Abstract
Type-1 diabetes mellitus (T1DM) is a chronic condition characterized by long-term hyperglycemia that results in several complications such as painful peripheral neuropathy, bone deterioration, and increased risk of bone fractures. Lithium, a first-line therapy for bipolar disorder, has become an attractive agent for attenuating peripheral neuropathy and menopause-induced bone loss. Therefore, our aim was to determine the effect of chronic lithium treatment on mechanical hypersensitivity and trabecular bone loss induced by T1DM in mice. T1DM was induced in male C57BL/6J mice by intraperitoneal injection of streptozotocin (STZ, 50 mg/kg/day, for 5 consecutive days). 12 weeks after T1DM-induction, mice received a daily intraperitoneal injection of vehicle, 30 or 60 mg/kg lithium (as LiCl) for 6 weeks. Throughout the treatment period, blood glucose levels and mechanical sensitivity were evaluated every 2 weeks. After lithium treatment, the femur and L5 vertebra were harvested for microcomputed tomography (microCT) analysis. T1DM mice showed significant hyperglycemia, mechanical hypersensitivity, and significant trabecular bone loss as compared with the control group. Chronic lithium treatment did not revert the hindpaw mechanical hypersensitivity nor hyperglycemia associated to T1DM induced by STZ. In contrast, microCT analysis revealed that lithium reverted, in a dose-dependent manner, the loss of trabecular bone associated to T1DM induced by STZ at both the distal femur and L5 vertebra. Lithium treatment by itself did not affect any trabecular bone parameter in non-diabetic mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a severe condition characterized by chronic hyperglycemia caused by defects in insulin production and/or its action (Petersmann et al. 2019; IDF 2021). The prevalence of DM is alarmingly increasing in the world; in 2019 it was reported that 463 million people were living with DM, and this number is expected to increase to 578 million by 2030 (IDF 2021). DM leads to several complications affecting the cardiovascular, renal, peripheral nervous, and skeletal systems (Mauricio et al. 2020; Romero-Díaz et al. 2021).
Recently, DM has also been associated with bone deterioration and to an increased risk of fracture (Vestergaard 2007; Thangavelu et al. 2020). Particularly, women and men with type-1 DM (T1DM) increased four and two times the risk of any fractures, respectively, compared with healthy individuals (Shah et al. 2015). In agreement with this, we and others have shown that chemically induced T1DM decreases bone mineral density and parameters of trabecular bone microarchitecture (Peng et al. 2016; Enríquez-Pérez et al. 2017; Martínez-Martínez et al. 2020) as well as decreases the strength of mouse bones (Fowlkes et al. 2013; Iyer et al. 2017; Maycas et al. 2017). Since the prevalence of T1DM is steadily increasing, it is expected that the incidence of its complications will also rise around the world (Patterson et al. 2019). Thus, there is a medical need to develop safer and more efficacious therapies to treat T1DM-associated peripheral neuropathies and osteoporosis.
Lithium is a mood stabilizer that has been a first-line therapy for bipolar disorder for several decades (Malhi et al. 2013; Volkmann et al. 2020). Patients on lithium maintenance therapy have been shown to improve bone mineral density (Zamani et al. 2009). Likewise, in vitro studies demonstrated that lithium stimulates osteoblast differentiation markers, decreased bone resorption, and increased calcium deposition (Wong et al. 2020). In addition, chronic lithium treatment has been shown to reverse trabecular bone loss in the femur and tibia in animal models of ovariectomy-induced osteoporosis (Jin et al. 2017; Bai et al. 2019), improves bone regeneration (Wang et al. 2015) and fracture healing (Klontzas et al. 2016).
Peripheral neuropathy is one of the most common complications of DM. It is mainly characterized by pain, hypersensitivity (tactile allodynia and hyperalgesia), paresthesia, and dysesthesia that generally affects the upper and lower extremities (Singh et al. 2014; Feldman et al. 2019). It has been reported that both acute and chronic lithium treatment reduces mechanical allodynia and hyperalgesia in models of neuropathic pain induced by paclitaxel (Pourmohammadi et al. 2012), partial sciatic nerve ligation (Banafshe et al. 2012), and sciatic nerve chronic constrictive injury (CCI) (Shimizu et al. 2000; Esu et al. 2021). However, to the best of our knowledge, the antinociceptive and osteoprotective effect of lithium under conditions of T1DM has not been studied. Thus, we aimed to determine the effect of chronic lithium treatment on mechanical sensitivity and trabecular bone loss induced by an experimental model of T1DM.
