Abstract
Heavy metal pollution is a global environmental problem, and the potential risks associated with heavy metals are increasing. The acid mine drainage (AMD) which is generated by mining activities at Dabaoshan Mine, the largest polymetallic mine in southern China, is harmful to local residents. A detailed regional survey of the ecological and human health risks of this polluted area is urgently needed. In this study, eight sediments and farmland samples were collected along the flow direction of tailing wastewater and Fandong Reservoir; the content of multiple heavy metals in these samples was determined by inductively coupled plasma mass spectrometry. The biological toxicity of water-soluble extracts from the samples was further assessed by referring to different endpoints of Caenorhabditis elegans (C. elegans). The relationship between specific heavy metals and biological toxicity was estimated by partial least squares regression. The results indicated that the risk of heavy metals in Dabaoshan mining area was very high (potential ecological risk index = 721.53) and was related to geographical location. In these samples, the carcinogenic risk (the probability that people are induced carcinogenic diseases or injuries when exposed to carcinogenic pollutants) of arsenic (As) for adults exceeded the standard value 1 × 10−4 and indicated that As presented a high carcinogenic risk to adults, while the high risk of non-carcinogenic effects (the hazard degree of human exposure to non-carcinogenic pollutants) in children was related to lead exposure (hazard index = 1.24). In addition, the heavy metals at high concentration in the water-soluble fraction of sediment and farmland soil extracts, which might easily distribute within the water cycle, inhibited the survival rate and growth of C. elegans. Gene expression and enzymatic activity related to oxidative stress were increased and genes related to apoptosis and metallothionein were also affected. In conclusion, the results of chemical analysis and biological assays provided evidence on the toxicity of soil and sediment extracts in the Dabaoshan mining area and advocated the control and remediation of heavy metal pollution around Dabaoshan Mine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution has attracted widespread attention because it is irreversible, persistent, concealed, and bioaccumulated. Mining activities are an important source of heavy metal pollution. Global metal ore mining reached 7.4 billion tons in 2010 (Zheng et al. 2018), while the scale of mining activities continues to grow (Ali et al. 2017). Although mineral resources play important roles in daily life and in industry, large volumes of tailings containing sulfide minerals are produced every year around the world. The tailings are discharged into tailing dams, which results in large amounts of acid mine drainage (AMD) characterized by strong acidity and high concentration of toxic heavy metals (Akcil and Koldas 2006). Improperly treated AMD can severely damage the environment such as soil and surface water and exposes local inhabitants to heavy metals via ingestion and skin absorption. The plants exposed to heavy metals can experience different degrees of disease, such as chlorosis, reduced biomass, decreased chlorophyll content, and even death (Vardhan et al. 2019). Additionally, human health can be threatened by conditions such as bone pain, Minamata disease, and increased mortality risk from cancer (Daraz et al. 2021). Thus, there is an urgent need to establish a more comprehensive environmental risk assessment method.
Various indexes have been used to evaluate the extent of heavy metal pollution in mining areas, such as the single factor index, geo-accumulation index, Hakanson potential ecological risk index, Nemerow index, enrichment factor, and health risk assessment based on the potential exposure pathways (i.e., ingestion) (Adewumi and Laniyan 2020; Lin et al. 2019). Previous studies have generally been limited to a few heavy metals, which does not reflect the real environmental risk of these contaminated sites (Chen et al. 2018b; Xiang et al. 2019). These studies were mainly based on the total amount of heavy metals, while the physicochemical properties of the environment such as pH, redox conditions, and organic matter content can affect the chemical form of heavy metals, which affects metal bioavailability, a prerequisite for toxicity (Schultz et al. 2004). Therefore, an accurate assessment of the health risks caused by heavy metal exposure should not be limited to studies of selected heavy metals or the toxic effects of total heavy metals in general.
Model organisms such as bacteria, plants, earthworms, and nematodes have previously been used to explore the biological effects of heavy metals in environmental samples (Chapman et al. 2013). Caenorhabditis elegans (C. elegans) is widely used in toxicology research because it has the following characteristics: short life cycle, simple and inexpensive propagation in the laboratory, ability to self-fertilize, transparent body, and its genes display high homology with human genes (60–80%) (Leung et al. 2008). Previous studies have used C. elegans to analyze the risks of different environmental samples including sediment, soil, wastewater, and particulate matter through different endpoints such as DNA damage, survival, lifespan, growth, and gene expression (Rai et al. 2019). In addition, C. elegans is acid-tolerant (pH range of tolerance: 3.1–11.9) and highly sensitive to metals by over-expressing metallothionein (Khanna et al. 1997). Therefore, C. elegans is a good model for studying the potential health risks of heavy metals in sulfide mining areas.
Dabaoshan Mine is the largest polymetallic mine in Guangdong Province, south China. The water bodies, sediments, soils, and food crops in the mining area are seriously polluted by heavy metals from AMD (Wang et al. 2019b). Under the stress of heavy metals in the acid soil of Dabaoshan Mine, the growth of tested Corymbiacitriodora var. variegata was significantly slowed down (Liu et al. 2009). In addition, the lead (Pb) concentrations in the hair of residents in the Dabaoshan mining area were 15–28 times higher than that of non-exposed people (Zhuang et al. 2014). Local residents are frequently reported to suffer from cancers such as esophageal and lung cancers probably because of long-term intake of fish, rice, and vegetables contaminated with heavy metals (Shu et al. 2018). The potential risks of heavy metal pollution in Dabaoshan Mine area have previously been evaluated mainly by chemical analysis, but there is a lack of relevant biological toxicity test data (Zhao et al. 2012; Zhou et al. 2007). Our study performed biological assays by using C. elegans besides chemical analysis to carry out a more comprehensive assessment. In this research, sediments and soils from around Dabaoshan Mine were collected and analyzed for heavy metal concentrations in the samples and in water-soluble extracts. At the same time, C. elegans was assayed to determine endpoints following exposure to water-soluble extracts. Additionally, the partial least squares regression (PLSR) model was applied to estimate the link between the endpoints and heavy metal concentrations in the extracts. The objectives of this study were as follows: (1) assess the potential ecological and human health risks of heavy metals in mining areas based on the total amount of heavy metals; (2) analyze the toxic effects and potential molecular mechanisms of nematode endpoints induced by heavy metals in extracts; and (3) determine the contribution of different heavy metals in the extracts to the changes in C. elegans biological indicators. Our results evaluated the toxic contribution of certain heavy metal to live organisms and humans in Dabaoshan Mine area and were valuable for the control and land restoration of such areas polluted by AMD.
