Abstract
Through multiple different pathways, the environmental multiple metals make their ways to the human bodies, where they induce different levels of the oxidative stress response. This study further investigated the impact of multiple-metal exposure on the risk of developing proliferative diabetic retinopathy (PDR). We designed a case–control study with type 2 diabetic patients (T2D), in which the case group was the proliferative diabetic retinopathy group (PDR group), while the control group was the non-diabetic retinopathy group (NDR group). Graphite furnace atomic absorption spectrometry (GFAAS) and inductively coupled plasma optical emission spectrometry (ICP-OES) were used to detect the metal levels in our participants’ urine samples. The least absolute shrinkage and selection operator (LASSO) regression approach was used to include these representative trace elements in a multiple exposure model. Following that, logistic regression models and Bayesian kernel machine regression (BKMR) models were used to describe the effect of different elements and also analyze their combined effect. In the single-element model, we discovered that lithium (Li), cadmium (Cd), and strontium (Sr) were all positively related to PDR. The multiple-exposure model revealed a positive relationship between Li and PDR risk, with a maximum quartile OR of 2.80 (95% CI: 1.10–7.16). The BKMR model also revealed that selenium (Se) might act as a protective agent, whereas magnesium (Mg), Li, and Cd may raise the risk of PDR. In conclusion, our study not only revealed an association between exposure to multiple metals and PDR risk but it also implied that urine samples might be a useful tool to assess PDR risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is a retinal condition resulted from retinal capillary damage caused by decreased glucose tolerance (Sinclair and Schwartz 2019). It is classified as an inflammatory neurovascular complication with neurological damage or malfunction, which can severely impair vision and eventually lead to blindness. According to the World Health Organization (WHO) in 2019, 146 million (34.6%) of the 422 million diabetes people worldwide have DR (World report on vision 2019). These estimates are expected to rise as the prevalence of DR increases in the middle- and low-income nations. Considering visual loss from DR is preventable, WHO has placed DR on the top of their priority list for ophthalmologic disorders. Although intravitreal injection of anti-VEGF drugs and vitrectomy are the common clinical treatment for DR, some problems exist, such as the need for repeated intraocular injections, transient vision improvement, and no-response to treatment in some patients for anti-VEGF treatment (Reddy et al. 2018). Therefore, advance intervention is important to reduce the incidence and delay the progression of DR.
Recent studies have shown that multiple-metal exposure may increase the risk of developing ocular diseases such as DR, age-related macular degeneration (AMD), cataracts and glaucoma (Wang et al. 2016; Bede-Ojimadu et al. 2021). Zhu and Hua have recently reported there are essential trace element (such as manganese (Mn) and zinc (Zn)) deficiencies, as well as toxic chemical (such as Cd and cesium (Cs)) accumulation in DR patients (Zhu and Hua 2020). Other scholars have also observed an association between urine barium (Ba), lead (Pb), molybdenum (Mo), Se, and Sr with the risk of diabetes (Feng et al. 2015). Trace elements play an essential role in the normal function of proteins, enzymes, and transcription factors. Toxic metals, however, may clash with necessary trace elements and in turn damage enzyme activities and other physiological processes. Recent research has shown that aberrant trace elements and toxic metal elements may cause microvascular complications in diabetic individuals, and the development of microvascular disorders in the fundus might impair vision in people with DR (Zhu and Hua 2020).
Modern society is a complex living environment with diverse levels of metal exposure. Metals are transported and excreted in the body through blood and urine. Whereas urine levels reflect long-term chronic exposure to metals, blood levels are more relevant to recent acute exposure. Therefore, urine can be used as a biomarker to assess the effects of multiple-metal exposure on humans. For urine metal levels can provide an indication of the absorption and toxicity of metals in the body (Pedersen et al. 2005).
The interactions between metals may also have some impacts on their effects to human health. The BKMR model has been used to evaluate the combined effects of mixed components by taking into consideration of any potential nonlinear effects and interactions (Zhong et al. 2021). It is widely agreed that blood levels are more pertinent to recent acute exposure, whereas urine levels represent long-term chronic exposure to a specific metal (Pedersen et al. 2005). By using BKMR models, Some scholars examined the impact of individual metal element and the combined effect of different metals. BKMR models are also used to examine the association of urine metal element levels with diabetes, hypertension, and other disorders (Zhou et al. 2021). Although related studies have preliminarily elucidated the effect of metals in the evolution of DR, there are still some limitations, such as lack of analysis of trace elements in urine and lack of systematic evaluation of the single-element effects or the combined effects of metal elements in the risk of DR development (Zhu and Hua 2020). Notably, the systematic evaluations are crucial to understand how multiple metals contribute to the onset of ocular disorders and to promote creation of new efficient guidelines to reduce the existing level of local environmental contamination. As a result, the objectives of this research are to (a) investigate the correlation between urinary metal levels and the risk of developing PDR in the presence of mixed multiple metal exposure; (b) provide new guidelines for the prevention and treatment of ocular diseases; (c) may provide an epidemiological basis for enhancing the understanding and detection of multiple metal contamination in the environment.