Materials and methods
Reagents
Streptozotocin (STZ, catalog #S0130) and lithium chloride (LiCl, catalog #62476-100G-F) were obtained from Sigma Aldrich (St. Louis, MO, USA). Citric acid monohydrate (catalog #0110-01) and sodium citrate dehydrate (catalog #3646-01) were obtained from J.T. Baker (Center Valley, PA, USA). All solutions were freshly prepared each day before using.
Animals
Fifty-two male C57BL/6J mice (11-weeks-old) with a body weight of 21–25 g at the beginning of the experiment were obtained from the breeding facility of Facultad de Medicina, UAEM. Mice were housed in a constant temperature (22 ± 2 °C) and humidity-controlled room under a 12-h light/dark cycle (lights on at 07:00 h), with free access to standard rodent chow and water. Procedures involving mice and their care were conducted in conformity with the Mexican Official Norm for Animal Care and Handling (NOM-062-ZOO-1999) and the Guide for the Care and Use of Laboratory Animals (NRC 2011). Moreover, ethics approval for the protocol was obtained by the Institutional Animal Care and Use Committee of the Facultad de Medicina, Universidad Autónoma del Estado de Morelos (FM/20/110).
Induction of type-1 diabetes mellitus
After 1 week of acclimation at our animal facility, mice were randomly divided into two groups: a control group and a T1DM group. The control group received one daily intraperitoneal (i.p.) injection of 0.1 M citrate buffer pH 4.5 (CIT, 0.1 mL/10 g body weight, i.p.) for five consecutive days. The T1DM group received five daily administrations of STZ (50 mg/kg, dissolved in CIT) (Motyl et al. 2012; Enríquez-Pérez et al. 2017; Martínez-Martínez et al. 2020). Both CIT and STZ were injected after a 12-h fasting period. To confirm the development and maintenance of T1DM model, blood glucose levels were determined at 1, 4, 8, and 12 weeks post-T1DM induction. Mice with blood glucose levels greater than 300 mg/dL were considered as diabetic and were included in the study. To ensure a high survival rate of diabetic mice, the cages were cleaned every day and the water supply was daily replaced.
Chronic lithium treatment
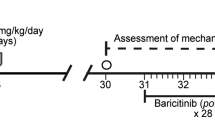
After 12 weeks post-T1DM induction, CIT-treated mice were subdivided by randomization using the GraphPad Prism software version 8 (GraphPad Software, Inc.) into the following groups: (1) CIT + Veh (n = 8), (2) CIT + Li 60 mg/kg (n = 9); while STZ-treated mice were distributed as follows: (3) STZ + Veh (n = 11), (4) STZ + 30 mg/kg Li (n = 12), and (5) STZ + 60 mg/kg Li (n = 12) (Fig. 1A). Mice were intraperitoneally injected with 30 or 60 mg/kg/day of lithium (LiCl) every day for 6 weeks. Mice from vehicle group received the same volume of distilled water (vehicle; 0.1/10 mg body weight) (Fig. 1A). The lithium doses used in this study have been reported to maintain safe serum lithium levels in mice (Gill et al. 2009). Furthermore, previous reports also showed that lithium treatment for 6 weeks, at similar dose is safe (Lewicki et al. 2006; Wang et al. 2015; Li et al. 2017; Kurgan et al. 2019).
Chronic treatment with lithium did not modify body weight or blood glucose levels. Schematic representation of the experimental design (A). T1DM mice showed a significant decrease in body weight as compared to CIT + Veh (B). Blood glucose levels from T1DM mice (STZ + Veh) were significantly higher as compared to glucose of CIT + Veh treated mice. However, both doses of lithium assayed did not affect body weight or blood glucose levels as compared with STZ + Veh (A, B). BL baselines, vF von Frey. Each point represents the mean ± SEM (n = 8–12); *p < 0.05 by two-way ANOVA on ranks followed by Dunn’s pairwise tests with Bonferroni adjustments
Assessment of lithium treatment on body weight and blood glucose levels
During the whole experiment, the body weight of mice was monitored once a week. Blood glucose levels were assessed before T1DM induction, every 4 weeks until week 12 post-T1DM induction, and biweekly after lithium treatment was started. In all cases, blood glucose measurement was performed after a 6-h fasting period. Blood samples were obtained from the tail vein to determine glucose concentration using a calibrated glucometer (Accu-Chek Performa, Roche Diagnostic, Germany).
