Abstract
It’s well-known that multiple metal elements can lead to the change of oxidative stress response levels in vivo. However, their relationship with age-related cataract (ARC) had not been well studied. We designed a case–control study including 210 individuals with ARC and 210 matched control group. The metal levels in their urine specimens were measured using graphite furnace atomic absorption spectrometry (GFAAS) and inductively coupled plasma optical emission spectrometry (ICP-OES). Least Absolute Shrinkage and Selection Operator (LASSO) regression was used to select representative metals into the multi-element model and reduce dimension. Multivariate logic analysis and Bayesian kernel machine regression (BKMR) were subsequently used to explore the association of ARC risk with multiple metal elements. We found that magnesium (Mg), chromium (Cr), arsenic (As), manganese (Mn), and selenium (Se) were positively associated with ARC in the single-element model. The multiple exposure model indicated a positive association between Mg and As, in which the OR in their highest quartile were 3.32 (95% CI: 1.24–8.89) and 7.09 (95% CI: 2.56–19.63). The BKMR model also showed the effect of As increased monotonically with its increasing concentration, and high levels of Mg and As had a significant positive effect on ARC risk. In conclusion, we found that exposure to multiple metals was associated with increased ARC risk. Further research is needed to verify these findings in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cataract remains to be the leading cause among many blind eye diseases in the world with the world’s population growing and aging increasing (Flaxman et al. 2017). ARC prevalence increased from 3.23 ~ 4.72% at age 45 ~ 49 to 65.78 ~ 74.03% at age 85 ~ 89 (Song et al. 2018). According to the data reported by World Health Organization in 2020, ARC caused 15.2 million blindness (45.5%) and 78.8 million visual damage (38.9%) in people aged 50 and above worldwide (GBD 2019). Visual impairment caused by lens opacity can affect patients’ independence and their quality of life. Meanwhile, global blindness and moderate or severe visual impairment (MSVI) have caused significant economic losses (Marques et al. 2021). While ophthalmic surgery is a common clinical method for cataract treatment with good postoperative results, but its complications, such as macular oedema and corneal opacity, can lead to postoperative visual loss (Gan et al. 2020).
Previous studies have shown that multiple risk factors, including aging, trauma, radiation, metabolic disorders, and malnutrition, were associated with ARC (Chang et al. 2011). Recently, some scholars reported that exposure to heavy metals such as lead (Pb) and cadmium (Cd) was associated with cataract, but relevant epidemiological studies were limited (Wang et al. 2016). Metal exposure is prevalent in our daily life and the environment, and they accumulate in the body as we age, which may cause a number of age-related eye diseases (Bede-Ojimadu et al. 2021). In addition to As and Cr that are toxic at low concentrations, some health-vital metals, such as copper (Cu) and iron (Fe), may become toxic in some cases due to their possible competition for metal-binding sites with essential ions, which may cause disturbances of metal homeostasis (Hartwig 2001; Valko et al. 2016). Furthermore, the disturbance of metal homeostasis can cause oxidative stress, DNA damage, lipid peroxidation, and other effects (Koedrith and Seo 2011). As we all know that a large proportion of the global population, especially those in developing countries, was still exposed to high levels of toxic metals (Naka et al. 2020). As a result, the risk of many diseases is increased, including cardiovascular disease, cancer, diabetes, cataract, and others (Jomova and Valko 2011). Although some countries had reported reductions in local toxic metal exposures, toxic damage and inadequate micronutrient intake may still affect the health of older adults (Frazzoli and Mantovani 2020; Grashow et al. 2015).
In recent years, many studies have made efforts to explore the effects of multi-pollutant mixtures on health. However, current methods of studying mixtures addressing some of these complexities have significant drawbacks. For example, clustering methods lose information by classifying continuous exposure concentrations. Statistical learning algorithms such as random forests can provide measures of variable importance for mixed components, but such measures cannot simply sum up the size or direction of the association (Breiman 2001). Hierarchical model formulae address highly correlated pollutants by narrowing individual effect estimates to population averages, but this approach also generally assumes a linear and additive association between each component and health (Thomas et al. 2007). In this paper, we introduce BKMR as a new method to estimate the health effects of mixtures. For this approach, we model the health outcomes as a smoothing function H of the exposure variables, expressed using a kernel, and adjusted for possible confounding factors. Since health outcomes may depend on only one component of the mixture, variable selection was performed to determine which components were responsible for the health effects of the mixture.
Our general population is exposed to various doses and different kinds of heavy metals daily. When exploring the effect of a single metal on cataract, the mixed effect of multiple metals should not be ignored. The BKMR model, considering potential nonlinear effects and interactions, was used to assess the combined effects of the mixed components (Zhong et al. 2021). Some scholars used the BKMR model to analyze the association between urine metal elements level and diseases such as diabetes or hypertension, and analyzed the role of a single metal element and the combined effects of multiple metals (Zhou et al. 2021). Therefore, the systematic and scientific evaluation of multiple element exposure using the BKMR model is of great significance to deepen our understanding of metal contamination in the risk of developing ARC.
Concerning the current environmental heavy metal pollution and the high prevalence of ARC (Wang et al. 2016), it is necessary to further explore the association between multiple metals exposure and ARC risk to deeply understand the cause of the disease. Therefore, the aims of this study are (1) to explore the associations between urinary metal element levels and ARC risk under mixed multi-metal exposure, and (2) to provide new ideas for the prevention and treatment of ARC.
