Abstract
Uruguay River is the most important river in western Rio Grande do Sul, separating Brazil from Argentina and Uruguay. However, its pollution is of great concern due to agricultural activities in the region and the extensive use of pesticides. In a long term, this practice leads to environmental pollution, especially to the aquatic system. The objective of this study was to analyze the physicochemical characteristics, metals and pesticides levels in water samples obtained before and after the planting and pesticides’ application season from three sites: Uruguay River and two minor affluents, Mezomo Dam and Salso Stream. For biomonitoring, the free-living nematode Caenorhabditis elegans was used, which were exposed for 24 h. We did not find any significant alteration in physicochemical parameters. In the pre- and post-pesticides’ samples we observed a residual presence of three pesticides (tebuconazole, imazethapyr, and clomazone) and metals which levels were above the recommended (As, Hg, Fe, and Mn). Exposure to both pre- and post-pesticides’ samples impaired C. elegans reproduction and post-pesticides samples reduced worms’ survival rate and lifespan. PCA analysis indicated that the presence of metals and pesticides are important variables that impacted C. elegans biological endpoints. Our data demonstrates that Uruguay River and two affluents are contaminated independent whether before or after pesticides’ application season. In addition, it reinforces the usefulness of biological indicators, since simple physicochemical analyses are not sufficient to attest water quality and ecological safety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aquatic environments are dynamic ecosystems, exhibiting great variability in the water quality. This variability can be partially attributed to the intrinsic characteristics of the system and to extrinsic variations such as climatic changes, droughts, and rainy seasons. However, most of these variations have been originated from pollution. Anthropogenic activities (i.e., industrial wastewater, transport, agriculture) release a wide variety of pollutants that are important threats to the conservation of the water resources (Ferreira et al. 2016).
The presence of pesticides in hydrological systems due to agricultural activities is the most common type of contamination. This is of particular concern because commercial formulations contain many different ingredients, such as potentially toxic metals and surfactants. They have potentially toxic properties to the living organisms, and due to their persistence in the environment, they are of high ecological risk (Gonzalez-Macias et al. 2006). In particular, Brazil has been one of the largest pesticide consumers since the 1970s. Notably, 30% of the pesticides applied in Brazil have been banned in Europe, which brings issues for commodities’ exportation. Among the most important agricultural commodities produced in Brazil and, particularly, in Rio Grande do Sul state (RS) is rice (Carneiro et al. 2015; Fao et al. 2019).
Rice (Oryza sativa L.) is a grain widely consumed in the basic diet of more than half of the world population, becoming the second largest crop after wheat. Rice crops are cultivated in more than 100 countries with 90% of the global production located in the Asians countries (Fukagawa and Ziska 2019; Kaur et al. 2016; Sun et al. 2017). Studies indicate that the consumption of rice, mainly those grown in flooded areas, are the main source of dietary exposure to arsenic (As) and residual pesticides (Fao et al. 2019; Khammanee et al. 2020; Kumarathilaka et al. 2018; Meharg et al. 2009; Zhao et al. 2013; Zhu et al. 2008).
In the southern Brazilian region, rice accounts for 10% of the crops, and according to local data, rice cultivation uses, on average, 10 L of pesticides per planted hectare, which is very high compared to other cultures (Primel et al. 2005). Notably, the municipality of Uruguaiana, located in the extreme west in the state Rio Grande do Sul, is well known as one of the top producers of irrigated rice in Brazil. The ideal climatic conditions, the vast lands, and the presence of a great river like the Uruguay River are the main reasons for this success. However, the amount of pesticides applied and the trafficking of illegal pesticides such as paraquat, which enters Brazil from the Uruguay and Argentina borders, are great concerns. There are many small dams and streams that were created to allow the water flow to and from rice crops. Therefore, pesticides may be dragged into the Uruguay River. The Uruguay River is very important for agriculture, fishing, and also for recreational activities in Brazil, Argentina, and Uruguay along its 1838 km. However, water quality has been neglected by regulatory agencies of these three countries. Erosion and silting of the banks and near extinction of some plant species are consequences of the pollution in this river (Arocena et al. 2018; Goncalves et al. 2020; Rojo et al. 2019; Speranza et al. 2020).
It is known that continuous monitoring is one of the most reliable practices for obtaining information on the quality of natural water resources. Different analyses have been used to detect variations in addition to the standardized physicochemical monitoring. However, most of these techniques cannot identify all the pollutants present in the aquatic environment. For this reason, ecotoxicological biomonitoring practices have increased in the last years and are have been added to the monitoring plans (Clavijo et al. 2016; Pignati et al. 2017; Ruan et al. 2009; Salem et al. 2016; Viarengo et al. 2007).