Assessment of lithium on mechanical sensitivity in mice with T1DM
To determine whether T1DM induced mechanical hypersensitivity, the 50% paw withdrawal threshold was assessed using von Frey filaments (Stoelting, IL, USA) following the up-down method previously described (Chaplan et al. 1994). Briefly, the mice were placed in individual acrylic boxes with a wire mesh floor for 30 min to allow them to acclimatize to their surroundings. The filaments were then pressed perpendicularly against the mid-plantar surface of both hindpaws for approximately 3–5 s. A response was considered positive when the mouse exhibited paw withdrawal after stimulation, along with a nociceptive behavior (flinching, licking, or guarding). The stimulation started with the 0.4 g filament, and a positive response was followed by the stimulation with the next lower force filament, while a negative response was followed by the stimulation with the next higher force filament in the set. This procedure continued until six responses were obtained. The pattern of positive-negative responses was used to calculate the 50% paw withdrawal threshold, expressed in grams. The 50% paw withdrawal thresholds were assessed in all animals at 0, 4, 8, and 12 weeks after T1DM induction. Then, to determine if lithium produced an antinociceptive effect, the 50% paw withdrawal thresholds were measured every 2 weeks (Fig. 1A). The investigator performing the evaluation was blinded to the experimental condition of the mice.
Assessment of lithium effect on bone loss in mice with T1DM
The day after the last lithium or vehicle injection (18 weeks post T1DM-induction), mice were anesthetized with a combination of ketamine and xylazine (100/10 mg/kg, i.p.) and perfused intracardially with 20 mL of phosphate buffered saline (PBS, 0.1 M pH 7.4 at 4 °C) followed by 20 mL of 4% formaldehyde in PBS (Fig. 1A). The hind limbs and L5 vertebra were removed and postfixed for 24 h with 4% formaldehyde and then stored in PBS at 4 °C until microcomputed tomography (microCT) analysis.
Trabecular bone was analyzed at the level of the distal femur and L5 vertebra using a microCT system (Skyscan 1272, Bruker, Belgium). The scanning process was performed at a 10 μm voxel size, 60 kVp x-ray power, and 166 µA with an integration time of 627 ms, according to the guidelines for microCT analysis for rodent bone structure (Bouxsein et al. 2010). All obtained images were reconstructed using NRecon Software (Bruker, Belgium). For distal femur analysis, the region of interest (ROI) was evaluated by selecting 1 mm in the vertical axis, after 0.2 mm from the growth plate (reference point). For the L5 vertebra, ROI was evaluated by selecting 2 mm in the vertical axis. CT analyzer program (Bruker, Belgium) was used to determine trabecular bone parameters. An automatic segmentation algorithm (CT analyzer) was applied to isolate trabecular bone from cortical bone. Hydroxyapatite calibration phantoms (250 and 750 mg/cm3) were used for calibrating bone density values. The parameters evaluated in trabecular bone were bone mineral density (BMD) and percent bone volume (BV/TV).
Statistical analysis
All values are presented as the mean ± SEM. A two-way repeated measures analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was used to compare each parameter between the different treatments and experimental groups at different time points. For microCT results, one-way ANOVA followed by Bonferroni’s post hoc test was used to compare each parameter between the different experimental groups. When normality test failed, a non parametric test was performed (two-way ANOVA on ranks followed by Dunn’s pairwise tests with Bonferroni adjustments). Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA). Throughout the study, p < 0.05 was considered to indicate a statistically significant difference.
Results
Chronic treatment with lithium did not affect body weight or blood glucose levels
At the beginning of the experiments, STZ- and CIT-injected mice had a similar body weight (Fig. 1B). Then, during the entire evaluation period (18 weeks), the results showed that STZ-injected mice did not increase body weight (Fig. 1B). Chronic treatment with lithium at 30 or 60 mg/kg did not change the body weight of STZ- or CIT-treated mice (Fig. 1B). Regarding glucose levels, both STZ- and CIT-injected mice displayed baseline blood glucose levels around 104.4 ± 1.8 mg/dL. However, after 12 weeks of T1DM induction, STZ-injected mice displayed blood glucose levels greater than 300 mg/dL. In contrast, the blood glucose levels of CIT-injected mice were 127.4 ± 2.8 mg/dL (Fig. 1C). The chronic treatment with lithium at both doses used did not significantly change the blood glucose levels in T1DM mice (Fig. 1C).