Materials and methods
Sampling details
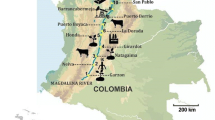
Dabaoshan Mine (24°34′28″N, 113°43′42″E), located at the junction of Qujiang and Wengyuan counties of Shaoguan, Guangdong Province, south China, is a polymetallic sulfide deposit. Surface sediments (E1, E2, E3, E4, E5) and soils (S1, S2, S3) near Dabaoshan Mine were collected in the summer of 2019 (rainy season). Detailed information about the locations of Dabaoshan Mine, Fandong Reservoir, and the sampling sites are shown in Fig. 1. Specifically, soil samples were collected from croplands at a depth of 0–20 cm in Liangqiao village which was about 3.6 km downstream of the tailing wastewater. Samples E1 and E2 were collected from Fandong Reservoir and its downstream (5.9 km), respectively. Samples E3 and E4 were collected from the tailing wastewater and its downstream (2.9 km), respectively. Sample E5 (about 6.7 km downstream of the wastewater) was collected at the confluence of the tailing wastewater and Fandong Reservoir water. These surface sediments (with a depth of 0–10 cm) which were scraped with a plastic shovel were collected from the river bed 3–5 m away from the river bank. Then, the collected surface sediments were stored in a sterile ziplock bag. Three samples were taken from each sampling point with an interval of 1 m, and mixed evenly as a mixed sample. All samples were freeze-dried, crushed, sieved with 100 mesh, and stored at − 20 °C for further heavy metal analysis and toxicity tests.
Heavy metal analysis
Samples for total heavy metal analysis were digested with a concentrated acid mixture (HNO3-HF). The digests were then supplemented with 7.5 mL H3BO3 (2.5% wt), and diluted to 50 mL with deionized water. Water-soluble metal extracts for toxicity tests were obtained after multiple steps according to a previous report (Hagner et al. 2018). Briefly, 2.5 g dry sample was mixed with 10 mL deionized water and shaken at a speed of 200 rpm for 24 h. After extraction, the mixture was centrifuged (12,000 rpm, 20 min) and the supernatant was collected into a polypropylene tube. Then, the collected supernatant was filtered through a 0.22-μm filter and stored at − 20 °C. The leachate was used for soluble heavy metal analysis and biological analysis. The pH of the elutriates is shown in Table S1.
The metals in acid digests and leachates were analyzed by inductively coupled plasma mass spectrometry (ICAPQ, Thermo Scientific). Replicates and blank samples were analyzed, and quality control was performed using certified reference materials (CRMs) including GSS-25 (GBW07454), Pacs-3 (National Research Council Canada), and Mess-4 (National Research Council Canada). The recoveries of heavy metals are shown in Table S2.
Assessment of potential ecological risk
The degree of heavy metal pollution in soils/sediments was defined as the potential ecological risk index (RI) by using the formulas below (Tejeda-Benitez et al. 2016).
where RI is calculated as the sum of Ei, which represents the potential ecological risks of multi-metal pollution; Ei represents the potential ecological risk index of individual elements; Ti is the metal toxicity coefficient (for Pb, Cu, and Ni, Ti is 5, it is 10 for As, 2 for V and Cr, and 1 for Zn and Mn) (Xu et al. 2008); fi is a metal pollution factor, calculated from Ci, which is the concentration of the metal in soils/sediments; and Cb is the reference value of the metal (V = 65.3 mg/kg, Cr = 50.5 mg/kg, Ni = 14.4 mg/kg, Cu = 17 mg/kg, As = 8.9 mg/kg, Pb = 36 mg/kg, Mn = 279 mg/kg, Zn = 47.3 mg/kg) (CNEMC 1990). By referring to a former study, RI was divided into five levels as shown in Table S3 (Zhu et al. 2018).
Health risk assessment
For soil contaminated with heavy metals, humans may be exposed to heavy metals in two ways, including direct intake and dermal absorption (Man et al. 2010). The exposure dose was calculated using Eqs. (4) and (5) (USEPA 1989).
Ingestion:
where ADII is the average daily intake by ingestion (mg/kg-day); SIR is the ingestion rate (mg/day); EF is the exposure frequency (day/per year); ED is the exposure duration (year); BW is the body weight (kg); and AT is the average time (day).
Dermal absorption:
where ADID is the average daily intake from dermal absorption (mg/kg-day); SA is the exposed skin area (cm2); AF is the adherence factor (mg/cm2-day); and ABS is the dermal absorption factor (unitless).
Health risks were reflected by carcinogenic and non-carcinogenic risks. The hazard quotient (HQ) reflects non-carcinogenic hazards determined by Eqs. (6) and (7). Specifically, HQABS and HQO represent the potential non-carcinogenic risks caused by dermal absorption and soil ingestion, respectively. Total potential non-carcinogenic effects (hazard index, HI) were calculated by Eq. (8). If the HQ was greater than 1, there might be non-carcinogenic effects. While HI > 1 suggested that it might have an adverse effect on human health (Li and Zhang 2010). Carcinogenic risks were calculated by Eqs. (9) and (10). The EPA accepts risks in the range of 10−6 to 10−4 (Jiang et al. 2017).
where ADI is the average daily intake; RfD is the reference dose (mg/kg-day); RfDABS is the dermally reference dose (mg/kg-day); RfDo is the oral reference dose (mg/kg-day); ABSGI is the gastrointestinal absorption factor (unitless); SF is the slope factor (per mg/kg-day); SFABS is the dermally slope factor (per mg/kg-day); and SFO is the oral slope factor (per mg/kg-day). The information on the relevant parameters in the formulae is shown in Table S4.
