Abstract
Fluopimomide is a novel pesticide intensively used in agricultural pest control; however, its excessive use may have toxicological effects on non-target organisms. In this study, Caenorhabditis elegans was used to evaluate the toxic effects of fluopimomide and its possible mechanisms. The effects of fluopimomide on the growth, pharyngeal pumping, and antioxidant systems of C. elegans were determined. Furthermore, the gene expression levels associated with mitochondria in the nematodes were also investigated. Results indicated that fluopimomide at 0.2, 1.0, and 5.0 mg/L notably (p < 0.001) decreased body length, pharyngeal pumping, and body bends in the nematodes compared to the untreated control. Additionally, fluopimomide at 0.2, 1.0, and 5.0 mg/L notably (p < 0.05) increased the content of malondialdehyde by 3.30-, 21.24-, and 33.57-fold, respectively, while fluopimomide at 1.0 and 5.0 mg/L significantly (p < 0.001) increased the levels of reactive oxygen species (ROS) by 49.14% and 77.06% compared to the untreated control. In contrast, fluopimomide at 1.0 and 5.0 mg/L notably reduced the activities of target enzyme succinate dehydrogenase and at 5.0 mg/L reduced the activities of antioxidant enzyme superoxide dismutase. Further evidence revealed that fluopimomide at 1.0 and 5.0 mg/L significantly inhibited oxygen consumption and at 0.2, 1.0, and 5.0 mg/L significantly inhibited ATP level in comparison to the untreated control. The expression of genes related to the mitochondrial electron transport chain mev-1 and isp-1 was significantly downregulated. ROS levels in the mev-1 and isp-1 mutants after fluopimomide treatments did not change significantly compared with the untreated mutants, suggesting that mev-1 and isp-1 may play critical roles in the toxicity induced by fluopimomide. Overall, the results demonstrate that oxidative stress and mitochondrial damage may be involved in toxicity of fluopimomide in C. elegans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluopimomide is a new fungicide to control oomycete pathogens (Zhang et al. 2014). Recent studies reported that fluopimomide exhibited a potential as nematicides against root-knot nematodes (Ji et al. 2020; Li et al. 2020a). Fluopimomide has a similar chemical structure to that of fluopyram, another fungicide having nematicidal activities (Jones et al. 2017). Results from many studies indicated that fluopyram had negative impacts on the non-target organisms (Li et al. 2020b; Tinwell et al. 2014). However, the potential risks of fluopimomide to non-target organisms remained largely unknown. Considering that exposure of fluopimomide may cause diseases to non-target organisms, it is necessary to evaluate its potential risk.
Caenorhabditis elegans is a powerful model in toxicology studies due to its considerable genetic homology to humans and conserved biology, and easy cultivation (Leung et al. 2008). The endpoints of C. elegans, such as growth, reproduction, locomotion, and reactive oxygen species (ROS), are usually used for evaluating the toxic effects of various xenobiotics (Kuhn et al. 2021; Li et al. 2022). Moreover, C. elegans has many useful endpoints that are highly sensitive to pesticides which can be used to evaluate their toxicity (Dai et al. 2019; Yin et al. 2021). Ruan et al. (2009) reported that cyhalothrin inhibited the head thrashes and body bends of the nematodes. Nidheesh et al. (2016) observed increased ROS content in C. elegans after monocrotophos exposure. Notably, it has been demonstrated that C. elegans can be predictive of toxicity in mammalian species in specific cases where they share the same mechanistic pathways and similar physiology (Hunt 2017).
Mitochondria are the center of cellular energy metabolism (Olsen and Gill 2017). Many reports demonstrated that mitochondria were key targets of environmental toxicants, including pesticides (Lee et al. 2021; Meyer et al. 2017). Mitochondrial dysfunction changes production of cellular energy, ROS, and other metabolite signaling (Bora et al. 2021). The production of excessive free radicals can cause oxidative damage if they are not cleared. And the imbalance between ROS and cellular stress defense mechanisms leads to a vicious cycle of further mitochondrial dysfunction, resulting in more ROS (Aguilar-López et al. 2016). Succinate dehydrogenase (SDH) is an important source of ROS in plants and mammals (Jardim-Messeder et al. 2015; Quinlan et al. 2012). It is known that fluopyram acts as SDH inhibitors in fungi and nematodes’ mitochondrial respiratory chain (Avenot and Michailides 2010). In the mitochondria, as the energy factories of the nematodes, the energy of nematode cells will be depleted when they are inhibited. As important genes of mitochondrial electron transport chain, mev-1 and isp-1 play crucial roles in regulating ROS and stress resistance (Soares et al. 2019). Our previous study suggested that fluopyram significantly inhibited the activity of SDH and increased the production of superoxide in mitochondria, leading to oxidative damage in C. elegans (Liu et al. 2021). Due to similar structure of fluopimomide and fluopyram, we hypothesize that fluopimomide may induce toxic effects by oxidative stress and mitochondrial damage in the nematodes.