Chronic treatment with lithium did not revert the mechanical hypersensitivity induced by T1DM
Before T1DM induction, there were no significant differences in the 50% withdrawal threshold values between the CIT- and STZ-treated mice (Fig. 2). However, after STZ injections, T1DM mice developed a progressive mechanical hypersensitivity in both hindpaws evidenced by a decrease in the 50% withdrawal threshold values until reaching their maximal reduction at week 12 post-STZ (0.43 ± 0.02 g). This mechanical hypersensitivity persisted until the end of the treatment period. Intraperitoneal chronic treatment with both 30 and 60 mg/kg lithium failed to revert the mechanical hypersensitivity developed in T1DM mice (Fig. 2).
Chronic lithium treatment did not revert mechanical hypersensitivity induced by T1DM. T1DM mice showed marked mechanical hypersensitivity 12 weeks post-STZ injection. 6 weeks of intraperitoneal lithium treatment at 30 or 60 mg/kg did not affect T1DM-induced mechanical hypersensitivity, on the left (A) or right (B) hindpaws. Data are presented as mean ± SEM (n = 8–12); *p < 0.05 by two-way ANOVA on ranks followed by Dunn’s pairwise tests with Bonferroni adjustments
Chronic treatment with lithium reversed trabecular bone loss induced by T1DM
To determine the effect of lithium on trabecular bone loss induced by T1DM, a microCT analysis at the distal femoral metaphysis (Fig. 3) and L5 vertebra (Fig. 4) was performed. The three-dimensional images of the distal femoral metaphysis showing the region of interest analyzed by microCT (dashed-line bracket, Fig. 3A) showed that mice from the STZ + Veh group (Fig. 3C) had a reduced number of trabeculae and greater trabeculae separation compared to control mice treated with CIT + Veh (Fig. 3B). Quantitative microCT analysis revealed a significant decrease in BMD (Fig. 3E) and BV/TV (Fig. 3F) in mice treated with STZ + Veh compared to mice treated with CIT + Veh. Chronic lithium treatment reversed, in a dose-dependent manner, the T1DM-induced loss in BMD and BV/TV; however, the effect of lithium on BMD was only statistically significant at the 60 mg/kg dose (Fig. 3D, E). Treatment with 30 or 60 mg/kg of lithium for 6 weeks significantly reversed the loss of BV/TV induced by T1DM (Fig. 3F).
Chronic lithium treatment reversed the loss of trabecular bone at the femoral distal metaphysis induced by T1DM. Longitudinal section of the distal femoral metaphysis showing the region of interest analyzed by microCT (dashed-line bracket, A). Representative three-dimensional reconstructions of the trabecular bone show a marked bone loss in mice treated with STZ + Veh (C) as compared to mice in the CIT + Veh (B) and STZ + Li (60 mg/kg, D) groups. Quantitative analysis revealed that lithium reversed, in a dose-dependent manner, the trabecular bone mineral density (BMD, E) and the percent bone volume rate (BV/TV, F) in T1DM mice. Data are presented as mean ± SEM (n = 8–12); *p < 0.05 by one-way ANOVA followed by Bonferroni’s post hoc test
Chronic lithium treatment reversed bone loss of trabecular bone at L5 vertebra induced by T1DM. Representative three-dimensional reconstructions showing the region of interest analyzed of the trabecular bone (dashed-line brackets), show a marked bone loss in STZ + Veh (B) as compared to CIT + Veh (A), and STZ + Li (60 mg/kg) treated mice (C). Quantitative analysis revealed that lithium reversed, in a dose-dependent manner, the trabecular bone mineral density (BMD, D) and percent bone volume rate (BV/TV, E) in T1DM mice. Data are presented as mean ± SEM (n = 8 each); *p < 0.05 by one-way ANOVA followed by Bonferroni’s post hoc test
Representative three-dimensional showing the region of interest trabecular bone in the L5 vertebra (dashed-line bracket), showed that mice treated with STZ + Veh (Fig. 4B) developed deterioration of the trabecular bone compared to those control mice treated with CIT + Veh (Fig. 4A). The quantitative analysis revealed a significant decrease of BMD (Fig. 4D), but not BV/TV (Fig. 4E) in T1DM mice as compared with CIT + Veh treated mice. The chronic lithium treatment reversed, in a dose-dependent manner, the T1DM-induced loss of BMD and BV/TV (Fig. 4D, E). Finally, a control CIT group was treated with the highest tested lithium dose (60 mg/kg) to determine whether lithium by itself did not affect bone parameters in naïve mice. Quantitative analysis showed that the lithium treatment, at the 60 mg/kg dose for 6 weeks, had no effect on any bone microarchitecture parameters in both bone types analyzed as compared with those in mice treated with CIT + Veh (data not shown).