C. elegans culturing and exposure conditions
N2 wild-type C. elegans were cultivated on nematode growth medium at 20 °C fed with Escherichia coli strain OP50 (Brenner 1974). To obtain synchronized nematodes, gravid nematodes were lysed with an alkaline hypochlorite solution (Chen et al. 2018a). Experiments were performed by using diluted extracts in K-medium (a solution containing 51 mM NaCl and 32 mM KCl) and K-medium was used as the control (Tejeda-Benítez et al. 2018). Based on the results of preliminary experiments (not shown here), S1–S3, E1, E2, and E5 extracts were diluted 20 times, E3 was diluted 20, 1000, 1500, and 2000 times, and E4 was diluted 20, 50, 100, and 200 times for the exposure assay. Specifically, the pH of E3 extract (1:1000 dilution) was 6.24, and the pH of E4 extract (1:50 dilution) was 10.28 (pH range of tolerance for C. elegans: 3.1–11.9).
Survival rate and body length of C. elegans
To determine the survival rate, the nematodes (n = 10) in L4 larvae were exposed for 24 h. The nematode was considered dead if it could not respond to the stimulus using a tiny metal needle (Xu et al. 2017). Four replicates were performed.
For assessing nematode growth, we measured the body length. As a previous study reported (Jiang et al. 2016), nematodes (n = 10) in L1 stage were exposed for 48 h, then body length was measured by Zeiss Discovery V20 microscope (Carl Zeiss AG, Oberkochen, Germany) and software Image J (National Institutes of Health, Bethesda, MD, USA). Three replicates were performed for each treatment.
Determination of enzymatic activity related to oxidative stress of C. elegans
To study the subacute toxic effect of the leachates, the L1 nematodes were ground in phosphate-buffered saline (PBS) and centrifuged following 72-h exposure to soluble extracts. The supernatants were assayed for superoxide dismutase (SOD) and catalase (CAT) activities by using commercial kits (Nanjing Jiancheng Institute, China) in accordance with the manufacturer’s instructions. Total protein was determined by the bicinchoninic acid (BCA) assay (Bradford 1976). The results were expressed in terms of enzymatic activity per unit protein.
Related gene expression quantification of C. elegans
The exposed L1-nematodes (72-h exposure) were collected in 1.5-mL RNase-free tubes and washed three times with PBS. Total RNA was isolated using Trizol reagent (Thermo Fisher) (Kamireddy et al. 2018). Only high-quality RNA was used for cDNA synthesis using the qScript cDNA synthesis kit (Bio-Rad). qRT-PCR assays were performed using iTaq Universal SYBR Green Supermix (Bio-Rad) on a CFX Connect™ Real-Time System (Bio-Rad, Hercules, CA, USA). The data were normalized using actin and analyzed using the comparative 2−ΔΔCt method (Wang et al. 2010b). Sequences and amplification efficiency of gene-specific primers are listed in Table S5.
Statistical analysis
The distribution map was created using ArcGIS 10.2 (Environmental Systems Research Institute Inc., CA, USA). Figures were presented using GraphPad Prism 5 (GraphPad Software, CA, USA) or Origin 2018 (OriginLab, MA, USA). By using the SPSS Statistics 25.0 (IBM SPSS, Chicago, USA), a one-way analysis of variance followed by LSD for multiple groups was used to determine the differences between groups when analyzing survival rate and length. A two-tailed Student’s t test was used in Microsoft Excel (Microsoft Corp., WA, USA) to test the difference between control (K-medium) and experimental groups when analyzing antioxidant enzymatic activity and gene expression. The difference was judged significant if the p-values were < 0.05. All values are expressed as means ± standard deviation (SE).
To analyze the relationship between metal concentrations in exposure liquid from soils/sediments and endpoints of nematodes, we adopted the partial least squares regression (PLSR) method in SIMCA 14.1 (Umetrics, Sweden), without considering the collinearity between explanatory variables. The independent variable is the concentration of heavy metals, while the dependent variable is the nematode-related biological indicators. When analyzing biological data, Q2 > 0.4 is acceptable (Westerhuis et al. 2008).
Results
Concentrations of heavy metals in soils, sediments, and water-soluble extracts of soils, sediments
After the soil and sediment samples were digested, we determined the total amount of heavy metals (V, Cr, Ni, Cu, As, Pb, Mn, and Zn) (Fig. 2). The concentrations of five heavy metals including Ni, Cu, As, Pb, and Zn at eight sites were respectively 17.36 to 58.17 mg/kg, 79.03 to 1911.65 mg/kg, 104.34 to 1726.66 mg/kg, 111.43 to 2351.72 mg/kg, and 116.48 to 3275.68 mg/kg, which all exceeded the corresponding soil background values of Guangdong Province (Ni: 14.4 mg/kg, Cu: 17 mg/kg, As: 8.9 mg/kg, Pb: 36 mg/kg, Zn: 47.3 mg/kg). Except in E4, V and Cr concentrations were all above the corresponding soil background levels. The concentrations of Mn at E1, E2, E4, and E5 were higher than the soil background value. The highest concentrations of Ni, Cu, Mn, and Zn were located at E5; those of As and Pb were at E3; and that of V was at S2. The lowest concentration of Cr was at E4. In addition, we also quantified the concentration of heavy metals (V, Cr, Ni, Cu, As Zn, Pb, and Mn) in the leachates and found the heavy metals in the water-soluble extracts were mainly Mn, Cu, Zn, Pb, As, and Ni (Table S6). The concentrations of these six heavy metals in the water-soluble extracts were highest at E3. We only detected the presence of Zn in the E3 and E4 extracts.