Methods
Subjects in research
The study was conducted at the First Affiliated Hospital of Anhui Medical University, Anhui Province, China, from December, 2017, to March, 2019. Anhui Province locates in the eastern part of China (31°52′N, 117°17′E). All participants were type 2 diabetes (T2D) patients from Anhui Province, China, and completed blood, urine, ophthalmological, and related systemic examinations. The case group (PDR group) was recruited from patients with proliferative diabetic retinopathy (PDR) who were requiring surgical treatment in the ophthalmology department, and the control group (NDR group) was recruited from patients with non-diabetic retinopathy (NDR) in the hospital. The diagnoses of DR and DM were made according to the American Academy of Ophthalmology guidelines (Flaxel et al. 2020) and the American Diabetes Association guidelines (American Diabetes 2020), separately.
Case group (PDR group) was defined as diabetic patients with any of the characteristic lesions of PDR in the fundus, including intraretinal hemorrhages, bead-like changes in fundus veins or microvascular abnormalities, retinal or optic disc neovascularisation, vitreous hemorrhages, or pre-retinal hemorrhages. The control group (NDR group) was diabetic patients without any characteristic lesions in the fundus of the eye with DR (Garoma et al. 2020). Exclusion criteria for both groups: having other retinal sicknesses (e.g., macular degeneration, retinal vein occlusion, pathological myopia); having other eye sicknesses (e.g., glaucoma, ocular trauma, infection and tumors); having mental illness, being pregnant, any systemic disease (e.g., acute infectious sickness, autoimmune ailment, severe liver or kidney dysfunction, malnutrition, cancer, etc.); employment or experience in unusual settings; taking vitamin or mineral supplements; family history of genetic disease, etc. (Liu et al. 2022; Zhu and Hua 2020). The pupils of participants’ eyes were suitably dilating with 1% tropicamide and the eyes were examined by the same experienced ophthalmologist.
Age (± 5 years for cases), diabetes duration (± 2 years for cases), and gender were matched 2:1 between the control and case groups. Ultimately, 60 participants from the PDR group and 120 participants from the NDR group were included in the research. All participants provided written informed consent, which was reviewed and approved by the Ethics Committee at Anhui Medical University (20170305).
Data gathering and definition of variables
Prior to the start of the survey, all participants completed an informed consent form, then completed our self-made structured questionnaire, which included information on gender, age, height, weight, quantity of sleep, frequency of exercise, total yearly family income, smoking status, and alcohol intake.
Three categories were used to categorize the quality of sleep: poor, general, and good. Physical activity levels were divided into two groups: < 2 times/week and ≥ 3 times/week. Three categories of annual family income were established: < 10,000 RMB, 10,000–60,000 RMB, and > 60,000 RMB. Participants who had smoked for a continuous or cumulative total of 6 months or more at the time of the survey were considered smokers. Participants who had shown alcoholic habits for 6 months in a row and at least once per week were considered alcohol drinkers.
The trained medical professionals used a height- and weight-measuring apparatus to determine the participants’ height and weight, which were then expressed in centimeters (cm) and kilograms (kg). The participants’ BMI was then determined, and according to Chinese adult norms, it was categorized into four categories: underweight BMI < 18.5 kg/m2, normal weight BMI 18.5–23.9 kg/m2, overweight BMI 24.0–27.9 kg/m2, and obese BMI ≥ 28.0 kg/m2 (Li et al. 2020).
Examination of urinary components
Participants had completed the study’s self-made questionnaire and provided their first clean mid-stream urine in the morning of the following day. The gathered samples were sent to the lab and kept there for further testing in a refrigerator at – 80 °C.
Urine samples were transferred from the ultra-low temperature refrigerator and thawed at room temperature. The extracted material was diluted with 5% nitric acid and then mixed homogeneously in a vortex mixer. The samples were microwaved in a microwave digester at 90 °C for 1 h before being centrifuged at room temperature. The aspirated supernatants were sent for further evaluation. An innocuous sample was included in the experiment alongside every 20 samples. The concentration of Cd in the urine was measured with the use of a Graphite Furnace Atomic Absorption Spectrometer (GFAAS, ZEEnit700P, Analytik Jena, Germany). The matrix modifier in the experiment was a 1% solvent of diammonium hydrogen phosphate. Inductively coupled plasma–optical emission spectrometry (ICP-OES 7000DV, PerkinElmer Corporation) was used to measure the concentrations of 10 different metals in urine. These metals included Ba, chromium (Cr), Pb, Li, Mg, Mn, Mo, iron (Fe), Se, and Sr.
The accuracy of the measurements was evaluated using the spiked recovery method with the spike-and-recovery experience ranging from 92 to 104%. The creatinine levels in the urine were measured by the BECKMAN DXC800 Biochemistry Analyzer, USA. Metal element concentrations in urine below the limit of detection (LOD) were replaced by LOD/√2 (Tellez-Plaza et al. 2013). The findings of the research used creatinine correction values [g/(g creatinine) or mg/(g creatinine)] to account for the influence of sample concentration status on metal element concentrations. All elements relying on LOD detection rates were more than 70%. In order to correct the impact of sample concentration status on metal element concentrations, the study’s results included metal element concentrations with creatinine adjustment values [μg/(g creatinine) or mg/(g creatinine)]. The detection rates for all LOD-dependent elements were more than 70% (Zhong et al. 2021).
Statistic evaluation
Demographic features of the case and control groups were compared using descriptive methods. We used paired samples t-test and Wilcoxon signed-rank test to describe normal and skewed distributions. The chi-square test (χ2) was employed to compare categories of information. All metal concentrations were adjusted for creatinine and then transformed to the natural logarithm.