Some criteria must be met for an organism to be adopted in biomonitoring: for example, must be sensitive to the toxic agent, easy to handle, and available throughout the year (Wah Chu and Chow 2002). Many authors have already demonstrated that the free-living nematode Caenorhabditis elegans (C. elegans) is a viable model as a bioindicator in ecological risk assessments as it meets the requirements (Clavijo et al. 2016). In addition, C. elegans is a globally accepted model for environmental impact analysis (Wah Chu and Chow 2002). Remarkably, this model is very attractive to assess aquatic toxicity because it has a short life cycle, a small body size, is easy to handle, and has low cost for maintenance. C. elegans has a high tolerance to pH variations, salinity, and water hardness and offers a wide range of ecological and toxicologically relevant parameters such as mortality, growth, and reproduction (Tejeda-Benitez et al. 2016).
Therefore, the objective of the present study was to evaluate the water quality from Uruguay River samples obtained before and after pesticides application in the rice crops, using C. elegans as bioindicator. In addition, we sought to correlate biological outcomes and limnological water analysis for future regulatory purposes.
Materials and methods
Sample collection
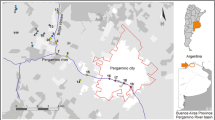
Samplings were carried out in two periods, before the planting and pesticides application period and after pesticides application in the crops. The sampling sites were selected based on the different proximities to the rice crops (Fig. 1): (A) the margin of the Uruguay River basin (U), which provides water for drinking and for crops irrigation, but also receives all kinds of wastes from the affluents (coordinates 29° 44′ 50.3″ S, 57° 05′ 19.6″ W); (B) Salso Stream (S) located at BR 472 (coordinates 29° 47′ 54.2″ S, 57° 05′ 31.6″ W), which receives water from the sewage treatment plant, which then drains back to the Uruguay River; (C) Mezomo Dam (M), chosen for receiving water from some irrigated rice plantation properties, draining this water back to the Uruguay River (coordinates 29° 59′ 14.5″ S, 57° 07′ 19.3″ W). Pre-pesticide water samples were collected in triplicates on August 19th, 20th, and 22nd/2016 (pre-D1, pre-D2, and pre-D3, respectively), and post-pesticide application samples on February 28th, March 2nd, and March 3rd/2017 (post-D1, post-D2, and post-D3, respectively) at same period of the day, between 11:00 and 12:00 a.m. using sterilized flasks, from the top layer to the water flow. The pesticide application occurred between December and February. Following limnologic analysis, samples were stored at − 20 °C.
Geographic locations from samples collection. Arrows indicate the exact point of water collections. a The margin of the Uruguay River basin, which receives tillage water and wastes from the region and also supplies water for drinking and irrigation (coordinates 29° 44′ 50.3″ S, 57° 05′ 19.6″ W). b Salso Stream located at BR 472, known for receiving the treated sewage material (coordinates 29° 47′ 54.2″ S, 57° 05′ 31.6″ W). c Mezomo Dam, located next to a rice farms (coordinates 29° 59′ 14.5″ S, 57° 07′ 19.3″ W). Source: Google Earth, scale 100 m
Physicochemical analysis
In order to determine the characteristics of the water samples, we have subjected them to physical and chemical analyses. Based on that, we evaluated dissolved oxygen, temperature, pH, and conductivity using a portable multiparameter; turbidity was analyzed with a turbidimeter and ammonia and nitrite by colorimetric assays.
Worms’ maintenance and synchronization
Wild-type N2 worms were used in all the experiments and were obtained from the Caenorhabditis Genetics Center (CGC). Animals were kept in 21–22 °C and grown on nematode growth medium (NGM) seeded with Escherichia coli/OP50 as food source. To obtain animals at the same larval stage, the population was synchronized by exposing gravid adult worms to a lysing solution (0.45 N NaOH, 2% HOCl). After 14 h, the eggs hatched, releasing L1 larvae that were used for the treatments (Brenner 1974).
Exposure to water samples
One thousand worms at L1 stage were placed in a 100-mL Erlenmeyer, containing 5 mL of the water samples and E. coli/OP50 as food source and left on constant agitation for 24 h (on orbital shaker at 70 RPM, in an incubator at 21 °C). K-medium (composed of 3 g of sodium chloride, 2.4 g of potassium chloride, and 1 L of H2O distilled) was used as control. After 24-h exposure, worms were pelleted and transferred to NGM plates seeded with E. coli/OP50 for recovery.
Survival and longevity assays
When worms reached L4 stage, the live ones were scored with the aid of a grid. This assay was performed only with animals exposed to post-pesticide samples. Afterwards, 20 treated animals were transferred to NGM plates seeded with E. coli/OP50, in duplicates. Survival was assessed daily until all the worms were dead. The worms were transferred daily during the reproductive period to avoid contamination of the progeny and then were transferred every 2 days. Each experiment was repeated at least three times.
Brood size
One worm from each treatment was individually transferred daily to NGM plates with E. coli/OP50 and reproduction was evaluated by counting the brood size until the end of the reproduction period. Each experiment was performed in triplicates and three independent experiments were performed.
