that further work on specific interactions between biochar type and rates, and cultivation measures is needed and quite promising.
References
Abideen Z, Koyro HW, Huchzermeyer B, Ansari R, Zulfiqar F, Gul B (2020) Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol 22:259–266. https://doi.org/10.1111/plb.13054
Abiven S, Hund A, Martinsen V, Cornelissen G (2015) Biochar amendment increases maize root surface areas and branching: a shovelomics study in Zambia. Plant Soil 395:45–55. https://doi.org/10.1007/s11104-015-2533-2
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmad S, Cui W, Kamran M, Ahmad I, Meng X, Wu X, Su W, Javed T, El-Serehy HA, Jia Z (2021) Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J Plant Growth Regul 40:1270–1283. https://doi.org/10.1007/s00344-020-10187-0
Akhtar SS, Li G, Andersen MN, Liu F (2014) Biochar enhances yield and quality of tomato under reduced irrigation. Agric Water Manag 138:37–44. https://doi.org/10.1016/j.agwat.2014.02.016
Akhtar SS, Andersen MN, Liu F (2015) Biochar mitigates salinity stress in potato. J Agron Crop Sci 201:368–378. https://doi.org/10.1111/jac.12132
An YM, Song LL, Liu YR, Shu YJ, Guo CH (2016) De novo transcriptional analysis of alfalfa in response to saline–alkaline stress. Front Plant Sci 7:1–14. https://doi.org/10.3389/fpls.2016.00931
Ashraf M, Orooj A (2006) Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain (Trachyspermum ammi [L.] Sprague). J Arid Env 64:209–220. https://doi.org/10.1016/j.jaridenv.2005.04.015
Aziz EE, Al-Amier H, Craker LE (2008) Influence of salt stress on growth and essential oil production in peppermint, pennyroyal, and apple mint. J Herbs Spices Med Plants 14:77–87. https://doi.org/10.1080/10496470802341375
Baghalian K, Haghiry A, Naghavi MR, Mohammadi A (2008) Effect of saline irrigation water on agronomical and phytochemical characters of chamomile (Matricaria recutita L.). Sci Hortic 116:437–441. https://doi.org/10.1016/0003-2697(71)90370-8
Banu MNA, Anamul Hoquea MD, Watanabe-Sugimoto M, Matsuoka K, Nakamaureaa Y, Shimoishia Y, Murata Y (2009) Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J Plant Physiol 166:146–156. https://doi.org/10.1016/j.jplph.2008.03.002
Bates LS, Waldron RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant and Soil 39: 205-217 https://doi.org/10.1007/BF00018060
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bistagni ZE, Hashemi M, DaCosta M, Craker L, Maggi F, Morshedloo MR (2019) Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind Crops Prod 135:311–320. https://doi.org/10.1016/j.indcrop.2019.04.055
Brantley K, Savin M, Brye K, Longer D (2016) Nutrient availability and corn growth in a poultry litter biochar amended loam soil in a greenhouse experiment. Soil Use Manag 32:279–288. https://doi.org/10.1111/sum.12296
Bukhat S, Manzoor H, Athar HUR, Zafar ZU, Azeem F, Rasul S (2019) Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J Plant Growth Regul 39:809–822. https://doi.org/10.1007/s00344-019-10024-
Chaganti VN, Crohn DM (2015) Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline–sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 259:45–55. https://doi.org/10.1016/j.geoderma.2015.05.005
Chrysargyris A, Michailidi E, Tzortzakis N (2018) Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front Plant Sci 9:489. https://doi.org/10.3389/fpls.2018.00489
Clough TJ, Condron LM, Kammann C, Muller C (2013) A review of biochar and soil nitrogen dynamics. Agron 3:275–293. https://doi.org/10.3390/agronomy3020275
Egamberdieva D, Ma H, Alaylar B, Zoghi Z, Kistaubayeva A, Wirth S, Bellingrath-Kimura SD (2021) Biochar amendments improve licorice (Glycyrrhiza uralensis Fisch) growth and nutrient uptake under salt stress. Plants 10:2135. https://doi.org/10.3390/plants10102135