In this work, the effects of fluopimomide on the growth, locomotion behavior, pharyngeal pumping, and enzyme activity and the expression level of genes associated with oxidative stress and mitochondrial damage of C. elegans were studied. The possible mechanisms of fluopimomide-induced toxicity in the nematodes were investigated. The results of the present study will help better understand the roles of oxidative stress and mitochondrial damage in the toxicity of fluopimomide in C. elegans.
Materials and methods
C. elegans culture
Nematode strains including the wild-type N2, TK22 [mev-1(kn1)], MQ887 [isp-1(qm150)], and Escherichia coli OP50 were provided by Caenorhabditis Genetics Center. Nematodes were cultivated on nematode growth medium (NGM) at 20°C containing E. coli OP50 as food (Brenner 1974). Age-synchronized nematodes were obtained using a bleaching solution. Briefly, gravid adults were washed with M9 buffer and incubated with freshly prepared bleach (2% NaOCl, 1 M NaOH). Then the mixture was shaken for 3 min, and the bleached eggs were centrifuged at 4000 rpm and washed thrice with M9 buffer. The eggs were left to hatch on the NGM plates, and the L4 nematodes were obtained for subsequent tests after 48 h of synchronization (Donkin and Dusenbery 1993).
Experimental chemicals
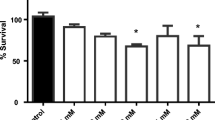
A stock solution of 104 mg/L of fluopimomide (98.6% purity, Sino-Agri Union, Jinan, China) was dissolved in dimethyl sulfoxide (DMSO) and then diluted in M9 buffer with the final DMSO concentration of 0.25%. A vehicle control with the same concentration was used. Fluopimomide at 0.5, 1.0, 2.0, 4.0, 8.0, 16, and 32 mg/L were used to determine the median-lethal concentration (LC50). Synchronized L4 N2 nematodes were transferred to 24-well plates seeded with an E. coli OP50 lawn with or without fluopimomide at 20°C, with about 50 worms per well (1 mL). Our results indicated fluopimomide at tested concentrations had no obvious effect on the growth of E. coli OP50 (data not shown). After acute exposure (24 h) to fluopimomide, survival of the nematodes was recorded by a microscope (Olympus SC180, Tokyo, Japan). The experiment was repeated three times. According to the LC50 value and our previous study, fluopimomide at 0.2, 1.0, and 5.0 mg/L were chosen for subsequent tests.
Physiological indicators assay
N2 nematodes at L4 stage were exposed under the stress of fluopimomide at 0.2, 1.0, and 5.0 mg/L at 20°C. Body length of N2 nematodes was determined according to Liu et al. (2022). After 24-h exposure, the worms were taken to a glass slide and added 50 μL M9 buffer. A flame of an alcohol lamp was used to heat the glass slide for 10 s and the body length was determined with a dissecting microscope (Olympus SZX10, Japan). For the number of pharyngeal pumping, the nematodes were picked onto the NGM plates after exposure and recorded under the microscope for 30 s. The pumping frequency of olfactory bulb every 30 s was the number of pharyngeal pumping of the nematodes (Collins et al. 2008). Frequencies of head thrash and body bend, represent locomotion behaviors of the nematodes, were measured according to Tsalik and Hobert (2003). The exposed worms were rinsed with M9 buffer and placed on a new NGM plate. After recovered for 1 min, the frequencies of head thrash and body bend were observed under the microscope within 30 s. The experiments were performed in triplicate with twenty nematodes each treatment.
MDA content, activities of target enzyme SDH, and antioxidant enzyme system
After the exposure, the worms were washed three times with M9 buffer, and mixed with phosphate-buffered saline solutions. Then the mixture was centrifuged at 5000 rpm at 4°C for 4 min. Malondialdehyde (MDA) content and SDH and superoxide dismutase (SOD) activities were evaluated using respective assay kits (Nanjing JianCheng, China). The MDA, SDH, and SOD contents were normalized by protein content. The experiments were repeated three times with 5000 worms each treatment.
Determination of ROS
The intracellular contents of ROS were determined by 2′,7′-dichlorodihydrofluoroscein diacetate (H2DCF-DA) (1 μM, Beyotime Biotechnology, China) (Yan et al. 2021). N2, TK22 [mev-1(kn1)], and MQ887 [isp-1(qm150)] nematodes were exposed as described above, then washed three times with M9 buffer and incubated with 1 μM of H2DCF-DA for 2.5 h. Then the worms were washed, and fixed by 1 mM levamisole and placed on 2% agar pads. Images were measured using a fluorescence microscope (Olympus IX73, Japan) and analyzed by ImageJ (NIH, Bethesda, MD). The tests were performed in triplicate with twenty nematodes each treatment.