By using logistic regression models, we calculated ORs and 95% confidence intervals (CIs) for PDR and divided each urinary metal level into quartiles. A reference was chosen based on the concentration distribution of the lowest quartile. To analyze the impact of each metal, we used two models. The conditional logistic regression model took gender and age (a continuous variable) into account while calculating metals in model 1. Additionally, model 2 made adjustments for BMI, sleep quality, physical activity, household annual income, smoking status, and alcohol consumption. The quartiles of urine element concentrations were entered into the models as an ordinal categorical variable to produce the linear trend p-values.
We created multi-metal models to assess the impact of multiple-metal mixed exposures on diabetic retinopathy, as well as a metal correlation heatmap to analyze metal correlations. Considering the diversity and correlation of elements, LASSO regression is used to select elements into the multiple-exposure model and accomplish dimensionality reduction (Alhamzawi and Ali 2018). LASSO regression can regress and penalize all variables and exclude relatively unimportant independent variables from the model (McEligot et al. 2020). The ORs and 95% CIs for multiple-metal exposure and PDR were then calculated using logistic regression models.
We used BKMR models to investigate individual metal effects and their interactions with one another in order to better understand the nonlinear effects of metal elements on the risk of PDR (Bobb et al. 2018). For brevity, below is the abbreviated form of the equation describing the BKMR model (Bobb et al. 2015):
where \({Y}_{i}\) denotes the health outcome, \(zi\) is a vector of exposure factors (e.g., air pollution constituents), \({\chi }_{i}^{T}\beta\) includes possible confounders, and \(\epsilon i\) stands for the residual. When considering environmental pollution mixed exposure, \(h(zi)\) has a high-dimensional exposure–response function, which may be nonlinear and/or involve interactions between mixture components. We created exposure matrices and variables and employed the kmbayes function to execute the Monte Carlo approach (Escobar et al. 2010). In this instance, the variables are the demographic traits and the exposure matrix comprises the metals chosen through LASSO regression.
Firstly, we performed variable selection for the exposure variable to fit the BKMR model and calculated the posterior inclusion probability (PIP) for the exposure variable (metals) (Valeri et al. 2017). And PIP indicates the relative importance of the impact on the outcome, with higher values indicating greater influence on the outcome. Then, when the other elements were set at their neutral concentrations, cross-sectional plots were then shown to demonstrate the respective individual-exposure response functions of the elements. When the levels of all other metal concentrations were set at a certain quartile (e.g., P25, P50, and P75), we also evaluated the variations in individual metal risk between their 75th and 25th percentiles. Following that, the cumulative impact of multiple-metal mixing exposure was evaluated by comparing exposure values when all metal were at a certain quantile with exposure values when all elements were at their median. Furthermore, this study explored the interactions between several metals and the risk of PDR and displayed bivariate exposures for each two components, with all other factors maintained at a certain quantile (median) (Bobb et al. 2018).
Data input and calibration checks were performed using EpiData 3.1 software. The follow-up analysis was conducted using SPSS, version 23.0 (SPSS Inc., Chicago, IL, USA), and R software (version 3.6.1; R Core Team). And α = 0.05 was chosen as the threshold for bilateral statistical significance.
Results
Population characteristics
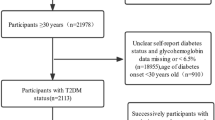
This study recruited a total of 60 PDR case groups and 120 matched NDR controls. The study groupings and experimental procedures are shown in Fig. 1. Table 1 provided the participants’ fundamental demographic details. The participants’ average ages were 54.20 ± 8.57 years for the NDR group and 53.48 ± 8.57 years for the PDR case group. Patients in the PDR group had a lower mean BMI than those in the control group (p < 0.05). However, when being categorized according to Chinese adult criteria, there was no discernible change in BMI between the PDR and control groups (p > 0.05). Additionally, there were no appreciable variations in the two groups’ levels of alcohol intake, physical activity, yearly family income, smoking status, or quality of sleep (p > 0.05).
Concentrations of metal elements in urine
Since the distribution of the initial metal concentrations in the urine of the case and control groups was skewed, we used percentiles (P25, P50, and P75) to describe the values. The distribution of the 11 urine metal elements is summarized in Table S1, where all metal elements were detected at > 70%. In comparison to the control group, Mg, Mo, Li, Sr, and Cd levels were all considerably higher (p < 0.05). The heat map of the Spearman correlation for urine metals is then shown in Fig. 2. The highest correlation was between Li and Sr with R = 0.59, followed by Fe and Mn with correlation coefficient of 0.58.
Single-element exposure model for PDR
The risk of PDR was categorized for each metal element concentration (adjusted for creatinine and converted by natural logarithm) into quartiles, with the lowest group (Q1) serving as a reference. Table 2 shows that Mg, Li, Sr, and Cd concentrations in model 1 were positively correlated with PDR risk. These correlations persisted in Li, Sr, or Cd after model 2’s correction for other possible confounders (p-trend < 0.05). And in the highest quartiles, we found a link between exposure to Li, Sr, or Cd (Q4 vs. Q1: OR = 3.12, 95% CI: 1.31–7.40; OR = 3.09, 95% CI: 1.13–8.49; OR = 2.45, 95% CI: 1.02–5.86).