Pesticides determination
The extraction was carried out using Strata X cartridges (500 mg/3 mL), 6 mL of methanol, 6 mL of ultrapure water, and 6 mL of acidified ultrapure water of pH 3 were used for the conditioning. Two liters of sample were percolated maintaining the flow rate of 1 mL/min, and subsequently, 3 mL of acidified pH 3 water was added to affect the cleaning of the cartridge. The analytes were eluted with 9 mL of methanol and under vacuum for another 10 min. The solvent was evaporated to dryness and the eluate was resuspended in 1.5 mL of methanol. Subsequently, the samples were filtered through a 0.22-μm syringe filter and analyzed by HPLC-DAD. The chromatographic system used was YL9100 (Young Lin, South Korea) equipped with a YL90 vacuum degasser YL9110, YL9150 autosampler, YL9131 column oven, and YL9160 diode array detector. The control of the equipment and the data acquisition were made through the YL-Clarity software. The acetonitrile and methanol used in the chromatographic analyses were HPLC grade (J.T. Baker, The Netherlands); ultrapure water was purchased from a Milli-Q system (Millipore, USA). Analyses were performed using Synergi 4 μ Fusion-RP 80 Å (250 × 4.6 mm) and pre-column Fusion-RP (4 × 3.0 mm) chromatography column (Phenomenex, USA). The method initially consists of acidified ultrapure water up to pH = 3, methanol, and acetonitrile (46/38/16, v/v) at the flow rate of 0.9 mL/min; after 10 min, the mobile phase, it became 40% of water pH 3 and 22% acetonitrile, maintaining the initial amount of methanol and with the flow rate of 1.0 mL/min, which is maintained up to 15 min. At 15 min, the mobile phase, it became 36% water pH 3 and 32% acetonitrile and methanol. After 30 min, the mobile phase went to 40% methanol, 36% acetonitrile, and 24% water pH 3 to 35 min. At 35 min, the mobile phase passed on a 44% methanol, 36% acetonitrile, and 20% water pH 3 and flow of 1.2 mL/min. The method was finished at 40 min with mobile phase in 48% methanol and 16% water pH 3. The wavelengths used were 220 nm for tebuconazole and clomazone and 248 nm for imazethapyr; the injection volume was 20 μL of sample. We have also analyzed the presence of diuron, 2,4D, bentazone, quinclorac, propanil, sulfentrazone, 3,4 DCA, and 3,5 DCA.
Metal analysis
Metals as arsenic, copper, iron, manganese, cadmium, zinc, aluminum, mercury, and lead were quantified through inductively coupled plasma mass spectrometry (ICP-MS/MS (Agilent 8800 ICP-QQQ)) as described before (Lohren et al. 2015; Meyer et al. 2018).
Statistical analysis
All assays were performed at least three individual times and GraphPad Prism 6 software was used to generate charts and statistical analysis. One or two-way ANOVA were used and p < 0.05 was considered statistically significant. Repeated measures one-way ANOVA was used for lifespan analysis. Post hoc tests were performed using Tukey post hoc test. Correlation and multiple linear regression were also performed.
Results
Physicochemical analyses
The data obtained from water samples collected pre- and post-pesticide application seasons in the crops are shown in Table 1. It can be observed that dissolved O2, turbidity, nitrite, ammonia, and conductivity were within the desired levels.
Pesticides and metal quantification
Table 2 depicts the amounts of pesticide residues of clomazone, imazethapyr, and tebuconazole that were found in all samples. There were no differences between pre- and post-samples, not even among locations. We have also detected metals as As and Hg as well as Cu, Fe, and Mn in all samples. Overall, pre-pesticide samples presented higher amounts of Mn, Cu, As, and Hg in relation to post-pesticides. No significant differences were found among locations, since they all presented similar metal levels. Most of the samples presented higher levels than the recommended for class II waters, according to the Brazilian regulatory agency CONAMA.
Brood size
In Fig. 2a and b, it can be observed that C. elegans reproductive capacity was impaired by exposure to all pre- and post-pesticide samples. When separately analyzing these data, we observed that some samples strongly contributed to these results (Supplementary Figure S1). For pre-pesticide samples, D1 Mezomo Dam and D2 Salso Stream and Uruguay River depicted significant results (S1 A and B, p < 0.05), whereas D1 post-pesticide samples from Salso and Mezomo had higher statistical power (S1 D, p < 0.05). These different effects can be attributed to the different metal, pesticides, and other contaminant composition in these samples that could be changed by factors such as flow dynamics, the possibility of residues addition in those particular collection days, or that the bottom of the River or the affluents could have been moved. Of note, samples were collected from the upper water column.