Oxygen consumption rate and ATP level tests
A Clark-type oxygen electrode (Hansatech, UK) was used to determine the oxygen consumption rates of the nematodes. The N2 nematodes were washed with M9 buffer, and transferred into the electrode chamber. Before analysis, the electrode chamber was stabilized with 1 mL air-saturated M9 buffer for 30 min. Oxygen consumption rates were obtained by monitoring oxygen concentration with oxygen electrode for 2–15 min (Gruber et al. 2015). Final oxygen consumption rates were normalized by protein concentration, which was determined using the DC-protein assay (Bio-Rad, Hercules, USA). For the ATP assay, the synchronized N2nematodes (~4000) were exposed to fluopimomide at 0.2, 1.0, and 5.0 mg/L. After thoroughly washing, worms were used to prepare the nematode homogenate by following the manufacturer’s guidelines of the assay kit (Shanghai MLBIO Biotechnology, China). ATP level was determined at 340 nm by a microplate reader. Both experiments were repeated three times.
qRT-PCR analysis
Total mRNA of the N2 worms (~8000) was extracted by Trizol following the manufacturer’s protocol (Accurate Biology, Changsha, China). cDNA was synthesized by a FastQuant RT kit (TIANGEN, China). qRT-PCR was conducted using StepOnePlus System (Applied Biosystems, USA). The mRNA expression levels of mev-1 and isp-1 related to mitochondrial damage and oxidative stress were tested. The relative gene expression were analyzed using 2−△△Ct method with tba-1 as a reference gene. Primer sequences are shown in Table S1. The experiments were repeated thrice.
Statistical analysis
Statistical analysis was performed by SPSS 23.0 (IBM, Chicago). Probit analysis was employed to calculate the LC50 values. Data were submitted to analysis of variance (ANOVA) with Fisher’s protected least significant difference (LSD) test at p = 0.05.
Results
Lethality of fluopimomide
A concentration-response manner existed between the mortality of worms and fluopimomide concentrations (Fig. 1). The LC50 value of fluopimomide on L4 stages nematodes was 5.35 mg/L.
Fluopimomide inhibited growth, pharyngeal pumping, and locomotive behavior of C. elegans
From Fig. 2(A), fluopimomide at tested concentrations notably (p < 0.05) decreased the body length by 15.01%, 19.66%, and 30.90% compared to the untreated control. As shown in Fig. 2(B), compared to the untreated control, fluopimomide at three concentrations significantly (p < 0.001) decreased the number of pharyngeal pumping, with a concentration-response manner. In addition, compared to the untreated control, fluopimomide at 5.0 mg/L notably (p = 0.001) decreased head thrashes in C. elegans by 30.07%, while fluopimomide at lower concentrations had no obvious differences (Fig. 2(C)). Moreover, compared to the untreated control, fluopimomide at three concentrations significantly (p < 0.001) reduced body bends in the nematodes (Fig. 2(D)).
Effects of fluopimomide on various physiological indicators in Caenorhabditis elegans. (A) Body length, (B) pharyngeal pumping, (C) head thrash, (D) body bend. The statistical significance of difference was analyzed by ANOVA with Fisher’s protected LSD test. Values followed by the same letter were not significantly different at p = 0.05
Fluopimomide increased MDA content and affected the activities of target enzyme SDH and antioxidant enzyme system
Nematodes exposed to fluopimomide notably (p < 0.05) increased MDA content by 3.30-, 21.24-, and 33.57-fold compared to the untreated control (Fig. 3(A)). Meanwhile, compared to the untreated control, fluopimomide at higher concentrations notably (p < 0.001) reduced the activity of SDH by 61.41% and 78.95%, respectively (Fig. 3(B)). Furthermore, compared to the untreated control, fluopimomide at 5.0 mg/L obviously (p < 0.001) suppressed SOD activity in the nematodes, whereas no obvious changes were observed between fluopimomide at lower concentrations (p = 0.251 and 0.054, respectively) (Fig. 3(C)).
Effects of fluopimomide on MDA content and antioxidant enzyme activities. (A) MDA content, (B) activity of SDH, (C) activity of SOD. The statistical significance of difference was analyzed by ANOVA with Fisher’s protected LSD test. Values followed by the same letter were not significantly different at p = 0.05
Fluopimomide increased ROS level in N2 nematodes and had no effects in mev-1 and isp-1 mutants
From Fig. 4, in comparison to the untreated control, fluopimomide at higher concentrations notably (p < 0.001) increased the levels of ROS by 45.37% and 109.90%, respectively, while no obvious effect was observed in fluopimomide at 0.2 mg/L. ROS levels in mev-1 and isp-1 mutant strains were further measured and the results showed that no significant changes were observed in the mutants after fluopimomide at tested concentrations compared with the untreated mutants (Fig. S1).