Multiple exposure model for PDR
We utilized LASSO regression to filter these 11 metal elements and included representative elements in the multiple-exposure model (Fig. 3a and b). According to the LASSO regression screening results, four metals (Mg, Li, Se, and Cd) were selected in the model. The study then investigated the relationship between PDR risk and mixed exposure to four metals, with adjustment for all potential covariates (Table 3). And the potential covariates include age, gender, BMI, sleep quality, physical activity, yearly family income, smoking status, passive smoking, and alcohol consumption. As observed, increased Li levels were positively correlated with PDR, with the highest quartile OR of 2.80 (95% CI: 1.10–7.16), respectively. Meanwhile, the results showed no association between Mg, Se, or Cd and PDR risk.
BKMR evaluations
The BKMR model was applied in this work to assess the effects of mixed exposure for the four metals chosen from the LASSO regression and shown in Fig. 4. We evaluated the PIP for the metal elements included in the BKMR model (Table S2). PIP indicates the relative importance of the impact on the outcome. Higher values (close to 1) imply greater relevance for the outcome, while lower values (close to 0) indicate less importance. Secondly, when the remaining components were maintained at their median amounts, we modeled separate exposure–response functions for each element (95% CI). In accordance with the findings of the single-element model, the exposure levels of three metals (Mg, Li, and Cd) were positively linked with the risk of developing PDR, as shown in Fig. 4a. Furthermore, we observed a negative correlation between Se and PDR risk.
The BKMR model of five metal elements evaluated the effect of mixed exposure on PDR risk. a The univariate exposure–response function and 95% CI for each metal element when the other elements were fixed at the median concentration (P50). b The overall effects of mixed-exposure in elements fixed to different percentiles as compared when they were at their medians (P50). c The effects of single-metal exposure between its 75th and 25th percentiles, when the remaining metals were fixed at different percentiles (25th, 50th, or 75th). d The bivariate cross-section effects of the exposure–response function of a single element where the second element was fixed at different percentiles (25th, 50th, or 75th)
Figure 4b shows the overall effect of the mixture. The combined effect (95% CI) of all factors was presented when the five elements were all fixed at specific percentile levels (P25 to P75, step value = 5th) compared to their median. In this case, the cumulative effect of all factors was statistically significant when they were above their 50th compared to the median. In this study, we observed a significant joint effect when the four metals (Mg, Li, Se, and Cd) were at or above 55th compared to their median values. The four metals’ combined impact was significantly and positively correlated with PDR risk. At the same time, the overall exposure had a negative impact on PDR risk when they were at the 40th and 45th percentiles (Table S3).
The distribution of separate exposures on the combined impact was then examined in this study. For example, when the remaining metal concentrations were fixed at different percentile levels (25th, 50th, and 75th), the difference in single-exposure risk when all of them were fixed to their 75th percentile versus when all of them were fixed to their 25th percentile was calculated (Est and 95% CI, Est = 0 indicates null). As shown in Fig. 4c, when the other remaining metal concentrations were fixed at the specified percentile, increased exposure to Cd or Li was positively linked with PDR risk (75th vs. 25th). Different Se or Mg concentrations had no statistically significant impact on the remaining elements, regardless of their concentration (Table S4).
The bivariate exposure–response results are shown in Fig. 4d, with a column denoting “exposure 1” and a row denoting “exposure 2.” When the concentrations of the other elements were kept fixed at their median values, “exposure 1” was the metal under investigation. “Exposure 2” was when they were at P25, P50, and P75. If the curves intersect, there may be an interaction, and the graph showed that there was no interaction between these variables on the outcome. Moreover, we found that the potential association of Cd, Li, or Mg with the other three metals increased the risk of PDR. The positive slope of Cd, Li, or Mg became steeper with increasing urine concentrations when the remaining metals were at their median.
Discussion
We found substantial disparities in metal levels between the two groups in our investigation. In the single-element model, after adjusting for other possible confounders, Li, Sr, and Cd were shown to be significantly positively linked with PDR risk compared to the lowest concentration group. Furthermore, in the multiple exposure model, rising quartiles of Li were significantly correlated with the incidence of PDR. Consistent to the findings in the multiple exposure model, the BKMR model similarly demonstrated a monotonic rise in the effect of Li corresponding with its increasing concentration. High concentrations of Mg, Li, and Cd significantly increase PDR risk, while Se has the opposite effect.
The existing studies showed that patients with type 2 diabetes at low BMI levels are at increased risk of developing DR and that higher BMI may have a protective effect against DR (Chen et al. 2022; Man et al. 2016). PDR is the progressive stage of DR, and the average BMI of patients with PDR in this study was lower than that of DR in the control group, also reflecting the risk role of low BMI levels.
Mg plays a crucial role in several enzymatic processes involved in maintaining glucose homeostasis. Numerous studies have shown that Mg deficiency leads to reduced tolerance to oxidative stress and accelerates the progression of diabetes-related complications, and Mg supplementation shows beneficial to diabetic patients. However, a study found that middle-aged population with low dietary Mg consumption did not have an increased risk of diabetes (Dubey et al. 2020). Some scholars found a direct correlation between urinary Mg and fasting blood glucose levels in T2D patients (Sales et al. 2011). The increased urinary Mg excretion in diabetic patients may be due to the effects of hyperglycemia, hyperfiltration, and the insulin effect on Mg renal channels (Ebrahimi Mousavi et al. 2021). In our study, elevated urine Mg levels were correlated with an increased incidence of PDR in T2D patients.