Lifespan assay
Next, we investigated the long-term effects of sample exposure to worms by analyzing the whole lifespan. We have found that only post-pesticides significantly reduced C. elegans longevity compared to the control group (Fig. 3a–c). The most powerful statistical difference was detected in Mezomo Dam data, in which worms exposed to post-pesticide samples had also a significant shorter lifespan in relation to pre-pesticide-exposed animals. The same was evidenced when analyzing separately these samples (Supplementary Figures 2 and 3).
Lifespan of worms exposed to samples pre- and post-pesticide application. a Mezomo Dam. b Salso Stream. c Uruguay River. * indicates significant differences in comparison to control and # indicate significant difference between pre- and post-samples. Data were analyzed by repeated measures one-way ANOVA followed by Tukey post hoc test (p < 0.05)
Survival
In order to detect short-term effects of sample exposure and considering that we previously hypothesized that post-pesticide samples would have been more contaminated and therefore would cause higher toxicity, we analyzed the survival rate 48 h after the end of exposure. We observed that worms exposed to all the water samples collected on D1 and D2 after the pesticides application season caused a reduced survival rate (Fig. 4, p < 0.05). However, worms exposed to D3 samples did not present reduced survival. This finding might be associated to the pesticides and metal levels, since all post-D3 samples depicted lower levels or below the detection levels of these contaminants in relation to D1 and D2 (Tables 2 and 3).
Correlation analyses
In order to determine whether physicochemical parameter or pesticides would be causing alterations in the biological endpoints, we have performed a correlation analysis (Fig. 5). We have observed that reproduction may be impaired by conductivity, nitrite, tebuconazole, and imazethapyr levels. Lifespan can be shortened by dissolved oxygen, conductivity, and ammonia in the samples. We have found positive correlations between turbidity and pesticide levels and a negative correlation between dissolved oxygen and pesticides, which indicate that pesticide presence impairs the water quality by reducing available O2 and changing the limpidity of the samples, possibly by stimulating anaerobic microorganism growth. The normality test demonstrated a great variation of the parameters in the samples, even those collected from the same sites in the same period, which may have impaired our analysis. As previously mentioned, these variations were expected, and in order to avoid inaccurate conclusions from a single sampling, we performed triplicate collections.
The principal component analysis (PCA) demonstrated a clearly separation between the data obtained from pre- and post-pesticide samples. Considering PC1 and PC2 composition, pre-pesticide samples are mostly influenced by metals, conductivity, ammonia, and dissolved O2, whereas post-pesticide samples are mostly influenced by turbidity, tebuconazole, imazethapyr, and clomazone levels (Fig. 6). This indicates that, despite the great variability among the samples, the presence of metals and pesticides are important variables that modify the biological outcomes in worms exposed to pre- and post-pesticide samples.
Discussion
The assessment of water quality is critical to water management policies. In the present study, the C. elegans nematode was used as a bioindicator to evaluate the quality of the Uruguay River and two affluents before and after pesticide application on rice crops. We have confirmed the presence of pesticide residues and toxic metals in both pre- and post-application samples. In addition, we have found that independent on whether samples were collected before or after pesticides applications, they caused physiological alterations in the worms, which indicate that samples had poor quality. One of the most important observations is that C. elegans reproduction and lifespan were impaired, even when the physicochemical data were within the acceptable levels. This aspect reinforces the concept that physicochemical data are not sufficient to attest water quality and that the use of bioindicators is a powerful tool. We hypothesize that these samples are constantly contaminated due to the repeated anthropogenic activities, especially agricultural activities.
Remarkably, we detected high levels of As, much higher than those recommended by CONAMA (Brazilian regulatory agency) and by the World Health Organization. Arsenic forms are easily absorbed, particularly in rice grains, which has been associated to the increased incidence of cardiovascular diseases in populations that are major rice consumers (Chanpiwat and Kim 2019; Fao et al. 2019; Majumder et al. 2020). Besides, As, Fe, Mn, and Hg were also found at higher then recommended levels. Remarkably, the damaging effects of the bioaccumulation of these metals to the aquatic ecosystems are of concern, considering their long-term effects (He et al. 2019; Vijver et al. 2004). In addition, some samples depicted conductivity levels above the recommended. There are no reference values in Brazilian legislation for conductivity; however, according to Von Sperling (2007), natural waters should have 10–100 μS/cm, whereas polluted environments may have up to 1000 μS/cm (Von Sperling 2005).
The presence of commonly used pesticides in rice crops as clomazone, imazethapyr, and tebuconazole (Glinski et al. 2018) was detected in the samples obtained prior and after pesticide application, mainly in the Uruguay River, which is the recipient of the crop affluents. We expected significantly higher levels of pesticides as well as higher toxicity in the post-application samples. However, there are 2 factors to consider: (i) we did not measure other pesticides used in the region because we did not have standards or standardized methods for quantification, and (ii) the persistence of the pesticides found and also those ones that could not be measured could be the cause of the toxicity found in pre-application samples. Recently, Gonçalves et al. demonstrated that another location of the Uruguay River presented other seven pesticides: atrazine, byspiribac-sodium, imidacloprid, malathion, propoxur, quinclorac, and simazine. Their presence were associated to alterations in several oxidative biomarkers in Astyanax jacuhiensis, a native species (Goncalves et al. 2020).