El-Banna MF, AL-Huqail AA, Farouk S, Belal BEA, El-Kenawy MA, Abd El-Khalek AF (2022) Morpho-physiological and anatomical alterations of salt-affected thompson seedless grapevine (Vitis vinifera L) to brassinolide spraying. Horticulturae 8:568. https://doi.org/10.3390/horticulturae8070568
El-Danasoury M, Al-Amier H, Helaly A, Aziz E, Craker L (2010) Essential oil and enzyme activity in spearmint under salt stress. J Herbs Spices Med Plants 16:136–145. https://doi.org/10.1080/10496475.2010.508975
El-Gamal SMA, Serag El-Din WM, Farouk S, Mokhtar NYO (2021) Integrated effects of biochar and potassium silicate on borage plant under different irrigation regimes in sandy soil. J Horti Sci & Ornam Plants 13:60–76. https://doi.org/10.5829/idosi.jhsop.2021.60.76
Elsheery NI, Cao KF (2008) Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol Plant 3:769–777. https://doi.org/10.1007/s11738-008-0179-x
FAO (2017) The future of food and agriculture: trends and challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, p 163
Farasaraei S, Moghaddam M, Ghasemi Pirbalouti A (2020) Changes in growth and essential oil composition of sweet basil in response of salinity stress and superabsorbents application. Sci Hortic 271:109465. https://doi.org/10.1016/j.scienta.2020.109465
Farhangi-Abriz S, Torabian S (2017) Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxico Environ Saf 137:64–70. https://doi.org/10.1016/j.ecoenv.2016.11.029
Farouk S, AL-Huqail AA (2022) Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 11:765. https://doi.org/10.3390/plants11060765
Farouk S, Elhindi KM, Alotaibi MA (2020) Silicon supplementation mitigates salinity stress on Ocimum basilicum L via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol Environ Saf 206:111396. https://doi.org/10.1016/j.ecoenv.2020.111396
Farouk S, AL-Huqail AA, El-Gamal SMA (2023) Potential role of biochar and silicon in improving physio-biochemical and yield characteristics of borage plants under different irrigation regimes. Plants 12:1605. https://doi.org/10.3390/plants12081605
Farrell M, Lynne M, Macdonald Butler G, Chirino-Valle I, Leo MC (2014) Biocharand fertilizer applications influence phosphorus fractionation and wheat yield. BiolFertil Soil 50:69–178. https://doi.org/10.1007/s00374-013-0845-z
Ge Y, Xl Li, Palviainen M, Zhou X, Heinonsalo J, Berninger F, Köster K, Sun H (2023) Response of soil bacterial community to biochar application in a boreal pine forest. J for Res 34:749–759. https://doi.org/10.1007/s11676-022-01509-x
Ghassemi-Golezani K, Farhadi N (2022) The efficacy of salicylic acid levels on photosynthetic activity, growth, and essential oil content and composition of pennyroyal plants under salt stress. J Plant Growth Regul 41:1953–1965. https://doi.org/10.1007/s00344-021-10515-y
Ghezzehei TA, Sarkhot DV, Berhe AA (2014) Biochar can be used to capture essential nutrients from dairy wastewater and improve soil physico-chemical properties. Solid Earth 5:953–962. https://doi.org/10.5194/se-5-953-2014
Ghorbanpour M, Hadian J, Hatami M, Salehi-Arjomand H, Aliahmadi A (2016) Comparison of chemical compounds and antioxidant and antibacterial properties of various Satureja species growing wild in Iran. Journal of Medicinal Plants 15(59):58–72
Hadian J, Mirjalili MH, Kanani MR, Salehnia A, Ganjipoor P (2011) Phytochemical and morphological characterization of Satureja khuzistanica Jamzad populations from Iran. Chem Biodivers 8:902–915. https://doi.org/10.1002/cbdv.201000249
Hadian J, Esmaeili H, Nadjafi F, Khadivi-Khub A (2014) Essential oil characterization of Satureja rechingeri in Iran. Ind Crops Prod 61:403–409. https://doi.org/10.1016/j.indcrop.2014.07.034