Li may cause hyperglycemia in the undertreated diabetic patients (Hermida et al. 1994). Chronic Li treatment did not alter body weight or blood glucose level in diabetic mice (Graniel-Amador et al. 2022). Li contamination has attracted a lot of attentions recently and chronic poisoning is the most common form of Li poisoning. Although Li toxicity may be related to oxidative stress response, the results from different research are very contradictory (Kiełczykowska et al. 2014). In a study, after 2 months of Li supplementation, the rats showed a remarkable rise in lipid peroxidation which resulted in a significant drop in the enzyme activities of reduced glutathione, catalase, glutathione transferase, and superoxide dismutase (SOD) (Malhotra and Dhawan 2008). A decrease in SOD levels was also observed in healthy subjects treated with Li (Khairova et al. 2012). In our study, the high level of Li exposure seemed to have a positive impact on the risk of PDR development in patients with T2D.
Cd exposure leads to excessive reactive oxygen species (ROS) production, increased oxidative stress response at the cellular level, and exhaustion of antioxidant defenses, which in turn causes biologically relevant molecules and cellular damage (Kar and Patra 2021). Numerous in vivo studies of short- and long-term Cd exposure shown that Cd can lead to hyperglycemia and disturb glucose homeostasis in lab animals (Nguyen et al. 2022). In patients with T2D, it was also observed that there is a significant association between elevated urinary Cd levels and elevated fasting glucose (Schwartz et al. 2003). In addition, Cd exposure increases the tendency for insulin resistance and endocrine dysfunction (Little et al. 2020). Furthermore, the increased levels of oxidative stress response, reduced antioxidant defenses, and a hyperglycemic state are all associated with the progression of DR. The other findings indicated a strong association between toxic metal deposition (Cd and Cs) and insufficiency of critical trace elements (Mn and Zn) (Zhu and Hua 2020). High levels of Cd and Pb, as well as low levels of Zn, Cr, and Mn, were also related to diabetic complications (Afridi et al. 2013). This finding may be helpful in the prevention and treatment of diabetic retinopathy. Consistent with the previous findings, this research discovered that high levels of Cd exposure were connected with PDR risk in T2D patients.
Se is involved in a complex system of anti-oxidative stress response through Se-dependent glutathione peroxidase and other selenoproteins. Se supplementation may have a protective effect on retinal pigment epithelium (RPE) against the chronic damaging effects from glucose during the progression of diabetic retinopathy (González et al. 2018). Although research has demonstrated a favorable correlation between dietary Se consumption or high blood Se levels and the incidence of diabetes, there are no recommendations so far for Se supplementation or restriction to prevent diabetes (Dubey et al. 2020). Urine Se levels in T2D patients with diabetic nephropathy and DR were considerably lower than those in T2D individuals without complications (Zhou et al. 2019). Similar to the above results, our study demonstrated a negative correlation between Se levels and the risk of developing PDR, which suggested a protective effect of Se.
As a heavy metal, Cr also generates toxic effects and pathophysiological damage after long-term exposure and bioaccumulation. It has been speculated that Cr toxicity is mainly caused by the oxidative stress response induced by increased ROS production, antioxidant imbalance, and genomic alterations, which eventually cause damage to organs and tissues of the body (Chakraborty et al. 2022). It was shown in lab animals that after 30 days of oral administration of potassium dichromate (K2Cr2O7), an imbalance appeared between antioxidants (SOD and glutathione peroxidase (GPxs)) and oxidative parameters, and the oxidative stress response was induced in the kidney and liver (Aline et al. 2019). Our study indicated that there is a positive correlation between increasing Cr urinary level and PDR risk in T2D, which suggested Cr exposure increased the incidence of PDR.
Over time, toxic metals from environmental sources accumulate in the RPE, causing damage to the adjacent external nerve retina when their concentration reached a critical point of damage (Pamphlett et al. 2020). Toxic metal elements can compete with essential trace elements in the body, disturbing numerous enzyme activities and interfering the homeostasis of metal elements. It then results in oxidative stress response, DNA damage, lipid peroxidation, etc. (Koedrith and Seo 2011). Numerous anti-oxidant enzymes are found in the retina. Impaired antioxidant defenses will lead to the production of retinal reactive oxygen and nitrogen species (ROS/RNS), and this condition may also be exacerbated with the change of tissue glucose levels (Madsen-Bouterse and Kowluru 2008; Al-Shabrawey and Smith 2010). Such micronutrient imbalances may lead to increased levels of oxidative stress and reduced antioxidant defense in diabetic patients, which ultimately causes the development of diabetic complications (Dubey et al. 2020). Microvascular changes in the fundus of the eye in DR patients may be caused by oxidative and metabolic stress response in the retina (Miller et al. 2020). Characterized by neovascularization of the fundus, PDR is a severe stage of DR that is commonly observed in patients with long duration of diabetes and unstable glycemic control. Choosing PDR patients as a case group may provide a better assessment of the impact of metal elements on the risk of developing DR. And PDR may be associated with inflammatory responses, chronic hypoxia, oxidative stress, and other reactions in the body (Aouiss et al. 2019). Therefore, exposure to metal elements may contribute to the progression of PDR by affecting the homeostasis of the metal environments and oxidative stress levels in the body.