Effects of fluopimomide on ROS level in Caenorhabditis elegans. (A) Microscopic fluorescence imaging of ROS, (B) fluorescence intensity of ROS. The statistical significance of difference was analyzed by one-way ANOVA with Fisher’s protected LSD test. Values followed by the same letter were not significantly different at p = 0.05
Fluopimomide inhibited oxygen consumption and ATP level in N2 nematodes
In Fig. 5(A), in comparison to the untreated control, fluopimomide at 1.0 and 5.0 mg/L significantly (p < 0.05) inhibited oxygen consumption by 41.73% and 54.11% respectively, while fluopimomide at 0.2 mg/L had no significant differences. In addition, nematodes exposed to fluopimomide notably (p < 0.05) inhibited ATP level by 21.79–64.10% compared to the untreated control (Fig. 5(B)).
Effects of fluopimomide on oxygen consumption and ATP level in Caenorhabditis elegans. (A) Oxygen consumption, (B) ATP level. The statistical significance of difference was analyzed by one-way ANOVA with Fisher’s protected LSD test. Values followed by the same letter were not significantly different at p = 0.05
Fluopimomide reduced the expression of genes related to mitochondrial damage and oxidative stress
In the N2 nematodes, in comparison to the untreated control, fluopimomide at higher concentrations notably (p < 0.05) decreased the gene expression of mev-1 by 36.73% and 40.23%, respectively, while no obvious effect (p = 0.956) observed in fluopimomide at 0.2 mg/L (Fig. 6). Fluopimomide at all tested concentrations obviously (p < 0.001) downregulated the isp-1 expression by 58.26%, 76.09%, and 67.77%, respectively, in comparison to the untreated control.
Effects of fluopimomide on expression of genes related to mitochondria. Gene expression values were normalized against the reference tba-1 gene and represented as the mean (n = 3) relative to the control. The statistical significance of difference was analyzed by one-way ANOVA with Fisher’s protected LSD test. Values followed by the same letter were not significantly different at p = 0.05
Discussion
Pesticides are an important class of organic pollutants posing a negative impact on the environment (Akash et al. 2022). Fluopimomide is a novel pesticide widely used in agricultural pest control, but its toxicological effects on non-target organisms are rarely studied. Moreover, so far as we know, the environmentally relevant concentrations of fluopimomide have not been reported yet. Based on the LC50 value and our previous study, fluopimomide at 0.2, 1.0, and 5.0 mg/L were used as the experimental concentrations in the present study. C. elegans was used to determine the toxicity of fluopimomide and its potential mechanisms.
In C. elegans, phenotypic changes are usually associated with toxicity induced by various poisons or stresses (Wang 2019). Body length, pharyngeal pumping, and locomotion behavior of C. elegans are classic endpoints for the assessment of nematode toxicity (Tejeda-Benitez and Olivero-Verbel 2016). Our results showed that fluopimomide had significantly reduced the body length of C. elegans (Fig. 2(A)). This is consistent with a previous study by Zeng et al. (2017), who reported carbofuran significantly reduced the body length of the nematodes. It was noted that the body length of the control N2 nematodes was smaller in comparison with many references in which the length was approximately 1100–1200 μm (Soares et al. 2023; Tang et al. 2023). Maybe it was the reason that the solvent (0.25% DMSO) was used in this study. DMSO has been widely adopted by many researchers (Maglioni et al. 2022; López-García et al. 2020). However, Xiong et al. (2017) reported that DMSO at 0.06% increased post-embryonic developmental time. Moreover, it has been reported that DMSO decreases pharyngeal pumping at concentrations greater than or equal to 0.25% (Calahorro et al. 2021). Therefore, whether DMSO affects the body length of C. elegans needs further study. The pharyngeal pumping also decreased in a concentration-dependent manner under fluopimomide exposure (Fig. 2(B)), which agreed with another study, in which malathion exposure significantly inhibited the swallowing ability of nematodes (Kamaladevi et al. 2016). Moreover, fluopimomide also inhibited the locomotion of nematodes (Fig. 2(C–D)). A previous study showed similar results that both of paichongding and its metabolite M1 reduced the locomotion of the nematodes (Bian et al. 2018). These results indicated that fluopimomide may cause impairment in neuronal and muscular function in C. elegans.