Hadian J, Hekmati M, Ghorbanpour M (2016) Agromorphological Variations and essential oil production of Satureja khuzestanica Jamzad under different planting densities. Journal of Essential Oil Bearing Plants. TEOP 19(5):1102 – 1110
Heidari M (2012) Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. Afr J Biotechnol 11:379–384. https://doi.org/10.1080/14620316.2020.1833764
Heydari SH, Pirzad A (2020) Efciency of Funneliformis mosseae and Tiobacillus sp. on the secondary metabolites (essential oil, seed oil and mucilage) of Lallemantia iberica under salinity stress. J Hortic Sci Biotechnol 96:249–259. https://doi.org/10.1080/14620316.2020.1833764
Hu L, Li H, Pang H, Fu J (2012) Responses of antioxidant gene, protein and enzymes to salinity stress in two genotypes of perennial ryegrass (Lolium perenne) differing in salt tolerance. J Plant Physiol 169:146–156. https://doi.org/10.1016/j.jplph.2011.08.020
Huang M, Zhang Z, Zhu C, Zhai Y, Lu P (2019) Effect of biochar on sweet corn and soil salinity under conjunctive irrigation with brackish water in coastal saline soil. Sci Hortic 250:405–413. https://doi.org/10.1016/j.scienta.2019.02.077
Ibrahim HM, Al-Wabel MI, Usman ARA, Al-Omran A (2013) Effect of Conocarpus biochar application on the hydraulic properties of a sandy loam soil. Soil Sci 178:165–173. https://doi.org/10.1097/SS.0b013e3182979eac
Jamzad Z (2011) Thyme and savory Iran. Publishing Research Institute of Forests and Rangelands, Tehran, Iran, p 172
Jeffery S, Verheijen FGA, Van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agri Ecosyst Environ 144:175–187. https://doi.org/10.1016/j.agee.2011.08.015
Jiang D, Lu B, Liu L, Duan W, Chen L, Li J, Zhang K, Sun H, Zhang Y, Dong H, Cundong L, Bai Z (2020) Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism. Peer J 8:e10486. https://doi.org/10.7717/peerj.10486
Khani S, Seyedjavadi SS, Zare-Zardini H, Mahmoodzadeh Hosseini H, Goudarzi M, Khatami S, Amani J, Imani Fooladi AA, Razzaghi Abyaneh M (2019) Isolation and functional characterization of an antifungal hydrophilic peptide, Skh-AMP1, derived from Satureja khuzistanica leaves. Phytochemisry 164:136–143. https://doi.org/10.1016/j.phytochem.2019.05.011
Kim HS, Kim RK, Yang JE, Ok YS, Owens G, Nehls T, Wessolek G, Kim KH (2016) Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 142:153–159. https://doi.org/10.1016/j.chemosphere.2015.06.041
Kishor PB, Sangam S, Amrutha RN (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. J Curr Sci 88:424–438
Lashari MS, Ye Y, Ji H, Li L, Kibue GW, Lu H, Zheng J, Pan G (2014) Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: a 2-year field experiment. J Sci Food Agrc 95:1321–1327. https://doi.org/10.1002/jsfa.6825
Lutts S, Almansouri M, Kinet JM (2004) Salinity and water stress have contrasting effects on the relationship between growth and cell viability during and after stress exposure in drum wheat callus. Plant Sci 167:9–18. https://doi.org/10.1016/j.plantsci.2004.02.014
Lv Y, Xu L, Guo X, Liu J, Zou B, Guo Y, Zhang Y, Li H, Zheng G, Guo Y et al (2023) Effect of biochar on soil physiochemical properties and bacterial diversity in dry direct-seeded rice paddy fields. Agronomy 13(1):4. https://doi.org/10.3390/agronomy13010004
Lyu S, Du G, Liu Z, Zhao L, Lyu D (2016) Effects of biochar on photosystem function and activities of protective enzymes in Pyrus ussuriensis Maxim. Under Drought Stress Acta Physiol Plant 38:220. https://doi.org/10.1007/s11738-016-2236-1
Majdi M, Malekzadeh-Mashhady A, Maroufi A, Crocoll C (2017) Tissue-specific gene-expression patterns of genes associated with thymol/carvacrol biosynthesis in thyme) Thymus vulgaris L.) and their differential changes upon treatment with abiotic elicitors. Plant Physiol Biochem 115:152–162. https://doi.org/10.1016/j.plaphy.2017.03.016