There are a few limitations in our study. First, this study included a limited variety of metal elements and could not include all metals that might be of relevance. Second, 24-h collection of urine should be taken into account in future research since single urine collection may not sufficiently represent the actual metal levels in the blood. Due to some limitations of the study data, this case–control study may not be able to fully reveal the causal relationship between metal elements and the risk of developing PDR. Subsequent studies should adopt a more rigorous design and adequate sample size to provide a more definitive relationship. Furthermore, this study only provides a preliminary data about the significance of abnormal urine metal levels; further research is required in order to fully understand the mechanisms of their toxicity and the microscopic damage caused by metal consumption.
There are several highlights in our research. First of all, our study may be the first one to evaluate the impact of multiple-metal exposure on the risk of developing PDR in patients with T2D, whereas other studies mainly focused on the effects of a specific metal or trace element in the diabetic development. Secondly, this work used the BKMR model to provide a systematic and scientific evaluation of multiple-metal exposure. Thirdly, the findings of this study may provide epidemiological evidence that multiple-metal exposure may affect the risk of developing PDR, which would help us to better understand the risk impact of metal pollution on human health.
Conclusions
In this work, we observed that exposure to multiple metals was correlated with the incidence of developing PDR in patients with T2D. Among them, Mg, Li, Cd and Cr may increase likelihood of getting PDR, while Se may have a protective effect. Our findings revealed a correlation between urine metal levels and PDR risk in T2D patients, suggesting that urine sample could serve as an useful tool in assessing PDR risk. Future studies could further explore the role of urine samples in assessing the risk of other eye diseases. Considering the limitations that only one time urine sample was obtained among relatively small number of study group, future research should increase the sample size and/or conduct multi-center studies to investigate the specific mechanisms of DR caused by multiple metals mixture exposure.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Afridi HI, Kazi TG, Brabazon D, Naher S, Talpur FN (2013) Comparative metal distribution in scalp hair of Pakistani and Irish referents and diabetes mellitus patients. Clin Chim Acta: Int J Clin Chem 415:207–214. https://doi.org/10.1016/j.cca.2012.10.029
Alhamzawi R, Ali HTM (2018) The Bayesian adaptive lasso regression. Math Biosci 303:75–82. https://doi.org/10.1016/j.mbs.2018.06.004
Aline BM, Caprara JF, de Franceschi ID, Feksa LR (2019) Effects of chronic exposure to hexavalent chromium in water on oxidative stress parameters in Wistar rats. Acta Sci: Biol Sci (1). https://doi.org/10.4025/actascibiolsci.v41i1.43771
Al-Shabrawey M, Smith S (2010) Prediction of diabetic retinopathy: role of oxidative stress and relevance of apoptotic biomarkers. EPMA J 1(1):56–72. https://doi.org/10.1007/s13167-010-0002-9
American Diabetes Association (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S14–S31. https://doi.org/10.2337/dc20-S002
Aouiss A, Anka Idrissi D, Kabine M, Zaid Y (2019) Update of inflammatory proliferative retinopathy: Ischemia, hypoxia and angiogenesis. Curr Res Transl Med 67(2):62–71. https://doi.org/10.1016/j.retram.2019.01.005
Bede-Ojimadu O, Orish CN, Bocca B, Ruggieri F, Frazzoli C, Orisakwe OE (2021) Trace elements exposure and risk in age-related eye diseases: a systematic review of epidemiological evidence. J Environ Sci Health Part C, Toxicol Carcinogenesis 39(3):293–339. https://doi.org/10.1080/26896583.2021.1916331
Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA (2015) Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics (oxford, England) 16(3):493–508. https://doi.org/10.1093/biostatistics/kxu058
Bobb JF, Claus Henn B, Valeri L, Coull BA (2018) Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health : a Global Access Science Source 17(1):67. https://doi.org/10.1186/s12940-018-0413-y
Chakraborty R, Renu K, Eladl MA, El-Sherbiny M, Elsherbini DMA, Mirza AK, Vellingiri B, Iyer M, Dey A, Valsala Gopalakrishnan A (2022) Mechanism of chromium-induced toxicity in lungs, liver, and kidney and their ameliorative agents. Biomed Pharmacother 151:113119. https://doi.org/10.1016/j.biopha.2022.113119
Chen J, Wan Y, Su J, Zhu Z, Pan E, Shen C, Wen J, Wang K, Yu H, Qin Y, Cui L, Zhou J, Wu M (2022) Association of generalized and abdominal obesity with diabetic retinopathy in Chinese type 2 diabetic patients. Acta Diabetol 59(3):359–367. https://doi.org/10.1007/s00592-021-01806-7
de González Vega R, García M, Fernández-Sánchez ML, González-Iglesias H, Sanz-Medel A (2018) Protective effect of selenium supplementation following oxidative stress mediated by glucose on retinal pigment epithelium. Metallomics : integrated biometal science 10(1):83–92. https://doi.org/10.1039/c7mt00209b