Metal concentrations in soils and sediments collected in the Dabaoshan mining area (the figure was created in GraphPad Prism 5). A V concentrations. B Cr concentrations. C Ni concentrations. D Cu concentrations. E As concentrations. F Pb concentrations. G Mn concentrations. H Zn concentrations. Dashed lines represent soil background values in Guangdong Province. Values are expressed as mean ± SE, mg/kg
Assessment of potential ecological risk
To assess the degree of heavy metal pollution in the Dabaoshan mining area, we calculated Ei and RI (Table 1). Overall, the average RI of metals in environmental samples around Dabaoshan Mine was classified as highly contaminated (RI = 721.53). RI was classified as very highly contaminated at E3, highly contaminated at E4 and E5, considerably polluted at S1, S3, and E1, and moderately polluted at S2 and E2. The pollution levels of the eight heavy metals decreased in the following order: As > Cu > Pb > Zn > Ni > V > Cr > Mn. As, Cu, and Pb respectively exhibited very high pollution, high pollution, and moderate pollution. The remaining heavy metals showed low pollution. E3 had the highest potential ecological risk (RI = 2517.94) and exhibited the most severe As pollution (Ei = 1940.07).
Health risk assessment
To further analyze the hazards of heavy metals in mining areas to humans, health risk assessment was carried out. In this study, three populations (men, women, and children) were considered. We calculated the average concentrations of heavy metals in three soil samples collected in Liangqiao Village and assessed the non-carcinogenic risk of six heavy metals (Cr, As, Pb, Ni, Cu, and Zn) and the carcinogenic risk of As. Table 2 presents the results of non-carcinogenic effects. The mean HQO and HQABS of all metals of adults were < 1, which indicated that these elements in soils posed a minimal risk to them. The mean HQO and HQABS of children were < 1 for most metals, although the mean HQO of Pb was > 1. We concluded that the highest risk of non-carcinogenic effects in children was related to Pb exposure (HQO = 1.09, HI = 1.24).
The results of carcinogenic risk related to As are also presented in Table 2. The carcinogenic risk values of As in adults were 1.46 × 10−4 (men) and 1.63 × 10−4 (women) which exceeded the standard value 1 × 10−4 and indicated that ingestion and dermal absorption of soils in Liangqiao Village over the human lifespan could increase the carcinogenic risk. However, the carcinogenic risk value of As for children was 7.06 × 10−5, which was between 1 × 10−4 and 1 × 10−6. Therefore, the carcinogenic risk for children depended on the situation and circumstances of exposure.
Survival rate and body length of C. elegans exposed to water-soluble extracts of soils and sediments
According to the above, it is not enough to analyze the risk of heavy metal pollution based only on the total amount of metals. Therefore, we carried out nematode toxicity experiments with water-soluble extracts to determine the ecotoxicity of the samples. The effect of the extracts on nematode growth and survival is shown in Fig. 3. After 24-h exposure, a significant decrease in survival rate was observed in E3 (1:20 dilution, 1:1000 dilution, 1:1500 dilution, 1:2000 dilution) and E4 (1:20 dilution, 1:50 dilution); this showed a possible dose-dependency (Fig. 3A, B, and C). At the same time, body length of C. elegans was significantly inhibited when exposed to E3 (1:1000 dilution, 1:1500 dilution, 1:2000 dilution) and E4 (1:50 dilution, 1:100 dilution) and exhibited a possible dose-dependency (Fig. 3D, E, and F). In addition, extracts of S3 (1:20 dilution) inhibited the growth of C. elegans. The remaining diluted sample extracts did not significantly affect survival rate and body length.
Survival rate (A, B, C) and body length (D, E, F) of C. elegans exposed to diluted extracts (the figure was created in GraphPad Prism 5). Except for E3 and E4 extracts, all other extracts were diluted 20 times. Values are expressed as means ± SE. *p < 0.05 and **p < 0.01, compared with the control (K-medium) by LSD in SPSS Statistics 25.0
Antioxidant enzymatic activity of C. elegans exposed to water-soluble extracts of soils and sediments
Because extracts from these samples were found to inhibit nematode growth, we further speculated that oxidative stress damage might occur in the exposed nematodes. Therefore, the activity of related enzymes including CAT and SOD was measured. The extracts for E3 (1:1500 dilution) and E4 (1:100 dilution) were chosen by referring to the above results. Other extracts were diluted 20 times for this test. When compared with the control, only E4 extracts (1:100 dilution) resulted in significantly increased CAT (2.38-fold) and SOD (0.65-fold) activities (Fig. 4).
Activity of the antioxidant enzymes CAT (A) and SOD (B) (the figure was created in GraphPad Prism 5). Except for E3 and E4 extracts, all other extracts were diluted 20 times. Values are expressed as means ± SE. *p < 0.05 and **p < 0.01, compared with the control (K-medium) by two-tailed Student’s t test in Microsoft Excel
Gene expression of C. elegans exposed to water-soluble extracts of soils and sediments
To further explore the underlying molecular mechanism, the expression of oxidative stress-related genes (sod-3, ctl-2, gst-4), as well as the expression levels of metallothionein-related genes (mtl-1, mtl-2) and a pro-apoptotic gene (egl-1), was analyzed in exposed C. elegans. As depicted in Fig. 5A and B, the expression of sod-3 was up-regulated after exposure to S1 (1:20 dilution) (2.06-fold) and S2 (1:20 dilution) (2.13-fold) extracts, and the expression of gst-4 was up-regulated after exposure to S1 (1:20 dilution) (0.99-fold) and E4 (1:100 dilution) (6.22-fold) extracts. No significant change on the expression of ctl-2 was observed following exposure to all leachates (Fig. 5C). The expression of egl-1 was up-regulated after exposure to S2 extracts (1:20 dilution) (3.82-fold) (Fig. 5D) and apoptosis was also observed (result not shown). The expression of mtl-1 was up-regulated after exposure to S1 (1:20 dilution) (4.80-fold) and S2 (1:20 dilution) (3.05-fold) extracts (Fig. 5E) and the expression of mtl-2 was up-regulated after exposure to S1 (1:20 dilution) (2.58-fold), E2 (1:20 dilution) (4.19-fold), E3 (1:1500 dilution) (6.40-fold), and E5 (1:20 dilution) (1.25-fold) extracts (Fig. 5F).