Mehdizadeh L, Moghaddam M, Lakzian A (2020) Amelioration of soil properties, growth and leaf mineral elements of summer savory under salt stress and biochar application in alkaline soil. Sci Hortic 267:109319. https://doi.org/10.1016/j.scienta.2020.109319
Mohammadi H, Amirikia F, Ghorbanpour M, Fatehi F, Hashempour H (2019) Salicylic acid induced changes in physiological traits and essential oil constituents in different ecotypes of Thymus kotschyanus and Thymus vulgaris under well-watered and water stress conditions. Ind Crops Prod 129:561–574. https://doi.org/10.1016/j.indcrop.2018.12.046
Munns R, Gilliham M (2015) Salinity tolerance of crops—what is the cost? New Phytol 208:668–673. https://doi.org/10.1111/nph.13519
Nefati M, Sriti J, Hamdaoui G, Kchouk ME, Marzouk B (2011) Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem 124:221–225. https://doi.org/10.1016/j.foodchem.2010.06.022
Neffati M, Marzouk B (2008) Changes in essential oil and fatty acid composition in coriander (Coriandrum sativum L.) leaves under saline conditions. Ind Crops Prod 28:137–142. https://doi.org/10.1016/j.indcrop.2008.02.005
Nooshkam A, Mumivand H, Hadian J, Alemardan A, Morshedloo MR (2017) Drug yield and essential oil and carvacrol contents of two species of Satureja (S. khuzistanica Jamzad and S. rechingeri Jamzad) cultivated in two different locations. J Appl Res Med Aromat Plants 6:126–130. https://doi.org/10.1016/j.jarmap.2017.04.002
Quartacci MF, Sgherri C, Frisenda S (2017) Biochar amendment affects phenolic composition and antioxidant capacity restoring the nutraceutical value of lettuce grown in a copper-contaminated soil. Sci Hortic 215:9–14. https://doi.org/10.1016/j.scienta.2016.12.002
Ran C, Gulaqa A, Zhu J, Wang X, Zhang S, Geng Y, Guo L, Jin F, Shao X (2019) Benefits of biochar for improving ion contents, cell membrane permeability, leaf water status and yield of rice under saline & and ash; sodic paddy field condition. J Plant Growth Regul 39:370–377
Rasool M, Akhter A, Haider MS (2021) Molecular and biochemical insight into biochar and Bacillus subtilis induced defense in tomatoes against Alternaria solani. Sci Hort 285:110203. https://doi.org/10.1016/j.scienta.2021.110203
Rehman M, Liu L, Bashir S, Saleem MH, Chen C, Peng D, Siddique KH (2019) Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L) grown as forage in aged copper-contaminated soil. Plant Physiol Biochem 138:121–129. https://doi.org/10.1016/j.plaphy.2019.02.021
Ren J, Ye J, Yin L, Li G, Deng X, Wang S (2020) Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agron 10:663. https://doi.org/10.3390/agronomy10050663
Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Horti 103:93–99. https://doi.org/10.1016/j.scienta.2004.04.009
Shariat A, Sefidkon F (2021) Tetraploid induction in savory (Satureja khuzistanica): cytological, morphological, phytochemical and physiological changes. Plant Cell Tiss Organ Cult 146:137–148. https://doi.org/10.1007/s11240-021-02053-y
Sheng H, Zeng J, Liu Y, Wang X, Wang Y, Kang H, Fan X, Sha L, Zhang H, Zhou Y (2020) Differential responses of two wheat varieties differing in salt tolerance to the combined stress of Mn and salinity. J Plant Growth Regul 39:795–808. https://doi.org/10.1007/s00344-019-10023-0
Shtereva LA, Vassilevska-Ivanova RD, Karceva TV (2015) Effect of salt stress on some sweet corn (Zea mays L. Var. saccharata) genotypes. Arc Biol Sci Belgrade 67:993–1000. https://doi.org/10.2298/ABS150121062S
Smirnoff N (1996) Botanical briefing: the function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669. https://doi.org/10.1006/anbo.1996.0175
Taarit MB, Msaada K, Hosni K, Marzouk B (2011) Physiological changes and essential oil composition of clary sage (Salvia sclarea L.) rosette leaves as affected by salinity. Acta Physiol Plant 33:153–162. https://doi.org/10.1007/s11738-010-0532-8