Discussion
Our results demonstrate that chronic lithium treatment reverted in a dose-dependent manner the trabecular bone loss (BMD and BV/TV parameters) at the distal femur and L5 vertebra induced by experimental T1DM. In agreement with this, several studies have reported that lithium acts as an osteoprotective agent in different models of bone loss. It has been shown that oral treatment with lithium at both low (20 mg/kg for 2 weeks) and high doses (150 mg/kg/2 days for three months) enhances fracture healing and bone formation in an osteoporosis model, respectively (Jin et al. 2017; Vachhani et al. 2018). Likewise, in a rat model of closed femoral shaft fracture, it was found that fracture healing was maximized with a daily oral lithium treatment (20 mg/kg for 2 weeks), initiated seven days after fracture (Bernick et al. 2014). Moreover, rats subjected to osteotomy and distraction osteogenesis chronically treated with LiCl (200 mg/kg/day, intragastric, for 10 weeks) showed higher BMD, more new mature bone tissue, and better regenerated bone mass continuity in the distraction gaps (Wang et al. 2015). In support of these preclinical studies, in clinical studies it has been observed that patients on lithium maintenance therapy (at therapeutic doses) had higher BMD at the lumbar spine and the proximal femur. Moreover, these patients also showed lower serum concentrations of bone turnover markers such as alkaline phosphatase (ALP), collagen type-1 C-telopeptide (CTX), and osteocalcin compared to age-matched controls, suggesting that lithium may enhance bone mass through by decreasing bone turnover (Zamani et al. 2009). In addition, a meta-analysis study found that lithium use was associated with a 20% decreased risk of fracture (Liu et al. 2019).
Although the exact mechanism underlying the osteoprotective effect of lithium remains unknown, it is possible to suggest that the treatment with the metal may cause an inhibition of the GSK-3β enzyme and activation of the Wnt/β-catenin signaling pathway. In support of this, a recent study demonstrated that treatment with a low dose of lithium (10 mg/kg/day) for 6 weeks inhibited GSK-3β, while activating Wnt/β-catenin in the bone of male C57BL/6J mice (Kurgan et al. 2019). Furthermore, studies have shown that modulating GSK-3β signaling stimulates osteoblast differentiation, increase bone mass, and reduce fracture risk (Hoeppner et al. 2009; Wang et al. 2009; Antika et al. 2017). Although most of the scientific evidence suggests that lithium positively affects osteoblasts and therefore increases bone formation, we cannot exclude the possibility that the osteoprotective effect of lithium observed in our study may result from osteoclast inhibition. Firstly, studies have shown that T1DM increased osteoclast activity and bone resorption in long bones of diabetic rats and mice, accompanied by increased protein expression of RANKL, TRAP (tartrate-resistant acid phosphatase), and cathepsin K (Hie et al. 2007; Yang et al. 2020). Second, in rats with osteoporosis induced by ovariectomy, 10 mM of LiCl locally injected into the distal femoral condyle on the 1st and 30th days after surgery significantly reduced TRACP-5b and CTX (Bai et al. 2019), essential markers of bone resorption. In addition, a study performed using a model of titanium-induced calvarial osteolysis in C57BL/6J mice reported that 50 or 200 mg/kg LiCl (i.p.) treatment for 2 weeks decreased the osteoclast number, pore number, and porosity area in mice calvaria (Hu et al. 2017). Finally, another potential explanation for the osteoprotective effect by lithium would be a possible ability to decrease diabetes progression and to indirectly revert the bone loss induced by STZ. However, this is less likely to occur as our results showed that chronic lithium treatment did not modify body weight or blood glucose levels in T1DM mice.
In the present study, it was shown that lithium treatment did not reverse the mechanical hypersensitivity observed in the T1DM mice. In contrast to our results, there is evidence showing that chronic lithium treatment reverts thermal hyperalgesia and mechanical allodynia in rat models of paclitaxel-induced neuropathic pain (Pourmohammadi et al. 2012) and sciatic nerve chronic constrictive injury (CCI) (Shimizu et al. 2000; Esu et al. 2021), respectively. Likewise, a study performed in male C57BL/6J mice using the sciatic nerve cuffing model reported that a single dose of LiCl 100 mg/kg reduced mechanical allodynia and thermal hyperalgesia, an effect that lasted about 24 h (Weinsanto et al. 2018). While the reasons behind these discrepancies are unknown, it is possible to suggest that the pathogenesis of the mechanical hypersensitivity associated with T1DM is different to those in other models of neuropathic pain.