Methods
Study population
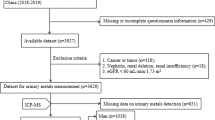
The current study was conducted in the Ophthalmology Department, First Affiliated Hospital of Anhui Medical University, Anhui, China. A total of 410 patients who came from Anhui and scheduled for surgical treatment from December 2017 to March 2019 in our hospital were selected for the study. The inclusion criteria of case group: newly diagnosed cataract patients with age older than 50 years and vision ≤ 0.4 in the affected eye (Abdellah et al. 2019). The inclusion criteria of control group: using convenience sampling method, control group was selected among patients diagnosed with diseases unrelated to cataract, corrected visual ≥ 0.6, and no lens opacities in either eye. The exclusion criteria for both the case and control groups are the following: history of any treatments or medical diagnosis associated with cataract or other diseases causing eye and vision problems (e.g., age-related macular degeneration, radiation therapy, diabetic retinopathy, glaucoma, previous ocular surgery, or acute or chronic uveitis); history of unhealthy dietary habit in the past year; history of work or exposure in special places; severe heart, liver, kidney, or digestive system diseases; and severe hearing and mental disorders (Movahedian et al. 2020; Sedaghat et al. 2017). Both groups were examined by the chief ophthalmologist (the author) in our department, and cataract diagnosis was made by slit-lamp biological microscopy.
Controls and cases were matched 1:1 based on sex and age (± 5 years of the cases). Finally, 210 pairs of cases and controls were enrolled in our study. The study had been approved by the Ethics Committee of Anhui Medical University (Approval No: 20170305), and the participants had signed written informed consent.
Variable collection and definition
In this study, the questionnaire had been developed through the preparation of the first draft, soliciting expert opinions, and pre-survey. The structured questionnaire included data on general conditions (e.g., gender, age, household annual income), lifestyle and behavioral habits (e.g., sleep quality, smoking status, alcohol consumption, physical activity), and diabetes-related conditions.
Sleep quality was divided into three categories (poor, general, and good). Smokers were those residents who smoked or accumulated continuously for 6 months or more at the time of the survey. Drinkers were those who had alcohol behavior at the time of the survey for 6 consecutive months and once a week. Household annual income was categorized into three groups (< 30,000 RMB, 30,000–120,000 RMB, and > 120,000 RMB). We divided the frequency of physical activity into four groups (never, 1–2 times, 3–4 times, and > 4 times per week) and asked the participants if they had diabetes. Body mass index (BMI) was classified into four categories following the standards of Chinese adults: < 18.5 kg/m2 (underweight), 18.5–23.9 kg/m2 (normal weight), 24.0–27.9 kg/m2 (overweight), and ≥ 28.0 kg/m2 (obesity) (Li et al. 2020).
Measurement of urinary elements
We collected, transported, and tested urine samples in accordance with standard operating specifications. All participants completed the self-made questionnaire and provided morning urine on the second day.
Participants’ first-morning urine was collected into a 50-ml polypropylene tube and subsequently placed in a cold closet for storage. The collected samples were sent to the laboratory within 1 day and stored at − 80 °C for subsequent testing. Inductively coupled plasma optical emission spectrometry (ICP-OES 7000DV, PerkinElmer Corporation) was used to detect the concentration of 14 metals: As, Barium (Ba), Cr, Cobalt (Co), Cu, Pb, Lithium (Li), Mg, Mn, Molybdenum (Mo), Fe, Se, Strontium (Sr), and Zinc (Zn) in the urine. Samples were removed from the cold closet before testing and thawed at room temperature. Three milliliters of urine was removed from the sample and transferred to a 15-ml polypropylene centrifuge tube. The removed urine samples were diluted with 9 ml of 5% (v/v) HNO3 and mixed evenly in a vortex. Digestion was performed with a microwave digestion instrument at 90 °C for 1 h and centrifuged at 4500 rpm for 8 min at room temperature, then aspirated the supernatant for analysis. Furthermore, graphite furnace atomic absorption spectrometry (GFAAS, ZEEnit700P, Analytik Jena, Germany) were used to detect the urinary Cd. The samples were diluted with 1% (v/v) HNO3. The accuracy of measurement was assessed by spiked recovery method during testing. And the spike-and-recovery experience range was 92–104%.
Urinary creatinine was tested using a BECKMAN DXC800 biochemical analyzer of the USA, and the kit was a human creatinine detection kit from BECKMAN Biotechnology Limited. The values of metal elements in urine below the limit of detection (LOD) were replaced with LOD /√2 (Tellez-Plaza et al. 2013). The metal element concentrations in the results of this study were all creatinine correction values [μg/(g creatinine) or mg/(g creatinine)]. And the detection rates of all elements were > 70% relied on the LODs.
Statistical analysis
The main baseline characteristics of ARC and non-cataract groups were described and compared using Wilcoxon signed-rank test and chi-square test. The values of all metal levels were corrected with creatinine and natural log-transformed.
We evaluated odds ratios (ORs) and 95% confidential intervals (95% CIs) for ARC risk by conditional logistic regression models and categorized each urine metal level into quartiles. The lowest quartile was taken as a reference based on its concentration distribution. We developed two models to analyze the effect of each metal in single-element model. Model 1 was only adjusted for sex and age, and Model 2 was additionally adjusted for other covariates: BMI, sleep quality, physical activity, household annual income, smoking status, alcohol consumption, and diabetes.
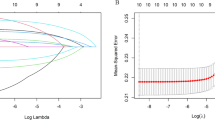
We also developed a multi-element model to assess the effects of multiple metals on ARC risk and a correlation heatmap of urine metals to compare the correlations. Considering the diversity of metals and mutual correlation, LASSO regression was used to select representative metals into the multi-element model and reduce dimension (Alhamzawi and Ali 2018). LASSO regression can regress and penalize all variables, changing the coefficients of relatively unimportant independent variables to 0 and excluding them from the model (McEligot et al. 2020). Then we used conditional logistic regression to calculate ORs and 95% CIs for multiple metals mixed-exposure and ARC risk.