SDH is a key enzyme located in the electron transport chain and is closely related to mitochondrial respiration (Olsen and Gill 2017). Fluopyram was reported to inhibit the mitochondrial complex II of the respiratory chain (Avenot and Michailides 2010). In this study, exposure to fluopimomide notably inhibited the activity of SDH in C. elegans (Fig. 3(B)). Similar results were obtained by Kamireddy et al. (2018), who reported that SDH activity in N2 nematodes was reduced after exposure to another classic neurotoxins 6-hydroxy dopamine (6OHDA). As sharing a similar structure, there is a possibility that fluopimomide has the same mode of action as fluopyram, a SDH inhibitor. Therefore, it is reasonable to speculate that fluopimomide is also a SDH inhibitor. Further investigations are needed to validate this hypothesis.
Mitochondria are known to be one of the main sites of ROS production in vivo, and mitochondrial dysfunction affects the production of ROS; the imbalance between ROS in C. elegans leads to a vicious cycle of further mitochondrial dysfunction (Aguilar-López et al. 2016; Bora et al. 2021). Guzy et al. (2008) reported that SDH played an important role in transporting electrons in the electron transport chain, and inhibition of SdhB function may increase the production of ROS in mammalian cells. Excessive increase of ROS can cause oxidative stress in the nematodes (Mates et al. 2008). Oxidative stress accelerates the peroxidation of polyunsaturated fatty acids, and MDA is one of the final products of peroxidation of polyunsaturated fatty acids (Islam 2017). In this work, fluopimomide increased levels of ROS and MDA (Fig. 3(A) and Fig. 4), suggesting that fluopimomide induced oxidative damages in the nematodes. These observations were in agreement with a previous study, which reported that exposure to paraquat significantly increased levels of ROS and MDA in the nematodes (Ji et al. 2022). Oxidative damage induced by fluopimomide in C. elegans was reported in a previous study (Zhang et al. 2022), and the toxic effect of fluopimomide on C. elegans mitochondria is also worth further study. The results of the present study provided an extensive and in-depth insight into the toxicity of fluopimomide to C. elegans. Oxygen consumption rate and ATP level are important indicators of mitochondrial function (Bailey et al. 2018). In this study, fluopimomide at higher concentrations significantly inhibited the oxygen consumption rate and ATP level of the N2 nematodes (Fig. 5), indicating that fluopimomide disturbed mitochondrial function. The results agreed with Bailey et al. (2018), who observed that glyphosate inhibited oxygen consumption and ATP level in the nematodes.
In addition, mev-1 and isp-1 are important genes of mitochondrial electron transport chain. The gene mev-1 encodes a homologue of the cytochrome b560 subunit of SDH of complex II, and the gene isp-1 encodes the “Rieske” iron-sulfur protein subunit of mitochondrial respiratory chain complex III in the nematodes (Ishii 2000). Mev-1 mutants have a dysfunction of SDH enzyme, leading to an abnormal energy metabolism with increased sensitivity to oxidative damage (Soares et al. 2019; Yu et al. 2014). On the other hand, isp-1 is a known mutation in a mitochondrial complex subunit that prolongs lifespan (Feng et al. 2001). In this work, fluopimomide notably inhibited the expression of mev-1 (except fluopimomide at 0.2 mg/L) and isp-1 (Fig. 6), indicating that both genes may play crucial roles in the toxicity of fluopimomide in C. elegans.
Conclusions
In this study, the toxicity of fluopimomide and its possible molecular mechanism were explored using C. elegans. The results showed that fluopimomide could not only inhibit the physiological activities of the nematodes, but also increase the ROS level and MDA content in the nematodes. Fluopimomide also inhibited the activity of SDH, oxygen consumption, ATP level, and expression of mev-1 and isp-1. These results indicated that fluopimomide could induce toxicity to C. elegans, and oxidative stress and mitochondrial damage might be the mechanism of fluopimomide toxicity. Overall, our results demonstrate that oxidative stress and mitochondrial damage may play vital roles in toxicity of fluopimomide in C. elegans.
Data availability
The datasets used and/or analyzed during the current study are available from authors on a reasonable request.