Dubey P, Thakur V, Chattopadhyay M (2020) Role of minerals and trace elements in diabetes and insulin resistance. Nutrients 12(6):1864. https://doi.org/10.3390/nu12061864
Ebrahimi Mousavi S, Ghoreishy SM, Hemmati A, Mohammadi H (2021) Association between magnesium concentrations and prediabetes: a systematic review and meta-analysis. Sci Rep 11(1):24388. https://doi.org/10.1038/s41598-021-03915-3
Escobar J, Varela-Nallar L, Coddou C, Nelson P, Maisey K, Valdés D, Aspee A, Espinosa V, Rozas C, Montoya M, Mandiola C, Rodríguez FE, Acuña-Castillo C, Escobar A, Fernández R, Diaz H, Sandoval M, Imarai M, Rios M (2010) Oxidative damage in lymphocytes of copper smelter workers correlated to higher levels of excreted arsenic. Mediators Inflamm 2010:403830. https://doi.org/10.1155/2010/403830
Feng W, Cui X, Liu B, Liu C, Xiao Y, Lu W, Guo H, He M, Zhang X, Yuan J, Chen W, Wu T (2015) Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in China. PLoS One 10(4):e0123742. https://doi.org/10.1371/journal.pone.0123742
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS (2020) Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 127(1):P66–P145. https://doi.org/10.1016/j.ophtha.2019.09.025
Garoma D, Merga H, Hiko D (2020) Determinants of diabetic retinopathy in Southwest Ethiopia: a facility-based case-control study. BMC Public Health 20(1):503. https://doi.org/10.1186/s12889-020-08652-2
Graniel-Amador MA, Torres-Rodríguez HF, Martínez-Mendoza RE, Vargas-Muñoz VM, Acosta-González RI, Castañeda-Corral G, Muñoz-Islas E, Jiménez-Andrade JM (2022) Effect of chronic lithium on mechanical sensitivity and trabecular bone loss induced by type-1 diabetes mellitus in mice. Biometals : an International Journal on the Role of Metal Ions in Biology, Biochemistry, and Medicine 35(5):1033–1042. https://doi.org/10.1007/s10534-022-00421-5
Hermida OG, Fontela T, Ghiglione M, Uttenthal LO (1994) Effect of lithium on plasma glucose, insulin and glucagon in normal and streptozotocin-diabetic rats: role of glucagon in the hyperglycaemic response. Br J Pharmacol 111(3):861–865. https://doi.org/10.1111/j.1476-5381.1994.tb14817.x
Kar I, Patra AK (2021) Tissue bioaccumulation and toxicopathological effects of cadmium and its dietary amelioration in poultry-a review. Biol Trace Elem Res 199(10):3846–3868. https://doi.org/10.1007/s12011-020-02503-2
Khairova R, Pawar R, Salvadore G, Juruena MF, de Sousa RT, Soeiro-de-Souza MG, Salvador M, Zarate CA, Gattaz WF, Machado-Vieira R (2012) Effects of lithium on oxidative stress parameters in healthy subjects. Mol Med Rep 5(3):680–682. https://doi.org/10.3892/mmr.2011.732
Kiełczykowska M, Kocot J, Kurzepa J, Lewandowska A, Żelazowska R, Musik I (2014) Could selenium administration alleviate the disturbances of blood parameters caused by lithium administration in rats? Biol Trace Elem Res 158(3):359–364. https://doi.org/10.1007/s12011-014-9952-4
Koedrith P, Seo YR (2011) Advances in carcinogenic metal toxicity and potential molecular markers. Int J Mol Sci 12(12):9576–9595. https://doi.org/10.3390/ijms12129576
Li G, Yao T, Wu XW, Cao Z, Tu YC, Ma Y, Li BN, Peng QY, Wu B, Hou J (2020) Novel and traditional anthropometric indices for identifying arterial stiffness in overweight and obese adults. Clinical Nutrition (edinburgh, Scotland) 39(3):893–900. https://doi.org/10.1016/j.clnu.2019.03.029
Little BB, Reilly R, Walsh B, Vu GT (2020) Cadmium is associated with type 2 diabetes in a superfund site lead smelter community in Dallas, Texas. Int J Environ Res Public Health 17(12):4558. https://doi.org/10.3390/ijerph17124558
Liu Z, Shao M, Ren J, Qiu Y, Li S, Cao W (2022) Association between increased lipid profiles and risk of diabetic retinopathy in a population-based case-control study. J Inflamm Res 15:3433–3446. https://doi.org/10.2147/JIR.S361613
Madsen-Bouterse SA, Kowluru RA (2008) Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord 9(4):315–327. https://doi.org/10.1007/s11154-008-9090-4
Malhotra A, Dhawan DK (2008) Zinc improves antioxidative enzymes in red blood cells and hematology in lithium-treated rats. Nutr Res (New York, N.Y.) 28(1):43–50. https://doi.org/10.1016/j.nutres.2007.11.002
Man RE, Sabanayagam C, Chiang PP, Li LJ, Noonan JE, Wang JJ, Wong TY, Cheung GC, Tan GS, Lamoureux EL (2016) Differential association of generalized and abdominal obesity with diabetic retinopathy in Asian patients with type 2 diabetes. JAMA Ophthalmol 134(3):251–257. https://doi.org/10.1001/jamaophthalmol.2015.5103
McEligot AJ, Poynor V, Sharma R, Panangadan A (2020) Logistic LASSO regression for dietary intakes and breast cancer. Nutrients 12(9):2652. https://doi.org/10.3390/nu12092652
Miller DJ, Cascio MA, Rosca MG (2020) Diabetic retinopathy: the role of mitochondria in the neural retina and microvascular disease. Antioxidants (basel, Switzerland) 9(10):905. https://doi.org/10.3390/antiox9100905