To accurately reflect the non-linear effects of metals on ARC risk, BKMR was used to further demonstrate the effects of metals and their interactions (Bobb et al. 2018). The corresponding BKMR model formula is as follows (Bobb et al. 2015):
where \({Y}_{i}\) is a health endpoint, \(zi\) is a vector of exposure variables (e.g., air pollution constituents), \({x}_{i}^{T}\) contains a set of potential confounders, and \({\in }_{i}\). In the context of environmental mixtures, \(h\left(zi\right)\) typically characterizes a high-dimensional exposure–response function that may incorporate non-linearity and/or interaction among the mixture components. We built an exposure matrix (including metals selected by LASSO regression) and a covariant (demographic characteristics), and implemented the Monte Carlo algorithm (using the kmbayes function) (Escobar et al. 2010). Firstly, we used variable selection to fit BKMR model and estimated the posterior inclusion probability (PIP) for each metal (Valeri et al. 2017). Then, the univariate exposure–response of each metal was showed with a cross-section plot when the other metals were fixed at the median. We also compared individual metal risk differences in ARC between its 75th percentile and 25th percentile, when all of other metals were fixed at a particular quantile (P25, P50, and P75). The cumulative effects of mixed-metals exposure were computed by comparing the value of the exposure while all elements were at a particular quantile as compared to when all of them are at their median. Besides, we further discussed the interaction between multiple metals and ARC risk, and visualized the bivariate exposure of each two factors, where all other factors were fixed on a specific quantile (median).
Data entry and calibration inspection were performed using EpiData 3.1 software. Analysis was performed using the Statistical Product and Service Solutions (SPSS), version 23.0 (SPSS Inc., Chicago, IL, USA) and R software (version 3.6.1; R Core Team). The two-sided statistical significance level was set at α = 0.05.
Results
Characteristics of study population
This study recruited 210 ARC cases and 210 matched non-cataract controls. Table 1 presented the demographic characteristics of the participants. The mean age of the cases was 59.2 ± 11.5 years, and 59.2 ± 11.8 years for controls. For the BMI, 7.7% cases were ≥ 28.0 kg/m2, which was lower than the 11.4% in controls. Diabetes was diagnosed in 16.7% of the case group and 15.2% in the control group. There were no significant differences in demographic characteristics including sex, age, BMI, sleep quality, physical activity, household annual income, smoking status, alcohol consumption, and diabetes (P > 0.05).
Urine elements and ARC risk in single-element model
We summarized the original metal concentrations of case and control groups in Table S1 and showed the Spearman correlation heatmap for urinary metals in Fig. S1. Then we applied a conditional logistic regression model to estimate the association between metal elements’ level and ARC risk. And grouped the concentration level by their quartiles and took the lowest group (Q1) as a reference. As Table 2 presented, the concentrations level of Mg, Cr, Mo, Zn, Fe, As, Cu, Mn, Pb, Se, and Cd were positively associated with ARC risk in Model 1. These associations remained present in five elements (Mg, Cr, As, Mn, Se) after adjusting for other potential confounders in Model 2. Positive associations were observed in the exposure of Mg, Cr, Mo, As, Mn and Se in highest quartiles (Q4 vs. Q1: OR = 3.09, 95% CI: 1.21–7.88; OR = 3.71, 95% CI: 1.52–9.08; OR = 2.78, 95% CI: 1.17–6.65; OR = 4.73, 95% CI: 2.07–10.78; OR = 3.84, 95% CI: 1.40–10.58; OR = 2.78, 95% CI: 1.20–6.47, respectively).
Multiple elements exposure model for ARC
Since there were many metal elements in this study, after considering the correlation of each metal, we adopted LASSO regression to select the metals (Fig. 1a and b). Finally, five metal elements (Mg, As, Mn, Se, and Fe) were included in the multiple exposure model. Table 3 showed the multivariable effect adjusted all potential covariates and used conditional logistic regression model to explore the correlation between five metal element mixed exposure and ARC risk. It was observed that a positive association of the gradually increasing quartile of Mg and As with ARC risk, with the OR in the highest quartile was 3.32 (95% CI: 1.24–8.89) and 7.09 (95% CI: 2.56–19.63). At the same time, we did not find any relationships between Mn, Se, and Fe and ARC risk.
BKMR analyses
We used the BKMR model to evaluate the effect of mixed exposure on the five metal elements selected by LASSO regression. Firstly, the individual exposure of each element was performed when the other elements were fixed at their median concentrations, which could reflect a positive association of five metal element exposure levels (Mg, As, Mn, Se, and Fe) with ARC risk, which was largely consistent with the results of the single-element model.
In Fig. 2b, the cumulative effect of all elements was showed when these five elements were fixed at different percentiles (P25 to P75, step value = 5th) as compared to their medians. And the joint effect of all elements was statistically significant when they were more than their 50th percentile as compared to when these elements were at medians as positive influences. Meanwhile, when they were in the 40th and 45th quartiles, overall exposure had a negative impact on ARC risk. We observed that the five metals (Mg, As, Mn, Se, and Fe) had significant joint effects compared to their median values when they were at or above their 55th percentile.