Changes in gene expression levels of nematodes exposed to diluted extracts (the figure was created in GraphPad Prism 5). A sod-3, B gst-4, C ctl-2, D mtl-1, E mtl-2, F egl-1. Except for E3 and E4 extracts, all other extracts were diluted 20 times. Values are expressed as means ± SE. *p < 0.05 and **p < 0.01, compared with the control (K-medium) by two-tailed Student’s t test in Microsoft Excel
Links between heavy metals in exposure liquids and endpoints of C. elegans
Combining the overall pollution level of heavy metals in the Dabaoshan mining area and related toxic effects of the polluted soils or sediments, PLSR analysis was performed to determine which heavy metals had the most impact on the nematode endpoints. To achieve strong prediction ability of the PLSR model for the eight endpoint changes (survival, length, SOD activity, CAT activity, sod-3, gst-4, egl-1, and mtl-2 gene expression), the number of components required is summarized in Table 3. In the survival model, we extracted three components that accounted for 99.2% of the survival variation. In models containing length, SOD activity, CAT activity, and sod-3 expression, two components were extracted that accounted for 89.1%, 85.5%, 93.3%, and 93.1% of the corresponding variability in endpoint changes. In gst-4, egl-1, and mtl-2 expression models, only one component was extracted. The models for gst-4, egl-1, and mtl-2 expression could explain 76.6%, 65.8%, and 78.8% of the corresponding variability in gene expression, respectively.
Variable importance for the projection (VIP) values and regression coefficients (RCs) in Fig. 6 reflect the relative importance of explanatory variables. Predictors with VIP values < 1 are not important for prediction purposes; therefore, the discussion is limited to variables with VIP values > 1. Pb had a significant negative impact on the nematodes survival and growth. The higher the Pb content, the greater the inhibitory effect on survival and growth, as reflected by the negative RCs (Fig. 6A and B). Cu content was also negatively correlated with nematode growth (Fig. 6B). In the SOD activity model, the increase in enzymatic activity was associated with high Pb, As, and Ni concentrations (Fig. 6C). However, the increase in CAT activity was associated with high Cu and Cr concentrations (Fig. 6D). In the gene expression models (Fig. 6E–H), higher expression of sod-3 was correlated with higher V and As concentrations; higher expression of gst-4 and egl-1 was both correlated with higher V, Cr, and As concentrations; and higher expression of mtl-2 was correlated with higher Cu and Pb concentrations.
The projected importance (VIP, bar graph) and regression coefficients (RCs, lines) of each predictor of the change in the biological endpoint of nematodes (the figure was created in Origin 2018). A Survival; B length; C SOD activity; D CAT activity; E sod-3; F gst-4; G egl-1; H mtl-2. All predictors were ranked, and the dotted line represents the threshold above which the predictors are considered to be important for explanatory purposes
Discussion
As a result of the long-term discharge of AMD into the river from Dabaoshan Mine, local agricultural water and drinking water have been contaminated with high levels of toxic heavy metals (Chen et al. 2007). In the current study, we analyzed eight river sediments and farmland samples along the flow direction of tailing wastewater and Fandong Reservoir to provide a full investigation into the pollution characteristics of the Dabaoshan mining area. Generally, the risk of heavy metals in the Dabaoshan mining area was very high due to serious As, Pb, and Cu pollution. We also found that the potential ecological risk degree of Pb, Cu, and Zn was ranked as Cu > Pb > Zn, which was consistent with a previous study (Shu et al. 2018). The concentration of Zn in the samples from this mining area was very high. However, the presence of Zn was only detected in the extracts of E3 and E4. We guessed this was related to the distance from AMD (E3 and E4 were located at AMD and its downstream (2.9 km), respectively). Local wastewater treatment might also play an important role. Additionally, the potential ecological risks of heavy metals in these samples were related to geographical location. First, E3 came from the pollution source of acid wastewater discharge; therefore, the pH of leachates was very low. The use of quicklime in the treatment process caused the alkalinity of E4 to increase. This was consistent with the highest ecological risk of E3. Second, the potential ecological risk of heavy metals at E3 and its downstream samples (E4 and E5) was higher than that of the Fandong Reservoir sample (E1) and its downstream sample (E2). Although the sewage treatment plant treated the wastewater from E3, its scale was too small, so the downstream sediments (E4 and E5) still had a high risk. Finally, Liangqiao Village was about 3.6 km away from the mining area, and its farmlands (S1, S2, S3) were moderately or heavily polluted, which was consistent with a previous study that showed farmland contamination was caused by irrigation of Hengshi River water which was polluted by AMD (Zhao et al. 2012). Although the total number of samples was relatively small, representative sampling points were selected according to the direction of water flow, and degree of heavy metal pollution of various heavy metals was analyzed in soils and sediments, providing valuable information for studying the migration of toxic heavy metals in AMD.
For plants, exposure to heavy metals can reduce chlorophyll content and seed germination rate (Zeng et al. 2012). For people, exposure to heavy metals can reduce energy levels and damage the function of the brain, lungs, kidneys, and liver, and even cause cancer (Jaishankar et al. 2014). Our research showed substantial health risks of As in these samples, especially in terms of carcinogenic risk, which was higher than that of a previous study which reported the carcinogenic risk of As was between 2 × 10−5 and 5 × 10−5 (the EPA accepts risks in the range of 10−6 to 10−4) (Li et al. 2014). These might be because our research area was smaller and closer to Dabaoshan Mine. Furthermore, we found that the non-carcinogenic risk for children was higher than that for adults from these samples. This might be caused by children’s behavioral and physiological characteristics, such as soil activity from hand to mouth, higher respiration rate, and increased gastrointestinal absorption of certain substances (Li et al. 2002). Therefore, it is recommended that children should reduce outdoor activities in these contaminated areas.