References
Aguilar-López JL, Laboy R, Jaimes-Miranda F, Garay E, Funes S (2016) Slm35 links mitochondrial stress response and longevity through TOR signaling pathway. Aging 8:3255–3271. https://doi.org/10.18632/aging.101093
Avenot HF, Michailides TJ (2010) Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot 29:643–651. https://doi.org/10.1016/j.cropro.2010.02.019
Bailey DC, Todt CE, Burchfield SL, Pressley AS, Denney RD, Snapp IB, Negga R, Traynor WL, Fitsanakis VA (2018) Chronic exposure to a glyphosate-containing pesticide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Environ Toxicol Pharmacol 57:46–52. https://doi.org/10.1016/j.etap.2017.11.005
Bian T, Zhu X, Guo J, Zhuang Z, Cai Z, Zhao X (2018) Toxic effect of the novel chiral insecticide IPP and its biodegradation intermediate in nematode Caenorhabditis elegans. Ecotox Environ Safe 164:604–610. https://doi.org/10.1016/j.ecoenv.2018.08.059
Bora S, Vardhan GSH, Deka N, Khataniar L, Gogoi D, Baruah A (2021) Paraquat exposure over generation affects lifespan and reproduction through mitochondrial disruption in C. elegans. Toxicology 447:152632. https://doi.org/10.1016/j.tox.2020.152632
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94. https://doi.org/10.1093/genetics/77.1.71
Calahorro F, Holden-Dye L, O'Connor V (2021) Impact of drug solvents on C. elegans pharyngeal pumping. Toxicol Rep 8:1240–1247. https://doi.org/10.1016/j.toxrep.2021.06.007
Collins JJ, Huang C, Hughes S, Kornfeld K (2008) The measurement and analysis of age-related changes in Caenorhabditis elegans. Wormbook 1:21. https://doi.org/10.1895/wormbook.1.137.1
Dai S, Zhang Y, Miao Y, Liu R, Pu Y, Yin L (2019) Intergenerational reproductive toxicity of chlordecone in male Caenorhabditis elegans. Environ Sci Pollut R 26:11279–11287. https://doi.org/10.1007/s11356-019-04519-1
Donkin SG, Dusenbery DB (1993) A soil toxicity test using the nematode Caenorhabditis elegans and an effective method of recovery. Arch Environ Con Tox 25:145–151. https://doi.org/10.1007/BF00212125
Feng J, Bussière F, Hekimi S (2001) Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell 1:633–644. https://doi.org/10.1016/S1534-5807(01)00071-5
Gruber J, Chen CB, Fong S, Ng LF, Teo E, Halliwell B (2015) Caenorhabditis elegans: What we can and cannot learn from aging worms. Antioxid Redox Sign 23:256–279. https://doi.org/10.1089/ars.2014.6210
Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT (2008) Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol 28:718–731. https://doi.org/10.1128/MCB.01338-07
Hunt PR (2017) The C. elegans model in toxicity testing. J Appl Toxicol 37:50–59. https://doi.org/10.1093/toxsci/kfn121
Ishii N (2000) Oxidative stress and aging in Caenorhabditis elegans. Free Radic Res 33:857–864. https://doi.org/10.1080/10715760000301371
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39:73–82. https://doi.org/10.1080/01616412.2016.1251711
Jardim-Messeder D, Caverzan A, Rauber R, de Souza FE, Margis-Pinheiro M, Galina A (2015) Succinate dehydrogenase (mitochondrial complex II) is a source of reactive oxygen species in plants and regulates development and stress responses. New Phytol 208:776–789. https://doi.org/10.1111/nph.13515
Ji X, Li J, Meng Z, Li N, Dong B, Zhang S, Qiao K (2020) Fluopimomide effectively controls Meloidogyne incognita and shows a growth promotion effect in cucumber. J Pest Sci 93:1421–1430. https://doi.org/10.1007/s10340-020-01247-1
Ji P, Li H, Jin Y, Peng Y, Zhao L, Wang X (2022) C. elegans as an in vivo model system for the phenotypic drug discovery for treating paraquat poisoning. PeerJ 10:e12866. https://doi.org/10.7717/peerj.12866
Jones JG, Kleczewski NM, Desaeger J, Meyer SL, Johnson GC (2017) Evaluation of nematicides for southern root-knot nematode management in lima bean. Crop Prot 96:151–157. https://doi.org/10.1016/j.cropro.2017.02.015
Kamaladevi A, Ganguli A, Balamurugan K (2016) Lactobacillus casei stimulates phase-II detoxification system and rescues malathion-induced physiological impairments in Caenorhabditis elegans. Comp Biochem Phys C 179:19–28. https://doi.org/10.1016/j.cbpc.2015.08.004
Kamireddy K, Chinnu S, Priyanka PS, Rajini PS, Giridhar P (2018) Neuroprotective effect of Decalepis hamiltonii aqueous root extract and purified 2-hydroxy-4-methoxy benzaldehyde on 6-OHDA induced neurotoxicity in Caenorhabditis elegans. Biomed Pharmacother 105:997–1005. https://doi.org/10.1016/j.biopha.2018.06.002