The BKMR model of five metal elements evaluated the effect of mixed exposure on ARC risk. (a) The univariate exposure response function (95% CI) for each metal element when the other elements were fixed at the median concentration (P50). (b) The overall effects of mixed-exposure in elements fixed to different percentiles as compared when they were at their medians (P50). (c) The effects of single metal exposure between its 75th and 25th percentile, when the remaining metals were fixed at different percentiles (25th, 50th, or 75th). (d) The bivariate cross-section effects of the exposure–response function of a single element where the second element was fixed at different percentiles (25th, 50th, or 75th)
Then we assessed the distribution of individual exposures to interaction effects to describe the univariate effects. Such as the risk difference of a single metal exposure between its 75th and 25th percentile, when the remaining metals were fixed at different percentiles (25th, 50th, or 75th). Figure 2c showed that increased As exposure was positively associated with ARC (75th vs. 25th) when the remaining metals were fixed to a particular quantile. When the remaining elements were fixed at P25 and P50, the difference value of Fe P75 − P25 was reduced (< 0). And when the remaining elements were fixed at P75, the effect increased and had statistical significance. No matter how the concentrations of other elements changed, the effects of different concentrations of Se, Mn, and Mg did not have any statistical significance.
Figure 2d explored the bivariate exposure–response consequences, with each column represented “exposure 1” and each row represented “exposure 2.” Exposure 1 was the studied metal and the others were fixed at their medians. Exposure 2 were located at their P25, P50, and P75. We observed that the potential association of Mg or As or Mn or Se or Fe with the other four metals increases the risk of ARC. When the other three metals were in the median, the positive slope of Mg, As, Mn, Fe, or Se became steeper with the increasing urinary concentration, and no significant interaction was found.
Sensitivity analysis
In this study, patients with diabetes and smoking status may be correlated with the risk of ARC, so we developed sensitivity analysis for sub-groups, such as non-smokers, or participants without diabetes. And we found no significant change in the results (Tables S2 and S3). We also conducted LASSO and Elastic net in terms of picking variables with no significant difference (Fig. S2).
Discussion
The development of modernization and the improvement of living standards have had a great impact on China’s natural environment. The Chinese government reported in 2014 that high levels of Cd, As, and Pb contamination in rice-growing areas were causing local farmers to contract Itai-itai disease (Takahashi 2016). In Anhui province, one of the major agricultural provinces in China, the heavy metal levels in rural kitchens, soil, air, crops, and water sources have a great impact on the health of local residents. As reported, the mining industry had caused metal pollution to the soil in Huainan and Tongling (Fang et al. 2015; Shen et al. 2019). Metal pollution has also been observed by some scholars in the Huai River (Bengbu section), with Pb and Hg pollution likely originating from local manufacturing, transportation, and agricultural emissions (Yang et al. 2017). Most of the time, we are susceptible to multiple types of heavy metal exposure in various ways (Sanaei et al. 2021). By far, there are few studies on the effect of multiple metal exposure on ARC risk.
It was generally accepted that urinary metal level reflects long-term chronic exposure of a certain metal, while blood level more correlates with recent acute exposure. In particular, for chronic diseases with long latency periods (such as cataract), urinary Cd level was considered a more appropriate biomarker to study the association of cataract with metal exposure, while using blood Cd level may attenuate risk estimates (V and A 2014; Wang et al. 2016). It is not uncommon clinically to test different biological samples for different purposes. For example, blood level of Se, Zn, and Mn was used to study the essential level of these trace elements in our body, while urine level was studied to understand the degree of absorption and intoxication of these metals (Pedersen et al. 2005). Therefore, the level changes of some metal elements in urine can potentially serve as good indicators in assessing cataract risk.
In this study, Mg, Cr, As, Mn, and Se were found to be positively associated with ARC risk in single-element model after controlling other potential confounders. Furthermore, the multiple exposure model showed a positive correlation of the gradually increasing quartile of Mg and As with ARC risk. Consistent with the multi-element model results, the BKMR model also shown that the effect of As increased monotonically with its increasing concentration, and high levels of Mg and As had a significant positive effect on ARC. Our analysis also provided supporting results for the association between Mn, Se, Fe, and ARC risk in the BKMR model, although the trends were not consistent in the multi-element model.
Long-term consumption of As-contaminated water and crops leads to chronic As poisoning, which disrupted the host immune system through mechanisms such as inducing apoptosis and oxidative stress (Dangleben et al. 2013). The poisoning mechanisms include increased ROS production, decreased superoxide dismutase (SOD), impaired structure and function of certain proteins (especially-SH proteins), and altered antioxidant defense systems and structural disorders of some cellular components (Rahman and Ley 2017). However, only very few literature reports had been reported on the associations between As exposure and ARC. A study of 349 residents in Taiwan villages with high incidence of As poisoning found that the intake of As had a positive relationship with cataract (especially posterior subcapsular cataract), and cumulative exposure to As and continuous drinking contaminated well water increased the risk of various types of lens opacity (See et al. 2007). In a carp model, after exposure to a certain concentration of As, carps developed cataract and skin damage (Mohanty et al. 2015). A study of smelting workers exposed to high concentration of As environment observed a positive correlation of worker urinary As levels with SOD activity and a negative correlation with serum vitamin E levels (Escobar et al. 2010). SOD and vitamin E are the main enzymatic and non-enzymatic mechanisms involved in the antioxidant reactions, respectively. Although short-time exposure to low concentrations of As could lead to increased antioxidant enzyme activity, long-term exposure caused a decline in various antioxidant enzyme activities (Valko et al. 2016). Similar to the above findings, this study suggested a significant positive association between As and ARC risk.