Remarkably, we observed that, collectively, exposure to pre- and post-pesticide samples impaired worms reproduction. In addition, post-pesticide exposure affected C. elegans survival in both short and long terms. Given all the factors that could modify worms’ biological responses, correlation and PCA analyses were performed (Figs. 5 and 6). We have observed that lifespan is negatively modulated by the reduction of dissolved oxygen in the medium. It is known that C. elegans can enter into an alternative larval stage known as “dauer” in response to environments with low oxygen concentrations and can even die under highly anaerobic conditions due to energy deficit (Kitazume et al. 2018; Miller and Roth 2009). However, the levels of dissolved oxygen were not as low to cause worms’ mortality; therefore, this parameter by itself cannot be used to explain reduced worms’ lifespan. Notably, as dissolved oxygen levels decreased, the levels of clomazone were increased, and this factor has been negatively correlated with worms’ longevity in our study. This herbicide has been widely used in various crops, including in rice. In 2009, clomazone was among the top ten most used herbicides in Brazil (Primel et al. 2005; Rebelo et al. 2010). Although very effective, it is known for contributing to environmental contamination due to its high solubility in water (1100 mg L−1) (Zanella et al. 2002). Previous studies have already described that clomazone caused increased mortality of Rhamdia quelen (LC50 7.32 μL L−1) (Crestani et al. 2007), and convulsions occurred after 30-min exposure to 20 and 50 mL/L (Miron et al. 2004). In addition, clomazone exposures have caused suppression of catalase antioxidant enzyme and increased lipid peroxidation in Rhamdia quelen, activation of glutathione transferase enzyme activity in Prochilodus lineatus (Pereira et al. 2013); and to cause high mortality, teratogenic effects, and underdevelopment in Danio rerio embryos, findings that evidence its toxicity (Stevanovic et al. 2017).
The correlation and PCA analysis have also indicated that tebuconazole presence may reduce longevity and egg viability in C. elegans. This fungicide toxicity in reproduction has already been reported in different animal models (Guimaraes et al. 2018; Li et al. 2020; Machado-Neves et al. 2018; Zanella et al. 2002), but not in this nematode. Tebuconazole is a fungicide that has been frequently detected in agriculture systems at concentrations that affect endocrine function in organisms. For instance, it has been found to cause oxidative stress and endocrine disruption in rats (Yang et al. 2018) and developmental toxicity associated to thyroid dysfunction in zebrafish (Li et al. 2020). Finally, imazethapyr has also been reported by causing metabolic alterations in Cyprinus carpio (Moraes et al. 2011), genotoxicity in tadpoles of Rhinella arenarum and Hypsiboas pulchellus species (Carvalho et al. 2019; Perez-Iglesias et al. 2017; Perez-Iglesias et al. 2015), and also in mammalian cells (Soloneski et al. 2017); however, the toxicity of herbicide is poorly characterized and needs to be further investigated.
We have found great variability of metals and pesticide levels among samples collected in the same site and same period. Of note, the samples were collected from the top layer of the water sources; therefore, the lower metal levels in post-pesticide samples could be attributed to sedimentation of these materials due to many days without rain (Ajima et al. 2015). Another explanation for this phenomenon is that the inert components present in pesticides and other contaminants may have decanted, sedimented, and accumulated, as reported by previous studies (Caballero-Gallardo et al. 2015; Kim et al. 2018; Tejeda-Benitez et al. 2016; Vallejo Toro et al. 2016). On the other hand, higher metal levels detected in 3rd day from all pre-pesticide samples could be attributed to movements in the bottom of the river or that some deposit was done on those sites in that day. We knew that these daily changes could occur since hydric systems are highly dynamic, and because of that, we have sampled in triplicates for each site, avoiding a unique collection and inaccurate conclusions.