Taffouo VD, Wamba OF, Yombi E, Nono GV, Akoa A (2010) Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranean (L.) verdc.) landraces grown under saline conditions. Int J Botany 6:53–58. https://doi.org/10.3923/ijb.2010.53.58
Taïbi KH, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312. https://doi.org/10.1016/j.sajb.2016.03.011
Tang JW, Zhang SD, Zhang XT, Chen JH, He XY, Zhang QZ (2020) Effects of pyrolysis temperature on soil plant microbe responses to Solidago canadensis L derived biochar in coastal saline-alkali soil. Sci Total Environ 731:13. https://doi.org/10.1016/j.scitotenv.2020.138938
Tavakkoli E, Rengasamy P, Mcdonald GK (2010) High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot 61:4449–4459. https://doi.org/10.1093/jxb/erq251
Trovato M, Mattioli R, Costantino P (2008) Multiple roles of proline in plant stress tolerance and development. Rend Lince 19:325–346. https://doi.org/10.1007/s12210-008-0022-8
Uddling J, Gelang-Alfredsson J, Piikki K, Pleijel H (2007) Evaluating the relationship between leaf chlorophyll concentration and spad-502 chlorophyll meter readings. Photosynth Res 91:37–46. https://doi.org/10.1007/s11120-006-9077-5
Xiong D, Chen J, Yu T, Gao W, Ling X, Li Y, Peng S, Huang J (2015) Spad-based leaf nitrogen estimation is impacted by environmental factors and crop leaf char-acteristics. Sci Rep 5:13389. https://doi.org/10.1038/srep13389
Xu G, Zhang Y, Sun J, Shao H (2016) Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci Total Environ 568:910–915. https://doi.org/10.1016/j.scitotenv.2016.06.079
Yang A, Akhtar SS, Li L, Fu Q, Li Q, Naeem MA, He X, Zhang Z, Jacobsen SE (2020) Biochar mitigates combined effects of drought and salinity stress in Quinoa. J Agron 10:912. https://doi.org/10.3390/agronomy10060912
Younis U, Athar M, Malik S, Reza Shah M, Mahmood S (2015) Biochar impact on physiological and biochemical attributes of Spinach (Spinacia oleracea L.) in nickel contaminated soil. Golbal J Environ Sci Manage 1:245–254. https://doi.org/10.7508/gjesm.2015.03.007
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang XZ (ed) Research Methodology of Crop Physiology. Agriculture Press, Beijing, pp 208–211
Zhang WM, Meng J, Wang JY, Fan SX, Chen WF (2013) Effect of biochar on root morphological and physiological characteristics and yield in rice. Acta Agron Sin 39:1445–1451. https://doi.org/10.3724/SP.J.1006.2013.01445
Zhang P, Liu L, Wang X, Wang Z, Zhang H, Chen J, Liu X, Wang Y, Li C (2021) Beneficial effects of exogenous melatonin on overcoming salt stress in sugar beets (Beta vulgaris L). Plants 10:886. https://doi.org/10.3390/plants10050886
Zulfiqar F, Chen J, Finnegan PM, Younis A, Nafees M, Zorrig W, Hamed KB (2021) Application of trehalose and salicylic acid mitigates drought stress in sweet basil and improves plant growth. Plants 10:1078. https://doi.org/10.3390/plants10061078
Acknowledgements
The authors thank the Research Council of Shahid Beheshti University for their financial support.
Author information
Authors and Affiliations
Contributions
Ghasem Eghlima: conceptualization, supervision, methodology, investigation, writing—original draft. Meisam Mohammadi: methodology, validation. Mohammad Hossein Mirjalili: validation, formal analysis, writing—review and editing. Mansour Ghorbanpour: supervision, validation, formal analysis, review and editing.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eghlima, G., Mohammadi, M., Mirjalili, M.H. et al. Exploring the Potential Impact of Biochar Amendments in Promoting Redox Reactions, Agro-Morphological, and Phytochemical Characteristics in Satureja khuzistanica Jamzad Under Salt Stress. J Soil Sci Plant Nutr 24, 190–202 (2024). https://doi.org/10.1007/s42729-023-01566-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01566-z