Recent studies mainly focused on the effect of hypomagnesemia on cataract and the beneficial effect of supplementary Mg2+ treatment for cataract. Mg deficiency caused increased oxidative stress and cataract production (Kaliaperumal et al. 2021). And restoration of the normal redox state of the lens was observed with taurine-magnesium supplementation in cataract animals (Choudhary and Bodakhe 2016). As an essential metal element of the human body, the physiological role of Mg cannot be ignored. Although some relevant data provided supporting evidence for the association of Mg deficiency with cataract, its role can only be highlighted as an association factor with several other factors (Agarwal et al. 2012). To the best of our knowledge, relevant experiments were mainly focus on the beneficial effects of Mg; very few clinical research has been conducted to reveal the consequence under high Mg exposure (Ajith 2019). Although some scholars have observed the inhibitory effect of high Mg concentrations on osteoblasts differentiation, further studies are still needed to explore the mechanism of toxicity with high level of Mg exposure (Wang et al. 2020). The results of this study showed that increased urinary Mg levels were significantly associated with ARC risk, which reflected the risk effect of increased Mg exposure in vivo.
Due to the narrow range between physiological and toxic level of Se, the results from previous studies regarding to Se as a dietary supplement are not completely consistent. While a significant 18% decrease in cataract development or progression was found in one study with 9-year randomized daily supplementation of Se-containing multivitamin (Clinical Trial of Nutritional et al. 2008), another scholar could not find any significant protective effect on cataract development with Se supplementation alone or in combination with vitamin E (Christen et al. 2015). Although appropriate dose of Se supplement can improve the antioxidant capacity, when Se supplement or exposure exceeds what is required for selenogenic protein synthesis, it will instead promote oxidative damage and cause cataract in experimental animals or ALS and cancer in humans (Vinceti et al. 2013). Therefore, the potential toxicity of Se should not be ignored when investigating its antioxidant effects. The poisoning mechanisms of Se include the production of ROS, enzyme inhibition, cellular dysfunction, interference with DNA expression and repair, and the production of inflammatory mediators (Zwolak 2020). With large oral intake of Se, acute intoxicity and even death may occur (Hadrup and Ravn-Haren 2020). Consistent with the results discussed above, this study found an association between high level exposure to Se with ARC risk.
Iron homeostasis plays an important role in many metabolic processes, while iron overload can be harmful to cells, tissues, and organs. Some studies reported that toxic metals may interfere the metabolism of essential metals, such as Fe, Cu, and Zn (López et al. 2004). Fe involved in various redox responses to ROS formation, especially when its homeostasis regulation was disturbed, causing oxidative stress and irreversible cellular damage (Valko et al. 2016). Increased Fe concentration in the lens and Fe-induced oxidative damage caused a variety of eye diseases including cataract (Levi et al. 1998). It was also commonly seen clinically patients with cataract caused by iron-containing foreign body or ocular siderosis (Kumagai et al. 2019). Furthermore, hereditary hyperferritinemia cataract syndrome (HHCS) was characterized by early-onset bilateral cataract. Also, patients with severe Fe deficiency anemia were reported to develop methemoglobinemia after enhanced Fe supplementation, and significant binocular cataract was observed about 2 years after the serum ferritin peak (Mehmet et al. 2021).
Mn is an essential trace element in the human and is involved in many metabolic reactions. Mn deficiency may increase oxidative stress by generating more ROS, while Mn supplementation can downregulate ROS production. But these effects were limited to low concentrations and appropriate exposure times (Zeinert et al. 2018). While the significant adverse health consequences caused by high level of Mn exposure had attracted the attention of many scholars, various experiments confirmed that Mn exposure could affect the function of the lung, liver, and kidney by activating various regulated oxidative stress, apoptosis, and inflammatory response molecular mechanism (Gandhi et al. 2022). It was also observed in experimental animals that excess Mn level inhibited the transcription of the potential antioxidant genes, which in turn reduced the corresponding protein levels and decreased the antioxidant capacity of the liver (Wang et al. 2021).
Several limitations of this study should be noted. First, new-diagnosed cases were used as case groups to ensure the representativeness of the sample. However, the sample size of paired designs was limited and may not adequately illustrate the causal relationship between urine trace elements and ARC risk. Second, ARC typing was not performed at the time of preliminary sample screening, so the relationship of urinary metal element content and the risk of different types of ARC needed further investigation. Third, a single urine sample may not fully represent causality, and 24 h of urine shall be considered in subsequent studies. Moreover, our study only demonstrated the possible significance of the differences in urinary metal level. The pharmacokinetics of intaking metals and the specific mechanism needs to be further explored.
There were some highlights in this study. First, while previous studies have mostly considered the role of individual metal elements or local trace elements in cataract onset, this study may be the first to evaluate the effect of multiple mixed metal exposures on ARC risk. Second, this study used BKMR model to systematically and scientifically evaluate the multi-element exposure situation. Third, this study used urine as a biomarker to evaluate ARC risk and collected meaningful results. Fourth, this study provided epidemiological evidence of the effect of metal pollution on ARC, which would deepen our understanding of metal pollution in the occurrence and development of ocular diseases.