The biological effects observed in the mixed metal exposure in the laboratory cannot be used to predict the biological toxicity effects of the complex environment directly (Kumar et al. 2015). C. elegans have been used for rapid assessment of heavy metal toxicity in acidic environments. Previous studies reported that heavy metals could induce the death of C. elegans and inhibit its growth, which was also observed in our study (Tiwari et al. 2020; Wang et al. 2009). In addition, the toxicity of the extracts was also related to geographic location, that was, the survival rates and body length of nematodes exposed to E3 leachate were lower than those exposed to E4 leachate. From the PLSR model of phenotype index (survival and body length), the key heavy metals that inhibited the survival and growth of nematodes were identified, and the explanation rate was as high as 90%. Pb had a significant negative effect on survival and growth, and growth was also regulated by Cu. When compared with a previous study where Spearman’s rank correlations were used to assess the association between metals and toxicity endpoints, our analysis was more comprehensive and empiric (Tejeda-Benitez et al. 2016).
SOD and CAT enzymes are the primary antioxidant enzymes in cells (Wang et al. 2019a). In our study, we found elevated activity of SOD and CAT enzymes in nematodes exposed to the extracts. Through the PLSR model, we further found that their activity increased with the increase in exposure concentration of corresponding heavy metals (SOD: Ni, As, Pb; CAT: Cu, Cr). A previous study reported that the increase in enzymatic activity was to quickly remove superoxide free radicals and H2O2 generated during the body’s metabolism to protect cells from stress damage (Song et al. 2014). In addition, the expression of oxidative stress-related genes was up-regulated and increased with the increase in heavy metal concentration. Many studies have combined and correlated ROS assays and gene expression changes when analyzing oxidative damage (Xiao et al. 2018), while our study measured related enzymatic activity and gene expression. The results of this study confirmed from the molecular level and biochemical level that the sample extracts contaminated by heavy metals could induce oxidative stress and produce toxic effects. Our PLSR model results were also consistent with experimental results. In addition to increasing the level of oxidative stress, the over-expression of heavy metal-induced metallothionein genes could protect cells from reactive oxygen species (Zeitoun-Ghandour et al. 2011) and the up-regulation of a pro-apoptotic gene could eliminate damaged cells (Craig et al. 2012). While the expression of mtl-2 was not induced by coal mining area samples in a previous study, which was different from our study (Turner et al. 2013). This might be because the concentration of heavy metals such as Cu that effectively induced the expression of metallothionein was at low levels in coal mining area samples.
In this study, we used C. elegans as a well-established model organism to evaluate ecological and human health risk of heavy metals in different locations of Dabaoshan mining area and applied PLSR models to analyze the relationship between the concentrations of heavy metals in extracts and toxicity endpoints of exposed C. elegans. As, Cu, and Pb respectively exhibited very high pollution, high pollution, and moderate pollution according to the potential ecological risk assessment result. In addition, a previous study reported that even if the well water in the mining area was diluted 51 times, the 24-h lethality rate of Daphnia carinata was 82% (Chen et al. 2007). The carcinogenic risk of As for adults was higher than 10−4, indicating that ingestion and dermal absorption from soils in Liangqiao Village over a long lifetime could increase the probability of cancer. While the high risk of non-carcinogenic effects in children was related to Pb exposure. PLSR results showed that the high-concentration heavy metals in the water-soluble extracts inhibited the survival rate and growth of C. elegans. Gene expression and enzymatic activity related to oxidative stress were increased. In addition, genes related to apoptosis and metallothionein were also affected. This study provided evidence on the toxicity of soil and sediment extracts in the Dabaoshan mining area and both provided a reliable method for environmental health risk assessment in similar regions.
Data availability
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request (Ling Wang: wangling4212@aliyun.com).
References
Adewumi AJ, Laniyan TA (2020) Contamination, sources and risk assessments of metals in media from Anka artisanal gold mining area, Northwest Nigeria. Sci Total Environ 718:137235. https://doi.org/10.1016/j.scitotenv.2020.137235
Akcil A, Koldas S (2006) Acid Mine Drainage (AMD): causes, treatment and case studies. J Clean Prod 14:1139–1145. https://doi.org/10.1016/j.jclepro.2004.09.006
Ali SH, Giurco D, Arndt N, Nickless E, Brown G, Demetriades A, Durrheim R, Enriquez MA, Kinnaird J, Littleboy A (2017) Mineral supply for sustainable development requires resource governance. Nature 543(7645):367. https://doi.org/10.1038/nature21359
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Chapman EEV, Dave G, Murimboh JD (2013) A review of metal (Pb and Zn) sensitive and pH tolerant bioassay organisms for risk screening of metal-contaminated acidic soils. Environ Pollut 179:326–342. https://doi.org/10.1016/j.envpol.2013.04.027