Nguyen J, Patel A, Gensburg A, Bokhari R, Lamar P, Edwards J (2022) Diabetogenic and obesogenic effects of cadmium in Db/Db mice and rats at a clinically relevant level of exposure. Toxics 10(3):107. https://doi.org/10.3390/toxics10030107
Pamphlett R, Cherepanoff S, Too LK, Kum Jew S, Doble PA, Bishop DP (2020) The distribution of toxic metals in the human retina and optic nerve head: Implications for age-related macular degeneration. PLoS One 15(10):e0241054. https://doi.org/10.1371/journal.pone.0241054
Pedersen EB, Jørgensen ME, Pedersen MB, Siggaard C, Sørensen TB, Mulvad G, Hansen JC, Asmund G, Skjoldborg H (2005) Relationship between mercury in blood and 24-h ambulatory blood pressure in Greenlanders and Danes. Am J Hypertens 18(5 Pt 1):612–618. https://doi.org/10.1016/j.amjhyper.2004.11.022
Reddy RK, Pieramici DJ, Gune S, Ghanekar A, Lu N, Quezada-Ruiz C, Baumal CR (2018) Efficacy of ranibizumab in eyes with diabetic macular edema and macular nonperfusion in RIDE and RISE. Ophthalmology 125(10):1568–1574. https://doi.org/10.1016/j.ophtha.2018.04.002
Sales CH, Pedrosa LF, Lima JG, Lemos TM, Colli C (2011) Influence of magnesium status and magnesium intake on the blood glucose control in patients with type 2 diabetes. Clin Nutr (edinburgh, Scotland) 30(3):359–364. https://doi.org/10.1016/j.clnu.2010.12.011
Schwartz GG, Il’yasova D, Ivanova A (2003) Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 26(2):468–470. https://doi.org/10.2337/diacare.26.2.468
Sinclair SH, Schwartz SS (2019) Diabetic retinopathy-an underdiagnosed and undertreated inflammatory, neuro-vascular complication of diabetes. Front Endocrinol 10:843. https://doi.org/10.3389/fendo.2019.00843
Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB, Navas-Acien A (2013) Cadmium exposure and incident cardiovascular disease. Epidemiology (Cambridge, Mass.) 24(3):421–429. https://doi.org/10.1097/EDE.0b013e31828b0631
Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, Kile ML, Quamruzzaman Q, Afroz S, Golam M, Amarasiriwardena C, Bellinger DC, Christiani DC, Coull BA, Wright RO (2017) The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ Health Perspect 125(6):067015. https://doi.org/10.1289/EHP614
Wang W, Schaumberg DA, Park SK (2016) Cadmium and lead exposure and risk of cataract surgery in U.S. adults. Int J Hygiene Environ Health 219(8):850–856. https://doi.org/10.1016/j.ijheh.2016.07.012
World report on vision. Geneva: World Health Organization (2019) Licence: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/publications/i/item/9789241516570
Zhong Q, Wu HB, Niu QS, Jia PP, Qin QR, Wang XD, He JL, Yang WJ, Huang F (2021) Exposure to multiple metals and the risk of hypertension in adults: a prospective cohort study in a local area on the Yangtze River, China. Environ Int 153:106538. https://doi.org/10.1016/j.envint.2021.106538
Zhou Q, Guo W, Jia Y, Xu J (2019) Serum and urinary selenium status in patients with the pre-diabetes and diabetes in Northeast China. Biol Trace Elem Res 191(1):61–69. https://doi.org/10.1007/s12011-018-1604-7
Zhou TT, Hu B, Meng XL, Sun L, Li HB, Xu PR, Cheng BJ, Sheng J, Tao FB, Yang LS, Wu QS (2021) The associations between urinary metals and metal mixtures and kidney function in Chinese community-dwelling older adults with diabetes mellitus. Ecotoxicol Environ Saf 226:112829. https://doi.org/10.1016/j.ecoenv.2021.112829
Zhu X, Hua R (2020) Serum essential trace elements and toxic metals in Chinese diabetic retinopathy patients. Medicine 99(47):e23141. https://doi.org/10.1097/MD.0000000000023141
Acknowledgements
Thanks to all the subjects participated in this research. This work was supported by The Project for Top Disciplinary Talents of Majors in Universities of Anhui Province [grant numbers gxbjZD09].
Funding
This work was supported by The Project for Top Disciplinary Talents of Majors in Universities of Anhui Province [grant number gxbjZD09].
Author information
Authors and Affiliations
Contributions
Yan-Qing Li, Si-Tian Zhang, Nai-Yu Ke, Fen Huang, and Yan-Feng Zhou contributed to the study conception and design. Material preparation and data collection were performed by Yan-Qing Li, Si-Tian Zhang, Nai-Yu Ke, and Wen-Lei Hu. Yan-Feng Zhou, Fen Huang, Wen-Lei Hu, and Guo-Ao Li analyzed and interpreted the data. Yan-Qing Li, Si-Tian Zhang, and Nai-Yu Ke wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study had been approved by the Ethics Committee of Anhui Medical University (Approval No: 20170305), and the participants had signed written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan-Qing Li, Si-Tian Zhang, Nai-Yu Ke, and Yan-Cheng Fang contributed equally to this work and should be considered co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, YQ., Zhang, ST., Ke, NY. et al. The impact of multiple metals exposure on the risk of developing proliferative diabetic retinopathy in Anhui, China: a case–control study. Environ Sci Pollut Res 30, 112132–112143 (2023). https://doi.org/10.1007/s11356-023-30294-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30294-1