Conclusion and policy recommendation
In this study, we found that exposure to multiple metals was associated with ARC risk. Therefore, local governments should detect metal content in the environment routinely and formulate corresponding policies to reduce metal pollution. The prevention and control of ARC caused by environmental pollution can start with the improvement of the living environment and lifestyle to properly control the intake and elimination of metals. Moreover, our results demonstrated the associations between urinary metal level and ARC, suggesting the urine may serve as a useful specimen for the assessment of ARC risk. Due to the limitations of the paired sample size and morning urine samples, this study may not adequately illustrate the causal relationship between urine trace elements and ARC risk. Future studies should expand the sample size, explore the representative of 24-h urine sample, and investigate the specific mechanism of ARC caused by poly-metallic mixed exposure.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdellah MM, Mohamed Mostafa E, Salama EH, Roshdy Mohamed E (2019) Association of serum 25-hydroxyl vitamin D deficiency and age-related cataract: a case-control study. J Ophthalmol 2019:9312929. https://doi.org/10.1155/2019/9312929
Agarwal R, Iezhitsa I, Agarwal P, Spasov A (2012) Magnesium deficiency: does it have a role to play in cataractogenesis? Exp Eye Res 101:82–89. https://doi.org/10.1016/j.exer.2012.05.008
Ajith TA (2019) Possible therapeutic effect of magnesium in ocular diseases. J Basic Clin Physiol Pharmacol. https://doi.org/10.1515/jbcpp-2019-0107
Alhamzawi R, Ali HTM (2018) The Bayesian adaptive lasso regression. Math Biosci 303:75–82. https://doi.org/10.1016/j.mbs.2018.06.004
Bede-Ojimadu O, Orish CN, Bocca B, Ruggieri F, Frazzoli C, Orisakwe OE (2021) Trace elements exposure and risk in age-related eye diseases: a systematic review of epidemiological evidence. J Environ Sci Health C Toxicol Carcinogenes. https://doi.org/10.1080/26896583.2021.1916331
Bobb JF, Valeri L, Claus HB, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA (2015) Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508. https://doi.org/10.1093/biostatistics/kxu058
Bobb JF, Claus Henn B, Valeri L, Coull BA (2018) Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17:67. https://doi.org/10.1186/s12940-018-0413-y
Breiman L (2001) Random forests. Mach Learn. https://doi.org/10.1023/A:1010933404324
Chang JR, Koo E, Agron E, Hallak J, Clemons T, Azar D, Sperduto RD, Ferris FL 3rd, Chew EY, Age-related eye disease study G (2011) risk factors associated with incident cataracts and cataract surgery in the age-related eye disease study (AREDS): AREDS report number 32. Ophthalmology 118:2113–2119. https://doi.org/10.1016/j.ophtha.2011.03.032
Choudhary R, Bodakhe SH (2016) Magnesium taurate prevents cataractogenesis via restoration of lenticular oxidative damage and ATPase function in cadmium chloride-induced hypertensive experimental animals. Biomed Pharmacother 84:836–844. https://doi.org/10.1016/j.biopha.2016.10.012
Christen WG, Glynn RJ, Gaziano JM, Darke AK, Crowley JJ, Goodman PJ, Lippman SM, Lad TE, Bearden JD, Goodman GE, Minasian LM, Thompson IM Jr, Blanke CD, Klein EA (2015) age-related cataract in men in the selenium and vitamin E cancer prevention trial eye endpoints study: a randomized clinical trial. JAMA Ophthalmol 133:17–24. https://doi.org/10.1001/jamaophthalmol.2014.3478
Clinical Trial of Nutritional S, Age-related Cataract Study G, Maraini G, Williams SL, Sperduto RD, Ferris F, Milton RC, Clemons TE, Rosmini F, Ferrigno L (2008) a randomized, double-masked, placebo-controlled clinical trial of multivitamin supplementation for age-related lens opacities. Clinical trial of nutritional supplements and age-related cataract report no. 3. Ophthalmology 115(4):599-607.e1. https://doi.org/10.1016/j.ophtha.2008.01.005
Dangleben NL, Skibola CF, Smith MT (2013) arsenic immunotoxicity: a review. Environ Health 12:73. https://doi.org/10.1186/1476-069X-12-73
Escobar J, Varela-Nallar L, Coddou C, Nelson P, Maisey K, Valdes D, Aspee A, Espinosa V, Rozas C, Montoya M, Mandiola C, Rodriguez FE, Acuna-Castillo C, Escobar A, Fernandez R, Diaz H, Sandoval M, Imarai M, Rios M (2010) Oxidative damage in lymphocytes of copper smelter workers correlated to higher levels of excreted arsenic. Mediators Inflamm 2010:403830. https://doi.org/10.1155/2010/403830
Fang T, Liu G, Zhou C, Lu L (2015) Lead in soil and agricultural products in the Huainan Coal Mining Area, Anhui, China: levels, distribution, and health implications. Environ Monit Assess 187:152. https://doi.org/10.1007/s10661-015-4368-y
Flaxman SR et al (2017) Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health 5:e1221–e1234. https://doi.org/10.1016/S2214-109X(17)30393-5
Frazzoli C, Mantovani A (2020) Toxicological risk factors in the burden of malnutrition: the case of nutrition (and risk) transition in sub-Saharan Africa. Food Chem Toxicol 146:111789. https://doi.org/10.1016/j.fct.2020.111789
Gan AT, Man RE, Cheung CMG, Kumari N, Fenwick EK, Sabanayagam C, Tham YC, Tan NY, Mitchell P, Wong TY, Cheng CY, Lamoureux EL (2020) Cataract surgery and the 6-year incidence of age-related macular degeneration in a multiethnic asian cohort. Asia Pac J Ophthalmol (phila) 9:130–136. https://doi.org/10.1097/APO.0000000000000275