Chen A, Lin C, Lu W, Wu Y, Ma Y, Li J, Zhu L (2007) Well water contaminated by acidic mine water from the Dabaoshan Mine, South China: chemistry and toxicity. Chemosphere 70:248–255. https://doi.org/10.1016/j.chemosphere.2007.06.041
Chen FJ, Wei CY, Chen QY, Zhang J, Wang L, Zhou Z, Chen MJ, Liang Y (2018a) Internal concentrations of perfluorobutane sulfonate (PFBS) comparable to those of perfluorooctane sulfonate (PFOS) induce reproductive toxicity in Caenorhabditis elegans. Ecotoxicol Environ Saf 158:223–229. https://doi.org/10.1016/j.ecoenv.2018.04.032
Chen YX, Jiang XS, Wang Y, Zhuang DF (2018b) Spatial characteristics of heavy metal pollution and the potential ecological risk of a typical mining area: a case study in China. Process Saf Environ Prot 113:204–219. https://doi.org/10.1016/j.psep.2017.10.008
CNEMC (1990) Background values of elements in soils of China. China Environmental Science Press, Beijing
Craig AL, Moser SC, Bailly AP, Gartner A (2012) Methods for studying the DNA damage response in the Caenorhabdatis elegans germ line. In: Rothman JH, Singson A (eds) Methods in Cell Biology. 107. Academic Press, pp. 321–352.https://doi.org/10.1016/B978-0-12-394620-1.00011-4
Daraz U, Li Y, Sun Q, Zhang M, Ahmad I (2021) Inoculation of Bacillus spp. Modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mine drainage. Chemosphere 263:128345.https://doi.org/10.1016/j.chemosphere.2020.128345
Hagner M, Romantschuk M, Penttinen OP, Egfors A, Marchand C, Augustsson A (2018) Assessing toxicity of metal contaminated soil from glassworks sites with a battery of biotests. Sci Total Environ 613–614:30–38.https://doi.org/10.1016/j.scitotenv.2017.08.121
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. https://doi.org/10.2478/intox-2014-0009
Jiang Y, Chen J, Wu Y, Wang Q, Li H (2016) Sublethal toxicity endpoints of heavy metals to the nematode Caenorhabditis elegans. PLoS ONE 11:e0148014–e0148014. https://doi.org/10.1371/journal.pone.0148014
Jiang YX, Chao SH, Liu JW, Yang Y, Chen YJ, Zhang AC, Cao HB (2017) Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 168:1658–1668. https://doi.org/10.1016/j.chemosphere.2016.11.088
Kamireddy K, Chinnu S, Priyanka PS, Rajini PS, Giridhar P (2018) Neuroprotective effect of Decalepis hamiltonii aqueous root extract and purified 2-hydroxy-4-methoxy benzaldehyde on 6-OHDA induced neurotoxicity in Caenorhabditis elegans. BiomedPharmacother 105:997–1005. https://doi.org/10.1016/j.biopha.2018.06.002
Khanna N, Cressman CP III, Tatara CP, Williams PL (1997) Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media. Arch Environ Contam Toxicol 32:110–114. https://doi.org/10.1007/s002449900162
Kumar R, Pradhan A, Khan FA, Lindström P, Ragnvaldsson D, Ivarsson P, Olsson PE, Jass J (2015) Comparative analysis of stress induced gene expression in Caenorhabditis elegans following exposure to environmental and lab reconstituted complex metal mixture. PLoS ONE 10:e0132896. https://doi.org/10.1371/journal.pone.0132896
Leung MCK, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN (2008) Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci 106:5–28. https://doi.org/10.1093/toxsci/kfn121
Li ZY, Ma ZW, van der Kuijp TJ, Yuan ZW, Huang L (2014) A review of soil heavy metal pollution from mines in China, Pollution and health risk assessment. Sci Total Environ 468–469:843–853. https://doi.org/10.1016/j.scitotenv.2013.08.090
Li SY, Zhang QF (2010) Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China. J Hazard Mater 181:1051–1058. https://doi.org/10.1016/j.jhazmat.2010.05.120
Lin WT, Wu KM, Lao ZL, Hu W, Lin BJ, Li YL, Fan HB, Hu JJ (2019) Assessment of trace metal contamination and ecological risk in the forest ecosystem of dexing mining area in northeast Jiangxi Province, China. Ecotoxicol Environ Saf 167:76–82. https://doi.org/10.1016/j.ecoenv.2018.10.001
Liu Y, Lin C, Ma Y, Lu W, Wu Y, Huang S, Zhu L, Li J, Chen A (2009) Toxic effects of two acid sulfate soils from the Dabaoshan Mine on Corymbia citriodora var. variegata and Daphnia carinata. J Hazard Mater 166:1162–1168. https://doi.org/10.1016/j.jhazmat.2008.12.017
Man YB, Sun XL, Zhao YG, Lopez BN, Chung SS, Wu SC, Cheung KC, Wong MH (2010) Health risk assessment of abandoned agricultural soils based on heavy metal contents in Hong Kong, the world’s most populated city. Environ Int 36:570–576. https://doi.org/10.1016/j.envint.2010.04.014
Rai N, Sjöberg V, Forsberg G, Karlsson S, Olsson PE, Jass J (2019) Metal contaminated soil leachates from an art glass factory elicit stress response, alter fatty acid metabolism and reduce lifespan in Caenorhabditis elegans. Sci Total Environ 651:2218–2227. https://doi.org/10.1016/j.scitotenv.2018.10.067
Schultz E, Joutti A, Räisänen ML, Lintinen P, Martikainen E, Lehto O (2004) Extractability of metals and ecotoxicity of soils from two old wood impregnation sites in Finland. Sci Total Environ 326:1–84. https://doi.org/10.1016/j.scitotenv.2003.12.008
Shu XH, Zhang Q, Lu GN, Yi XY, Dang Z (2018) Pollution characteristics and assessment of sulfide tailings from the Dabaoshan Mine, China. Int Biodeterior Biodegrad 128:122–128. https://doi.org/10.1016/j.ibiod.2017.01.012
Song SJ, Zhang XY, Wu HH, Han Y, Zhang JZ, Ma EB, Guo YP (2014) Molecular basis for antioxidant enzymes in mediating copper detoxification in the nematode Caenorhabditis elegans. PLoS ONE 9(9):e107685. https://doi.org/10.1371/journal.pone.0107685
Tejeda-Benitez L, Flegal R, Odigie K, Olivero-Verbel J (2016) Pollution by metals and toxicity assessment using Caenorhabditis elegans in sediments from the Magdalena River, Colombia. Environ Pollut 212:238–250. https://doi.org/10.1016/j.envpol.2016.01.057