In summary, this study demonstrated that pesticides and metals are present in the Uruguay River and two of its affluents, with particular attention to metals above the recommended levels. Furthermore, the biological data demonstrated that the worms suffered negative consequences from the exposure to contaminated samples, therefore indicating that aquatic organisms would be impaired as well. We did find correlation between these biological effects and the presence of the analyzed metals and pesticides; however, we did not find a correlation between pre- and post-pesticide application season, therefore indicating that Uruguay River and affluents are constantly contaminated. In addition, the presence of other contaminants and their association must be taking into consideration. The regulatory authorities from Brazil, Uruguay, and Argentina must be aware of the presence of these contaminants in this important River and take actions to reduce the ecotoxicological outcomes to all live forms that depend on it.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ajima MN, Nnodi PC, Ogo OA, Adaka GS, Osuigwe DI, Njoku DC (2015) Bioaccumulation of heavy metals in Mbaa River and the impact on aquatic ecosystem. Environ Monit Assess 187:768
Arocena R, Chalar G, Pacheco JP (2018) Agriculture and elevation are the main factors for Pampasic stream habitat and water quality. Environ Monit Assess 190:254
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Caballero-Gallardo K, Guerrero-Castilla A, Johnson-Restrepo B, de la Rosa J, Olivero-Verbel J (2015) Chemical and toxicological characterization of sediments along a Colombian shoreline impacted by coal export terminals. Chemosphere 138:837–846
Carneiro FF, Rigotto RM, Augusto LGS, Friedrich K, Burigo AC (2015) Dossiê ABRASCO: um alerta sobre os impactos dos agrotóxicos na saúde. Oswaldo Cruz Foundation-1st edn. Expressão Popular Publishing, São Paulo, p 624 (in Portuguese)
Carvalho WF, Ruiz de Arcaute C, Perez-Iglesias JM, Laborde MRR, Soloneski S, Larramendy ML (2019) DNA damage exerted by mixtures of commercial formulations of glyphosate and imazethapyr herbicides in Rhinella arenarum (Anura, Bufonidae) tadpoles. Ecotoxicology 28:367–377
Chanpiwat P, Kim KW (2019) Arsenic health risk assessment related to rice consumption behaviors in adults living in Northern Thailand. Environ Monit Assess 191:674
Clavijo A, Kronberg MF, Rossen A, Moya A, Calvo D, Salatino SE, Pagano EA, Morabito JA, Munarriz ER (2016) The nematode Caenorhabditis elegans as an integrated toxicological tool to assess water quality and pollution. Sci Total Environ 569-570:252–261
Crestani M, Menezes C, Glusczak L, dos Santos MD, Spanevello R, Silveira A, Goncalves FF, Zanella R, Loro VL (2007) Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 67:2305–2311
Fao N, Nascimento S, de La Cruz AH, Calderon D, Rocha R, Saint’Pierre T, Gioda A, Thiesen FV, Brucker N, Emanuelli T, Garcia SC (2019) Estimation of total arsenic contamination and exposure in Brazilian rice and infant cereals. Drug Chem Toxicol 2:1–9
Ferreira AS, da Silva HC, Rodrigues HO, Silva M, Junior A (2016) Occurrence and spatial-temporal distribution of herbicide residues in the Ipojuca River sub-basin, Pernambuco, Brazil. Rev Bras Eng Agríc Ambient 20:1124–1128
Fukagawa NK, Ziska LH (2019) Rice: importance for global nutrition. J Nutr Sci Vitaminol 65:S2–s3
Glinski DA, Purucker ST, Van Meter RJ, Black MC, Henderson WM (2018) Analysis of pesticides in surface water, stemflow, and throughfall in an agricultural area in South Georgia, USA. Chemosphere 209:496–507
Goncalves C, Marins AT, do Amaral AMB, Nunes MEM, Muller TE, Severo E, Feijo A, Rodrigues CCR, Zanella R, Prestes OD, Clasen B, Loro VL (2020) Ecological impacts of pesticides on Astyanax jacuhiensis (Characiformes: Characidae) from the Uruguay river, Brazil. Ecotoxicol Environ Saf 205:111314
Gonzalez-Macias C, Schifter I, Lluch-Cota DB, Mendez-Rodriguez L, Hernandez-Vazquez S (2006) Distribution, enrichment and accumulation of heavy metals in coastal sediments of Salina Cruz Bay, Mexico. Environ Monit Assess 118:211–230
Guimaraes B, Bandow C, Amorim MJB, Kehrer A, Coors A (2018) Mixture toxicity assessment of a biocidal product based on reproduction and avoidance behaviour of the collembolan Folsomia candida. Ecotoxicol Environ Saf 165:284–290
He X, Deng H, Hwang HM (2019) The current application of nanotechnology in food and agriculture. J Food Drug Anal 27:1–21
Kaur B, Ranawana V, Henry J (2016) The glycemic index of rice and rice products: a review, and table of GI values. Crit Rev Food Sci Nutr 56:215–236