Gandhi D, Rudrashetti AP, Rajasekaran S (2022) The impact of environmental and occupational exposures of manganese on pulmonary, hepatic, and renal functions. J Appl Toxicol 42:103–129. https://doi.org/10.1002/jat.4214
GBD (2019) Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study (2021) Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health 9(2):e144–e160. https://doi.org/10.1016/S2214-109X(20)30489-7
Grashow R, Sparrow D, Hu H, Weisskopf MG (2015) Cumulative lead exposure is associated with reduced olfactory recognition performance in elderly men. The normative aging study. Neurotoxicology 49:158–164. https://doi.org/10.1016/j.neuro.2015.06.006
Hadrup N, Ravn-Haren G (2020) Acute human toxicity and mortality after selenium ingestion: a review. J Trace Elem Med Biol 58:126435. https://doi.org/10.1016/j.jtemb.2019.126435
Hartwig A (2001) Zinc finger proteins as potential targets for toxic metal ions: differential effects on structure and function. Antioxid Redox Signal 3(4):625–634. https://doi.org/10.1089/15230860152542970
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87. https://doi.org/10.1016/j.tox.2011.03.001
Kaliaperumal R, Venkatachalam R, Nagarajan P, Sabapathy SK (2021) Association of serum magnesium with oxidative stress in the pathogenesis of diabetic cataract. Biol Trace Elem Res 199:2869–2873. https://doi.org/10.1007/s12011-020-02429-9
Koedrith P, Seo YR (2011) Advances in carcinogenic metal toxicity and potential molecular markers. Int J Mol Sci 12:9576–9595. https://doi.org/10.3390/ijms12129576
Kumagai T, Matsumoto CS, Kimura I, Shinoda K (2019) Electroretinograms before and after extraction of large intraocular iron foreign body. Am J Ophthalmol Case Rep 15:100463. https://doi.org/10.1016/j.ajoc.2019.100463
Levi S, Girelli D, Perrone F, Pasti M, Beaumont C, Corrocher R, Albertini A, Arosio P (1998) Analysis of ferritins in lymphoblastoid cell lines and in the lens of subjects with hereditary hyperferritinemia-cataract syndrome. Blood 91:4180–4187
Li G, Yao T, Wu X-w, Cao Z, Tu Y-c, Ma Y, Li B-n, Peng Q-y, Wu B, Hou J (2020) Novel and traditional anthropometric indices for identifying arterial stiffness in overweight and obese adults. Clin Nutr 39:893–900. https://doi.org/10.1016/j.clnu.2019.03.029
López AM, Prieto MF, Miranda M, Castillo C, Hernández J, Luis BJ (2004) Interactions between toxic (As, Cd, Hg and Pb) and nutritional essential (Ca Co, Cr, Cu, Fe, Mn, Mo, Ni, Se, Zn) elements in the tissues of cattle from NW Spain. Biometals 17(4):389–397. https://doi.org/10.1023/b:biom.0000029434.89679.a2
Marques AP, Ramke J, Cairns J, Butt T, Zhang JH, Muirhead D, Jones I, Tong B, Swenor BK, Faal H, Bourne RRA, Frick KD, Burton MJ (2021) Global economic productivity losses from vision impairment and blindness. EClinicalMedicine 35:100852. https://doi.org/10.1016/j.eclinm.2021.100852
McEligot AJ, Poynor V, Sharma R, Panangadan A (2020) Logistic LASSO Regression for Dietary Intakes and Breast Cancer. Nutrients 12(9):2652. https://doi.org/10.3390/nu12092652
Mehmet E, Onur O, Eray A (2021) Cataract secondary to iatrogenic iron overload in a severely anemic patient. Indian J Ophthalmol Case Rep. https://doi.org/10.4103/IJO.IJO_2872_20
Mohanty BP, Banerjee S, Sadhukhan P, Chowdhury AN, Golder D, Bhattacharjee S, Bhowmick S, Manna SK, Samanta S (2015) Pathophysiological changes in rohu (Labeo rohita, Hamilton) fingerlings following arsenic exposure. Natl Acad Sci Lett 38:315–319. https://doi.org/10.1007/s40009-014-0345-1
Movahedian M, Thomas J, Rahmani J, Clark CCT, Rashidkhani B, Ghanavati M (2020) Association between dietary glycemic index and glycemic load, insulin index and load with incidence of age-related cataract: results from a case-control study. Diab Metab Syndr 14:199–204. https://doi.org/10.1016/j.dsx.2020.02.013
Naka KS, de Cássia Dos Santos Mendes L, de Queiroz T, Costa B, de Jesus IM, de Magalhães Câmara V, de Oliveira Lima M (2020) A comparative study of cadmium levels in blood from exposed populations in an industrial area of the Amazon Brazil. Sci Total Environ 698:134309. https://doi.org/10.1016/j.scitotenv.2019.134309
Pedersen EB, Jorgensen ME, Pedersen MB, Siggaard C, Sorensen TB, Mulvad G, Hansen JC, Asmund G, Skjoldborg H (2005) relationship between mercury in blood and 24-h ambulatory blood pressure in Greenlanders and Danes. Am J Hypertens 18:612–618. https://doi.org/10.1016/j.amjhyper.2004.11.022
Rahman MT, De Ley M (2017) Arsenic induction of metallothionein and metallothionein induction against arsenic cytotoxicity. Rev Environ Contam Toxicol 240:151–168. https://doi.org/10.1007/398_2016_2
Sanaei F, Amin MM, Alavijeh ZP, Esfahani RA, Sadeghi M, Bandarrig NS, Fatehizadeh A, Taheri E, Rezakazemi M (2021) Health risk assessment of potentially toxic elements intake via food crops consumption: Monte Carlo simulation-based probabilistic and heavy metal pollution index. Environ Sci Pollut Res Int 28:1479–1490. https://doi.org/10.1007/s11356-020-10450-7
Sedaghat F, Ghanavati M, Nezhad Hajian P, Hajishirazi S, Ehteshami M, Rashidkhani B (2017) Nutrient patterns and risk of cataract: a case-control study. Int J Ophthalmol 10:586–592. https://doi.org/10.18240/ijo.2017.04.14