The present study has some limitations. First, the biomechanical properties of the bones were not assessed, so further studies are needed to determine the biomechanical strength of bone after lithium treatment under T1DM conditions. Secondly, serum lithium levels were not measured in our study. However, similar doses to those used in our study in mice have been shown to reach therapeutic levels compared with patients on treatment for bipolar disorder (Gill et al. 2009). We do recognize that experiments evaluating biomechanical properties of bone and measurement of serum lithium level would significantly improve this study. However, due to lack of infrastructure and restricted resources in our laboratory we were not able to perform these experiments. Thirdly, we suggest that with the lithium doses used in this study, osteoblast differentiation and activity are promoted based on the results from previous reports, further studies are needed to clarify the exact mechanisms behind this effect.
Conclusions
Chronic lithium treatment did not reverse the mechanical hypersensitivity induced by STZ-induced T1DM. In contrast, microCT analysis revealed that lithium reverted, in a dose-dependent manner, the trabecular bone loss induced by T1DM at both the distal femur and L5 vertebra. These results suggest that lithium treatment could represent a therapeutic alternative for bone loss in patients with T1DM.
Data availability
The data supporting the findings of the article is available within the article.
Code availability
Not applicable.
References
Antika LD, Lee E-J, Kim Y-H, Kang M-K, Park S-H, Kim DY, Oh H, Choi Y-J, Kang Y-H (2017) Dietary phlorizin enhances osteoblastogenic bone formation through enhancing β-catenin activity via GSK-3β inhibition in a model of senile osteoporosis. J Nutr Biochem 49:42–52
Bai J, Xu Y, Dieo Y, Sun G (2019) Combined low-dose LiCl and LY294002 for the treatment of osteoporosis in ovariectomized rats. J Orthop Surg Res 14:177. https://doi.org/10.1186/s13018-019-1210-1
Banafshe HR, Mesdaghinia A, Arani MN, Ramezani MH, Heydari A, Hamidi GA (2012) Lithium attenuates pain-related behavior in a rat model of neuropathic pain: possible involvement of opioid system. Pharmacol Biochem Behav 100(3):425–430. https://doi.org/10.1016/j.pbb.2011.10.004
Bernick J, Wang Y, Sigal IA, Alman BA, Whyne CM, Nam D (2014) Parameters for lithium treatment are critical in its enhancement of fracture-healing in rodents. J Bone Joint Surg Am 96(23):1990–1998. https://doi.org/10.2106/jbjs.n.00057
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res 25(7):1468–1486
Chaplan SR, Bach F, Pogrel J, Chung J, Yaksh T (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Enríquez-Pérez IA, Galindo-Ordoñez KE, Pantoja-Ortíz CE, Martínez-Martínez A, Acosta-González RI, Muñoz-Islas E, Jiménez-Andrade JM (2017) Streptozocin-induced type-1 diabetes mellitus results in decreased density of CGRP sensory and TH sympathetic nerve fibers that are positively correlated with bone loss at the mouse femoral neck. Neurosci Lett 655:28–34. https://doi.org/10.1016/j.neulet.2017.06.042
Esu KD, Bakare AO (2021) Effects of co-administration of vitamin E and lithium chloride on chronic constriction injury-induced neuropathy in male Wistar rats: focus on antioxidant and anti-inflammatory mechanisms. Pain Pract. https://doi.org/10.1111/papr.13064
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V (2019) Diabetic neuropathy. Nat Rev Dis Primers 5(1):41. https://doi.org/10.1038/s41572-019-0092-1
Fowlkes JL, Nyman JS, Bunn RC, Jo C, Wahl EC, Liu L, Cockrell GE, Morris LM, Lumpkin CK Jr, Thrailkill KM (2013) Osteo-promoting effects of insulin-like growth factor I (IGF-I) in a mouse model of type 1 diabetes. Bone 57(1):36–40
Gill A, Kidd J, Vieira F, Thompson K, Perrin S (2009) No benefit from chronic lithium dosing in a sibling-matched, gender balanced, investigator-blinded trial using a standard mouse model of familial ALS. PLoS ONE 4(8):e6489