Khammanee N, Qiu Y, Kungskulniti N, Bignert A, Meng Y, Zhu Z, Lekew Teffera Z (2020) Presence and health risks of obsolete and emerging pesticides in paddy rice and soil from Thailand and China. Int J Environ Res Public Health 17(11):3786
Kim SW, Moon J, Jeong SW, An YJ (2018) Development of a nematode offspring counting assay for rapid and simple soil toxicity assessment. Environ Pollut 236:91–99
Kitazume H, Dayi M, Tanaka R, Kikuchi T (2018) Assessment of the behaviour and survival of nematodes under low oxygen concentrations. PLoS One 13:e0197122
Kumarathilaka P, Seneweera S, Meharg A, Bundschuh J (2018) Arsenic accumulation in rice (Oryza sativa L.) is influenced by environment and genetic factors. Sci Total Environ 642:485–496
Li S, Jiang Y, Sun Q, Coffin S, Chen L, Qiao K, Gui W, Zhu G (2020) Tebuconazole induced oxidative stress related hepatotoxicity in adult and larval zebrafish (Danio rerio). Chemosphere 241:125129
Lohren H, Bornhorst J, Galla HJ, Schwerdtle T (2015) The blood-cerebrospinal fluid barrier--first evidence for an active transport of organic mercury compounds out of the brain. Metallomics 7:1420–1430
Machado-Neves M, Neto MJO, Miranda DC, Souza ACF, Castro MM, Sertorio MN, Carvalho TF, Matta SLP, Freitas MB (2018) Dietary exposure to tebuconazole affects testicular and epididymal histomorphometry in frugivorous bats. Bull Environ Contam Toxicol 101:197–204
Majumder B, Das S, Pal B, Biswas AK (2020) Evaluation of arsenic induced toxicity based on arsenic accumulation, translocation and its implications on physio-chemical changes and genomic instability in indica rice (Oryza sativa L.) cultivars. Ecotoxicology 29:13–34
Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu Y-G, Feldmann J, Raab A, Zhao F-J, Islam R, Hossain S, Yanai J (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43:1612–1617
Meyer S, Markova M, Pohl G, Marschall TA, Pivovarova O, Pfeiffer AFH, Schwerdtle T (2018) Development, validation and application of an ICP-MS/MS method to quantify minerals and (ultra-)trace elements in human serum. J Trace Elem Med Biol 49:157–163
Miller DL, Roth MB (2009) C. elegans are protected from lethal hypoxia by an embryonic diapause. Curr Biol 19:1233–1237
Miron DS, Silva LVF, Golombieski JI, Machado SLO, Marchezan E, Baldisserotto B (2004) Lethal concentration of clomazone, metsulfuron-metil, and quinclorac for silver catfish, Rhamdia quelen, fingerlings. Cienc Rural 34:1465–1469
Moraes BS, Clasen B, Loro VL, Pretto A, Toni C, de Avila LA, Marchesan E, Machado SL, Zanella R, Reimche GB (2011) Toxicological responses of Cyprinus carpio after exposure to a commercial herbicide containing imazethapyr and imazapic. Ecotoxicol Environ Saf 74:328–335
Pereira L, Fernandes MN, Martinez CB (2013) Hematological and biochemical alterations in the fish Prochilodus lineatus caused by the herbicide clomazone. Environ Toxicol Pharmacol 36:1–8
Perez-Iglesias JM, Soloneski S, Nikoloff N, Natale GS, Larramendy ML (2015) Toxic and genotoxic effects of the imazethapyr-based herbicide formulation Pivot H(R) on montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 119:15–24
Perez-Iglesias JM, Ruiz de Arcaute C, Natale GS, Soloneski S, Larramendy ML (2017) Evaluation of imazethapyr-induced DNA oxidative damage by alkaline Endo III- and Fpg-modified single-cell gel electrophoresis assay in Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol Environ Saf 142:503–508
Pignati WA, Lima FANS, Lara SS, Correa MLM, Barbosa JR, Leão LHC, Pignatti MG (2017) Distribuição espacial do uso de agrotóxicos no Brasil: uma ferramenta para a Vigilância em Saúde. Cien Saude Colet 22:3281–3293
Primel EG, Zanella R, Kurz MHS, Gonçalves FF, Machado SO, Marchezan E (2005) Poluição das águas por herbicidas utilizados no cultivo do arroz irrigado na região central do estado do Rio Grande do Sul, Brasil: predição teórica e monitoramento. Quim Nova 28:605–609
Rebelo RM, Vasconcelos R, Buys B, Rezende J, Moraes K, Oliveira R (2010) Produtos agrotóxicos e afins comercializados em 2009 no Brasil: uma abordagem ambiental. Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA), Brasília, Brazil
Rojo M, Alvarez-Munoz D, Domanico A, Foti R, Rodriguez-Mozaz S, Barcelo D, Carriquiriborde P (2019) Human pharmaceuticals in three major fish species from the Uruguay River (South America) with different feeding habits. Environ Pollut 252:146–154
Ruan Q-L, Ju J-J, Li Y-H, Liu R, Pu Y-P, Yin L-H, Wang D-Y (2009) Evaluation of pesticide toxicities with differing mechanisms using Caenorhabditis elegans. J Toxic Environ Health A 72:746–751
Salem FB, Said OB, Aissa P, Mahmoudi E, Monperrus M, Grunberger O, Duran R (2016) Pesticides in Ichkeul Lake-Bizerta Lagoon Watershed in Tunisia: use, occurrence, and effects on bacteria and free-living marine nematodes. Environ Sci Pollut Res Int 23:36–48