Kuhn E, Jacques M, Teixeira D, Meyer S, Gralha T, Roehrs R, Camargo S, Schwerdtle T, Bornhorst J, Ávila D (2021) Ecotoxicological assessment of Uruguay River and affluents pre-and post-pesticides’ application using Caenorhabditis elegans for biomonitoring. Environ Sci Pollut R 28:21730–21741. https://doi.org/10.1007/s11356-020-11986-4
Lee H, Ko E, Shin S, Choi M, Kim KT (2021) Differential mitochondrial dysregulation by exposure to individual organochlorine pesticides (OCPs) and their mixture in zebrafish embryos. Environ Pollut 277:115904. https://doi.org/10.1016/j.envpol.2020.115904
Leung M, Williams P, Benedetto A, Au C, Helmcke K, Aschner M, Meyer J (2008) Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci 106:5–28. https://doi.org/10.1093/toxsci/kfn121
Li CJ, Yuan SF, Jiang F, Xie YF, Guo YH, Yu H, Cheng YL, Qian H, Yao WR (2020b) Degradation of fluopyram in water under ozone enhanced microbubbles: kinetics, degradation products, reaction mechanism, and toxicity evaluation. Chemosphere 258:127216. https://doi.org/10.1016/j.chemosphere.2020.127216
Li J, Meng Z, Li N, Dong B, Ji X, Zhang S, Qiao K (2020a) Evaluating a new non-fumigant nematicide fluopimomide for management of southern root-knot nematodes in tomato. Crop Prot 129:105040. https://doi.org/10.1016/j.cropro.2019.105040
Li X, Yang Q, Wang L, Song C, Chen L, Zhang J, Liang Y (2022) Using Caenorhabditis elegans to assess the ecological health risks of heavy metals in soil and sediments around Dabaoshan Mine, China. Environ Sci Pollut R 29:16332–16345. https://doi.org/10.1007/s11356-021-16807-w
Liu Y, Zhang WP, Wang Y, Liu HM, Zhang SA, Ji XX, Qiao K (2021) Oxidative stress, intestinal damage, and cell apoptosis: toxicity induced by fluopyram in Caenorhabditis elegans. Chemosphere 286:131830. https://doi.org/10.1016/J.chemosphere.2021.131830
Liu S, Wu Q, Zhong Y, He Z, Wang Z, Li R, Wang M (2022) Fosthiazate exposure induces oxidative stress, nerve damage, and reproductive disorders in nontarget nematodes. Environ Sci Pollut R 30:1–10. https://doi.org/10.1007/s11356-022-23010-y
López-García G, Cilla A, Barberá R, Genovés S, Martorell P, Alegría A (2020) Effect of plant sterol and galactooligosaccharides enriched beverages on oxidative stress and longevity in Caenorhabditis elegans. J Funct Foods 65:103747
Maglioni S, Arsalan N, Hamacher A, Afshar S, Schiavi A, Beller M, Ventura N (2022) High-content C. elegans screen identifies natural compounds impacting mitochondria-lipid homeostasis and promoting healthspan. Cells 11(1):100
Mates JM, Segura JA, Alonso FJ, Marquez J (2008) Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol 82:273–299. https://doi.org/10.1007/s00204-008-0304-z
Meyer JN, Leuthner TC, Luz AL (2017) Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 391:42–53. https://doi.org/10.1016/j.tox.2017.07.019
Nidheesh T, Salim C, Rajini PS, Suresh PV (2016) Antioxidant and neuroprotective potential of chitooligomers in Caenorhabditis elegans exposed to Monocrotophos. Carbohyd Polym 135:138–144. https://doi.org/10.1016/j.carbpol.2015.08.055
Quinlan C, Orr A, Perevoshchikova I, Treberg J, Ackrell B, Brand M (2012) Mitochondrial complex II can generate reactive oxygen species at highrates in both the forward and reverse reactions. J Biol Chem 287:27255–27264. https://doi.org/10.1074/jbc.M112.374629
Ruan Q, Ju J, Li Y (2009) Evaluation of pesticide toxicities with differing mechanisms using Caenorhabditis elegans. J Toxicol Env Heal A 72:746–751. https://doi.org/10.1080/15287390902841532
Soares A, Rodrigues L, Salgueiro W, Dal Forno A, Rodrigues C, Sacramento M, Franco J, Alves D, Oliveira R, Pinton S, Ávila D (2019) Organoselenotriazoles attenuate oxidative damage induced by mitochondrial dysfunction in mev-1 Caenorhabditis elegans mutants. J Trace Elem Med Bio 53:34–40. https://doi.org/10.1016/j.jtemb.2019.01.017
Soares GC, Müller L, Josende ME, Ventura-Lima J (2023) Biochemical and physiological effects of multigenerational exposure to spheric polystyrene microplastics in Caenorhabditis elegans. Environ Sci Pollut R 30:69307–69320. https://doi.org/10.1007/s11356-023-27162-3