See LC, Chiou HY, Lee JS et al (2007) Dose-response relationship between ingested arsenic and cataracts among residents in Southwestern Taiwan. J Environ Sci Health A Tox Hazard Subst Environ Eng 42(12):1843–1851. https://doi.org/10.1080/10934520701566884
Shen Z, Xu D, Li L, Wang J, Shi X (2019) Ecological and health risks of heavy metal on farmland soils of mining areas around Tongling City, Anhui, China. Environ Sci Pollut Res Int 26:15698–15709. https://doi.org/10.1007/s11356-019-04463-0
Song P, Wang H, Theodoratou E, Chan KY, Rudan I (2018) The national and subnational prevalence of cataract and cataract blindness in China: a systematic review and meta-analysis. J Glob Health 8:010804. https://doi.org/10.7189/jogh.08-010804
Takahashi G (2016) Damage and heavy metal pollution in China’s farmland: reality and solutions. J Contemp East Asia Stud. https://doi.org/10.1080/24761028.2016.11869089
Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB, Navas-Acien A (2013) Cadmium exposure and incident cardiovascular disease. Epidemiology 24:421–429. https://doi.org/10.1097/EDE.0b013e31828b0631
Thomas DC, Witte JS, Greenland S (2007) Dissecting effects of complex mixtures: who’s afraid of informative priors? Epidemiology 18:186–190. https://doi.org/10.1097/01.ede.0000254682.47697.70
V AS, A NP (2014) Cadmium blood and urine concentrations as measures of exposure: NHANES 1999–2010. J Eposure Sci Environ Epidemiol 24:163–170. https://doi.org/10.1038/jes.2013.55
Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, Kile ML, Quamruzzaman Q, Afroz S, Golam M, Amarasiriwardena C, Bellinger DC, Christiani DC, Coull BA, Wright RO (2017) The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from Rural Bangladesh. Environ Health Perspect 125:067015. https://doi.org/10.1289/EHP614
Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90:1–37. https://doi.org/10.1007/s00204-015-1579-5
Vinceti M, Crespi CM, Bonvicini F, Malagoli C, Ferrante M, Marmiroli S, Stranges S (2013) The need for a reassessment of the safe upper limit of selenium in drinking water. Sci Total Environ 443:633–642. https://doi.org/10.1016/j.scitotenv.2012.11.025
Wang W, Schaumberg DA, Park SK (2016) Cadmium and lead exposure and risk of cataract surgery in U.S. adults. Int J Hyg Environ Health 219:850–856. https://doi.org/10.1016/j.ijheh.2016.07.012
Wang N, Maskomani S, Meenashisundaram GK, Fuh JYH, Dheen ST, Anantharajan SK (2020) A study of titanium and magnesium particle-induced oxidative stress and toxicity to human osteoblasts. Mater Sci Eng C Mater Biol Appl 117:111285. https://doi.org/10.1016/j.msec.2020.111285
Wang X, Shen X, Chen L, Yu Q, Xiong S, Tian K, Xie Y, Zeng R, Zhou Y (2021) Hepatic oxidative damage and Nrf2 pathway protein changes in rats following long-term manganese exposure. Toxicol Ind Health 37:251–259. https://doi.org/10.1177/0748233721993311
Yang Y, Jin Q, Fang JM, Fq L, Li AM, Tandon P, Shan AD (2017) Spatial distribution, ecological risk assessment, and potential sources of heavy metal(loid)s in surface sediments from the Huai River within the Bengbu section, China. Environ Sci Pollut Res Int 24(12):11360–11370. https://doi.org/10.1007/s11356-017-8732-z
Zeinert R, Martinez E, Schmitz J, Senn K, Usman B, Anantharaman V, Aravind L, Waters LS (2018) Structure-function analysis of manganese exporter proteins across bacteria. J Biol Chem 293:5715–5730. https://doi.org/10.1074/jbc.M117.790717
Zhong Q, Wu HB, Niu QS, Jia PP, Qin QR, Wang XD, He JL, Yang WJ, Huang F (2021) Exposure to multiple metals and the risk of hypertension in adults: a prospective cohort study in a local area on the Yangtze River. China Environ Int 153:106538. https://doi.org/10.1016/j.envint.2021.106538
Zhou TT, Hu B, Meng XL et al (2021) The associations between urinary metals and metal mixtures and kidney function in Chinese community-dwelling older adults with diabetes mellitus. Ecotoxicol Environ Saf 226:112829. https://doi.org/10.1016/j.ecoenv.2021.112829
Zwolak I (2020) The role of selenium in arsenic and cadmium toxicity: an updated review of scientific literature. Biol Trace Elem Res 193:44–63. https://doi.org/10.1007/s12011-019-01691-w
Acknowledgements
Thanks to all the subjects participated in this research.
Funding
This work was supported by The Project for Top Disciplinary Talents of Majors in Universities of Anhui Province [grant numbers gxbjZD09].
Author information
Authors and Affiliations
Contributions
Yan-Qing Li, Qian Wang, Ran Liu, Fen Huang, and Yan-Feng Zhou contributed to the study conception and design. Material preparation and data collection were performed by Yan-Qing Li, Qian Wang, Ran Liu, and Guo-Ao Li. Yan-Feng Zhou, Fen Huang, Guo-Ao Li, and Jia-Liu He analyzed and interpreted the data. Yan-Qing Li, Qian Wang, and Guo-Ao Li wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study had been approved by the Ethics Committee of Anhui Medical University (Approval No: 20170305), and the participants had signed written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, YQ., Wang, Q., Liu, R. et al. Associations of exposure to multiple metals with the risk of age-related cataract in Anhui, China: a case–control study. Environ Sci Pollut Res 30, 4680–4693 (2023). https://doi.org/10.1007/s11356-022-22494-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22494-y