Soloneski S, Ruiz de Arcaute C, Nikoloff N, Larramendy ML (2017) Genotoxicity of the herbicide imazethapyr in mammalian cells by oxidative DNA damage evaluation using the Endo III and FPG alkaline comet assays. Environ Sci Pollut Res Int 24:10292–10300
Speranza ED, Colombo M, Heguilor S, Tatone LM, Colombo JC (2020) Alterations in the sterol signature of detritivorous fish along pollution gradients in the Rio de la Plata basin (Argentina): from plant to sewage-based diet. Environ Res 184:109351
Stevanovic M, Gasic S, Pipal M, Blahova L, Brkic D, Neskovic N, Hilscherova K (2017) Toxicity of clomazone and its formulations to zebrafish embryos (Danio rerio). Aquat Toxicol 188:54–63
Sun XD, Su P, Shan H (2017) Mycotoxin contamination of rice in China. J Food Sci 82:573–584
Tejeda-Benitez L, Flegal R, Odigie K, Olivero-Verbel J (2016) Pollution by metals and toxicity assessment using Caenorhabditis elegans in sediments from the Magdalena River, Colombia. Environ Pollut 212:238–250
Vallejo Toro PP, Vasquez Bedoya LF, Correa ID, Bernal Franco GR, Alcantara-Carrio J, Palacio Baena JA (2016) Impact of terrestrial mining and intensive agriculture in pollution of estuarine surface sediments: spatial distribution of trace metals in the Gulf of Uraba, Colombia. Mar Pollut Bull 111:311–320
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol Toxicol Pharmacol 146:281–300
Vijver MG, Van Gestel CA, Lanno RP, Van Straalen NM, Peijnenburg WJ (2004) Internal metal sequestration and its ecotoxicological relevance: a review. Environ Sci Technol 38:4705–4712
Von Sperling M (2005) Principles of biological treatment of residual waters. In: Von Sperling M (ed) Introduction to water quality and sewage treatment, 3rd edn. Federal University of Minas Gerais Publishing, Belo Horizonte, p 452
Von Sperling M (2007) Biological wastewater treatment series: basic principles of wastewater treatment, 1st edn. IWA Publishing, London
Wah Chu K, Chow KL (2002) Synergistic toxicity of multiple heavy metals is revealed by a biological assay using a nematode and its transgenic derivative. Aquat Toxicol 61:53–64
Yang JD, Liu SH, Liao MH, Chen RM, Liu PY, Ueng TH (2018) Effects of tebuconazole on cytochrome P450 enzymes, oxidative stress, and endocrine disruption in male rats. Environ Toxicol 33:899–907
Zanella R, Primel E, Oliveira M, Gonçalves F, Marchezan E (2002) Monitoring of the herbicide clomazone in environmental water samples by solid-phase extraction and high-performance liquid chromatography with ultraviolet detection. Chromatographia 55:573–577
Zhao FJ, Zhu YG, Meharg AA (2013) Methylated arsenic species in rice: geographical variation, origin, and uptake mechanisms. Environ Sci Technol 47:3957–3966
Zhu YG, Williams PN, Meharg AA (2008) Exposure to inorganic arsenic from rice: a global health issue? Environ Pollut 154:169–171
Funding
This study was funded in part by CNPq (Universal Grants- 453963/2014-5), FAPERGS/PqG # 18/2551-0000434-0, CNPq/FAPERGS/DECIT/SCTIE-MS/PRONEM #16/2551-0000248-7, PROPPI/ UNIPAMPA, and the German Research Foundation Research Unit TraceAge (FOR 2558). Authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. D.S.A is recipient of researcher fellowship (PQ2/CNPq).
Author information
Authors and Affiliations
Contributions
E.C.K conceptualization, investigation, methodology, data curation, formal analysis, writing- original draft; M.T.J conceptualization, supervision, methodology, formal analysis, writing - original draft; D.T methodology, data curation, formal analysis, writing; S.M methodology, data curation, formal analysis; T.G methodology, data curation, formal analysis, writing; R.R data curation, formal analysis, supervision, funding acquisition, writing; S. C data curation, formal analysis, writing- original draft; T. S methodology, data curation, formal analysis, resources, writing; J. B methodology, data curation, formal analysis, writing; D.S.A conceptualization, funding acquisition, investigation, methodology, project administration, data curation, formal analysis, resources, supervision, writing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent to publish
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Chris Lowe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 304 kb)
Rights and permissions
About this article
Cite this article
Kuhn, E.C., Jacques, M.T., Teixeira, D. et al. Ecotoxicological assessment of Uruguay River and affluents pre- and post-pesticides’ application using Caenorhabditis elegans for biomonitoring. Environ Sci Pollut Res 28, 21730–21741 (2021). https://doi.org/10.1007/s11356-020-11986-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11986-4