Tejeda-Benitez L, Olivero-Verbel J (2016) Caenorhabditis elegans, a biological model for research in toxicology. Rev Environ Contam T 237:1–35. https://doi.org/10.1007/978-3-319-23573-8_1
Tinwell H, Rouquié D, Schorsch F, Geter D, Bars R (2014) Liver tumor formation in female rat induced by fluopyram is mediated by CAR/PXR nuclear receptor activation. Regul Toxicol Pharm 70:648–658. https://doi.org/10.1016/j.yrtph.2014.09.011
Tsalik E, Hobert O (2003) Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol 56:178–197. https://doi.org/10.1002/neu.10245
Wang DY (2019) Molecular toxicology in Caenorhabditis elegans. Springer Nature Singapore Pte Ltd
Xiong H, Pears C, Woollard A (2017) An enhanced C. elegans based platform for toxicity assessment. Sci Rep 7:9839. https://doi.org/10.1038/s41598-017-10454-3
Yan C, Wu X, Cao X, Li M, Zhou L, Xiu G, Zeng J (2021) In vitro and in vitro toxicity study of diesel exhaust particles using BEAS-2B cell line and the nematode Caenorhabditis elegans as biological models. Environ Sci Pollut R 28:60704–60716. https://doi.org/10.1007/s11356-021-14908-0
Yin J, Jian Z, Zhu G, Yu X, Pu Y, Yin L, Wang D, Bu Y, Liu R (2021) Male reproductive toxicity involved in spermatogenesis induced by perfluorooctane sulfonate and perfluorooctanoic acid in Caenorhabditis elegans. Environ Sci Pollut R 28:1443–1453. https://doi.org/10.1007/s11356-020-10530-8
Yu C, Wei C, Liao VH (2014) Curcumin-mediated oxidative stress resistance in Caenorhabditis elegans is modulated by age-1, akt-1, pdk-1, osr-1, unc-43, sek-1, skn-1, sir-2.1, and mev-1. Free Radic Res 48:371–379. https://doi.org/10.3109/10715762.2013.872779
Zeng R, Yu X, Tan X, Ye S, Ding Z (2017) Deltamethrin affects the expression of voltage-gated calcium channel α1 subunits and the locomotion, egg-laying, foraging behavior of Caenorhabditis elegans. Pestic Biochem Phys 138:84–90. https://doi.org/10.1016/j.pestbp.2017.03.005
Zhang R, Wang H, Xu H, Wang J, Wang K (2014) Uptake and transportation behavior of a new fungicidal agent LH-2010A in cucumber plants. J Pestic Sci 39:43–47. https://doi.org/10.1584/jpestics.D13-017
Zhang WP, Liu HM, Fu GH, Li YJ, Ji XX, Zhang SA, Wei M, Qiao K (2022) Exposure to fluopimomide at sublethal doses causes oxidative stress in Caenorhabditis elegans regulated by insulin/insulin-like growth factor 1-like signaling pathway. Environ Toxicol 10:2529–2539. https://doi.org/10.1002/tox.23616
Akash S, Sivaprakash B, Rajamohan N, Pandiyan CM, Vo DVN (2022) Pesticide pollutants in the environment-a critical review on remediation techniques, mechanism and toxicological impact. Chemosphere 134754. https://doi.org/10.1016/j.chemosphere.2022.134754
Olsen A, Gill MS (2017) Ageing: Lessons from C. elegans. https://doi.org/10.1007/978-3-319-44703-2
Tang J, Qin J, Kuerban G, Li J, Zhou Q, Zhang H, Sun R, Yin L, Pu Y, Zhang J (2023) Effects of tri-n-butyl phosphate (TnBP) on neurobehavior of Caenorhabditis elegans. Environ Sci Pollut R. https://doi.org/10.1007/s11356-023-28015-9
Funding
This work was supported by Shandong Provincial Natural Science Foundation (ZR2021MC065), Shandong Innovation Capability Enhancement Project for Technological Small and Medium sized Enterprises (2023TSGC0613), Shandong Province Modern Agricultural Technology System Peanut Innovation Team, China (SDAIT-04-08), and National Natural Science Foundation of China (31601661).
Author information
Authors and Affiliations
Contributions
KQ and XJ conceived and designed the research. HL, GF, LB, and WL conducted the experiments and analyzed data. All authors contributed to discussion of the results. KQ and SZ supervised the research, and reviewed and revised the manuscript. KQ wrote the paper. All authors read this manuscript and approved its submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors agree that the article will be published by the journal after acceptance.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Chris Lowe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 317 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Fu, G., Li, W. et al. Oxidative stress and mitochondrial damage induced by a novel pesticide fluopimomide in Caenorhabditis elegans. Environ Sci Pollut Res 30, 91794–91802 (2023). https://doi.org/10.1007/s11356-023-28893-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28893-z