Hie M, Shimono M, Fujii K, Tsukamoto I (2007) Increased cathepsin K and tartrate-resistant acid phosphatase expression in bone of streptozotocin-induced diabetic rats. Bone 41(6):1045–1050. https://doi.org/10.1016/j.bone.2007.08.030
Hoeppner LH, Secreto FJ, Westendorf JJ (2009) Wnt signaling as a therapeutic target for bone diseases. Expert Opin Ther Targets 13(4):485–496. https://doi.org/10.1517/14728220902841961
Hu X, Wang Z, Shi J, Guo X, Wang L, Ping Z, Tao Y, Yang H, Zhou J, Xu Y, Geng D (2017) Lithium chloride inhibits titanium particle-induced osteoclastogenesis by inhibiting the NF-κB pathway. Oncotarget 8(48):83949–83961. https://doi.org/10.18632/oncotarget.20000
IDF (2021) Diabetes atlas, 10th edn. International Diabetes Federation, Brussels
Iyer S, Han L, Ambrogini E, Yavropoulou M, Fowlkes J, Manolagas SC, Almeida M (2017) Deletion of FoxO1, 3, and 4 in Osteoblast Progenitors Attenuates the Loss of Cancellous Bone Mass in a Mouse Model of Type 1 Diabetes. J Bone Miner Res 32(1):60–69. https://doi.org/10.1002/jbmr.2934
Jin Y, Xu L, Hu X, Liao S, Pathak JL, Liu J (2017) Lithium chloride enhances bone regeneration and implant osseointegration in osteoporotic conditions. J Bone Miner Metab 35(5):497–503. https://doi.org/10.1007/s00774-016-0783-6
Klontzas M, Kenanidis I, MacFarlane EJ, Michail R, Potoupnis TE, Heliotis M, Mantalaris M, Tsiridis E (2016) Investigational drugs for fracture healing: preclinical & clinical data. Expert Opin Investig Drugs 25(5):585–596
Kurgan N, Bott KN, Helmeczi WE, Roy BD, Brindle ID, Klentrou P, Fajardo VA (2019) Low dose lithium supplementation activates Wnt/β-catenin signalling and increases bone OPG/RANKL ratio in mice. Biochem Biophys Res Commun 511(2):394–397
Lewicki M, Paez H, Mandalunis PM (2006) Effect of lithium carbonate on subchondral bone in sexually mature Wistar rats. Exp Toxicol Pathol 58(2–3):197–201
Li L, Peng X, Qin Y, Wang R, Tang J, Cui X, Wang T, Liu W, Pan H, Li B (2017) Acceleration of bone regeneration by activating Wnt/β-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci Rep 7(1):1–12
Liu B, Wu Q (2019) Lithium use and risk of fracture: a systematic review and meta-analysis of observational studies. Osteoporos Int 30:257–266. https://doi.org/10.1007/s00198-018-4745-9
Malhi GS, Tanious M, Das P, Coulston CM, Berk M (2013) Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs 27(2):135–153
Martínez-Martínez A, Muñoz-Islas E, Ramírez-Rosas MB, Acosta-González RI, Torres-Rodríguez HF, Jiménez-Andrade JM (2020) Blockade of the colony-stimulating factor-1 receptor reverses bone loss in osteoporosis mouse models. Pharmacol Rep 72(6):1614–1626. https://doi.org/10.1007/s43440-020-00091-5
Mauricio D, Alonso N, Gratacòs M (2020) Chronic Diabetes Complications: The Need to Move beyond Classical Concepts. Trends Endocrinol Metab 31(4):287–295. https://doi.org/10.1016/j.tem.2020.01.007
Maycas M, McAndrews KA, Sato AY, Pellegrini GG, Brown DM, Allen MR, Plotkin LI, Gortazar AR, Esbrit P, Bellido T (2017) PTHrP-Derived Peptides Restore Bone Mass and Strength in Diabetic Mice: Additive Effect of Mechanical Loading. J Bone Miner Res 32(3):486–497. https://doi.org/10.1002/jbmr.3007
Motyl KJ, McCauley LK, McCabe LR (2012) Amelioration of type I diabetes-induced osteoporosis by parathyroid hormone is associated with improved osteoblast survival. J Cell Physiol 227(4):1326–1334
National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th edn. The National Academies Press, Washington, DC
NOM (1999) NOM-062-ZOO-1999 Especificaciones ténicas para la producción, cuidado y uso de los animales de laboratorio. Diario Oficial de la Federación México
Patterson CC, Karuranga S, Salpea P, Saeedi P, Dahlquist G, Soltesz G, Ogle GD (2019) Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 157:107842
Peng J, Hui K, Hao C, Peng Z, Gao QX, Jin Q, Lei G, Min J, Qi Z, Bo C, Dong QN, Bing ZH, Jia XY, Fu DL (2016) Low bone turnover and reduced angiogenesis in streptozotocin-induced osteoporotic mice. Connect Tissue Res 57(4):277–289. https://doi.org/10.3109/03008207.2016.1171858
Petersmann A, Müller-Wieland D, Müller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E (2019) Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 127:01. https://doi.org/10.1055/a-1018-9078
Pourmohammadi N, Alimoradi H, Mehr SE, Hassanzadeh G, Hadian MR, Sharifzadeh M, Bakhtiarian A, Dehpour AR (2012) Lithium attenuates peripheral neuropathy induced by paclitaxel in rats. Basic Clin Pharmacol Toxicol 110(3):231–237. https://doi.org/10.1111/j.1742-7843.2011.00795.x
Romero-Díaz C, Duarte-Montero D, Gutiérrez-Romero SA, Mendivil CO (2021) Diabetes and bone fragility. Diabetes Ther 12(1):71–86. https://doi.org/10.1007/s13300-020-00964-1
Shah VN, Shah CS, Snell-Bergeon JK (2015) Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med 32(9):1134–1142. https://doi.org/10.1111/dme.12734
Shimizu T, Shibata M, Wakisaka S, Inoue T, Mashimo T, Yoshiya I (2000) Intrathecal lithium reduces neuropathic pain responses in a rat model of peripheral neuropathy. Pain 85(1–2):59–64. https://doi.org/10.1016/s0304-3959(99)00249-3
Singh R, Kishore L, Kaur N (2014) Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res 80:21–35
Thangavelu T, Silverman E, Akhter MP, Lyden E, Recker RR, Graeff-Armas LA (2020) Trabecular bone score and transilial bone trabecular histomorphometry in type 1 diabetes and healthy controls. Bone 137:115451. https://doi.org/10.1016/j.bone.2020.115451
Vachhani K, Whyne C, Wang Y, Burns DM (2018) Low-dose lithium regimen enhances endochondral fracture healing in osteoporotic rodent bone. J Orthop Res 36:1783–1789. https://doi.org/10.1002/jor.23799
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 18(4):427–444. https://doi.org/10.1007/s00198-006-0253-4
Volkmann C, Bschor T, Köhler S (2020) Lithium treatment over the lifespan in bipolar disorders. Front Psychiatry 11:377. https://doi.org/10.3389/fpsyt.2020.00377
Wang F-S, Ko J-Y, Weng L-H, Yeh D-W, Ke H-J, Wu S-L (2009) Inhibition of glycogen synthase kinase-3β attenuates glucocorticoid-induced bone loss. Life Sci 85(19–20):685–692
Wang X, Zhu S, Jiang X, Li Y, Song D, Hu J (2015) Systemic administration of lithium improves distracted bone regeneration in rats. Calcif Tissue Int 96(6):534–540
Weinsanto I, Mouheiche J, Laux-Biehlmann A, Aouad M, Maduna T, Petit-Demouliere N, Chavant V, Poisbeau P, Darbon P, Charlet A (2018) Lithium reverses mechanical allodynia through a mu opioid-dependent mechanism. Mol Pain 14:1744806917754142
Wong SK, Chin KY, Ima-Nirwana S (2020) The Skeletal-Protecting Action and Mechanisms of Action for Mood-Stabilizing Drug Lithium Chloride: Current Evidence and Future Potential Research Areas. Front Pharmacol 11:430. https://doi.org/10.3389/fphar.2020.00430
Yang J, Chen S, Zong Z, Yang L, Liu D, Bao Q, Du W (2020) The increase in bone resorption in early-stage type I diabetic mice is induced by RANKL secreted by increased bone marrow adipocytes. Biochem Biophys Res Commun 525(2):433–439. https://doi.org/10.1016/j.bbrc.2020.02.079
Zamani A, Omrani GR, Nasab MM (2009) Lithium’s effect on bone mineral density. Bone 44(2):331–334. https://doi.org/10.1016/j.bone.2008.10.001
Funding
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT) project CB 2017–2018/A1-S-27869 (EMI) and project No. 316211 (RIAG).
Author information
Authors and Affiliations
Contributions
JMJA conceived the project. MAGA, HFTR, REMM, and VMVM performed the experiments. JMJA, RIAG and EMI analyzed and interpreted the data. JMJA, MAGA, HFTR, REMM, RIAG, GCC and EMI wrote and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Protocol was obtained by the Institutional Animal Care and Use Committee of the Facultad de Medicina, Universidad Autónoma del Estado de Morelos (FM/20/110).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Graniel-Amador, M.A., Torres-Rodríguez, H.F., Martínez-Mendoza, R.E. et al. Effect of chronic lithium on mechanical sensitivity and trabecular bone loss induced by type-1 diabetes mellitus in mice. Biometals 35, 1033–1042 (2022). https://doi.org/10.1007/s10534-022-00421-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-022-00421-5