Tejeda-Benítez L, Noguera-Oviedo K, Aga DS, Olivero-Verbel J (2018) Toxicity profile of organic extracts from Magdalena River sediments. Environ Sci Pollut Res 25:1519–1532. https://doi.org/10.1007/s11356-017-0364-9
Tiwari S, Tambo F, Agarwal R (2020) Assessment of lead toxicity on locomotion and growth in a nematode Caenorhabditis elegans. J Appl Nat Sci 12:36–41. https://doi.org/10.31018/jans.v12i1.2227
Turner EA, Kroeger GL, Arnold MC, Thornton BL, Di Giulio RT, Meyer JN (2013) Assessing different mechanisms of toxicity in mountaintop removal/valley fill coal mining-affected watershed samples using Caenorhabditis elegans. PLoS ONE 8:e75329. https://doi.org/10.1371/journal.pone.0075329
USEPA (1989) Risk assessment guidance for superfund. Human Health evaluation manual, (part A) [R], vol. 1. Washington, DC: Office of emergency and remedial response. [EPA/540/1–89/002]
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 290:111197. https://doi.org/10.1016/j.molliq.2019.111197
Wang Q, Liu BZ, Yang HS, Wang XY, Lin ZH (2009) Toxicity of lead, cadmium and mercury on embryogenesis, survival, growth and metamorphosis of Meretrix meretrix larvae. Ecotoxicology 18:829–837. https://doi.org/10.1007/s10646-009-0326-1
Wang XF, Miao JJ, Pan LQ, Li Y, Lin YF, Wu JY (2019a) Toxicity effects of p-choroaniline on the growth, photosynthesis, respiration capacity and antioxidant enzyme activities of a diatom, Phaeodactylum tricornutu. Ecotoxicol Environ Saf 169:654–661. https://doi.org/10.1016/j.ecoenv.2018.11.015
Wang Y, Dong R, Zhou YZ, Luo X (2019b) Characteristics of groundwater discharge to river and related heavy metal transportation in a mountain mining area of Dabaoshan, Southern China. Sci Total Environ 679:346–358. https://doi.org/10.1016/j.scitotenv.2019.04.273
Westerhuis JA, Hoefsloot HCJ, Smit S, Vis DJ, Smilde AK, van Velzen EJJ, van Duijnhoven JPM, van Dorsten FA (2008) Assessment of PLSDA cross validation. Metabolomics 4:81–89. https://doi.org/10.1007/s11306-007-0099-6
Xiang L, Liu PH, Jiang XF, Chen PJ (2019) Health risk assessment and spatial distribution characteristics of heavy metal pollution in rice samples from a surrounding hydrometallurgy plant area in No. 721 uranium mining, East China. J GeochemExplor 207:106360. https://doi.org/10.1016/j.gexplo.2019.106360
Xiao GS, Zhao L, Huang Q, Yang JN, Du HH, Guo DQ, Xia MX, Li GM, Chen ZX, Wang DY (2018) Toxicity evaluation of Wanzhou watershed of Yangtze Three Gorges Reservior in the flood season in Caenorhabditis elegans. Sci Rep 8:6734–6734. https://doi.org/10.1038/s41598-018-25048-w
Xu ZQ, Ni SJ, Tuo XG, Zhang CJ (2008) Calculation of heavy metal’s toxicity coefficient in the evaluation of Potential Ecological Risk Index. Environ Sci Technol 31:112–115 (In Chinese with English abstract)
Xu TT, Zhang MK, Hu JN, Li ZH, Wu TP, Bao JN, Wu SY, Lei LL, He DF (2017) Behavioral deficits and neural damage of Caenorhabditis elegans induced by three rare earth elements. Chemosphere 181:55–62. https://doi.org/10.1016/j.chemosphere.2017.04.068
Zeitoun-Ghandour S, Leszczyszyn O, Blindauer C, Geier F, Bundy J, Stürzenbaum S (2011) C. elegans metallothioneins: response to and defence against ROS toxicity. Mol BioSyst 7:2397–2406. https://doi.org/10.1039/c1mb05114h
Zeng FR, Qiu BY, Wu XJ, Niu SZ, Wu FB, Zhang GP (2012) Glutathione-mediated alleviation of chromium toxicity in rice plants. Biol Trace Elem Res 148:255–263. https://doi.org/10.1007/s12011-012-9362-4
Zhao HR, Xia BC, Fan C, Zhao P, Shen SL (2012) Human health risk from soil heavy metal contamination under different land uses near Dabaoshan Mine, Southern China. Sci Total Environ 417–418:45–54. https://doi.org/10.1016/j.scitotenv.2011.12.047
Zheng XZ, Wang RR, Wood R, Wang C, Hertwich EG (2018) High sensitivity of metal footprint to national GDP in part explained by capital formation. Nat Geosci 11:269–273. https://doi.org/10.1038/s41561-018-0091-y
Zhou JM, Dang Z, Cai MF, Liu CQ (2007) Soil heavy metal pollution around the Dabaoshan Mine, Guangdong Province, China. Pedosphere 17:588–594. https://doi.org/10.1016/S1002-0160(07)60069-1
Zhu DW, Wei Y, Zhao YH, Wang QL, Han JC (2018) Heavy metal pollution and ecological risk assessment of the agriculture soil in Xunyang Mining Area, Shaanxi Province, Northwestern China. Bull Environ Contam Toxicol 101:178–184. https://doi.org/10.1007/s00128-018-2374-9
Zhuang P, Lu H, Li Z, Zou B, McBride MB (2014) Multiple exposure and effects assessment of heavy metals in the population near mining area in South China. PLoS ONE 9:e94484. https://doi.org/10.1371/journal.pone.0094484
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant number: 21906069 and 21777061) and the Open Fund of Hubei Key Laboratory of Environmental and Health Effects of Persistent Toxic Substance (PTS2020-3).
Author information
Authors and Affiliations
Contributions
Xin Li performed experiments and statistical analysis and wrote original draft. Qingqing Yang performed chemical analysis. Ling Wang designed experiments and revised the draft. Chuxin Song performed biological experiments. Lufeng Chen provided technical and editorial assistance. Jie Zhang conceived and designed the experiment. Yong Liang conceived the experiment. And all authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Our research does not involve animal or human experiments, so ethics approval is not applicable.
Consent for publication
Our manuscript does not contain data from any individual person and its publication is approved by all authors. Therefore, the section “Consent for publication” is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Yang, Q., Wang, L. et al. Using Caenorhabditis elegans to assess the ecological health risks of heavy metals in soil and sediments around Dabaoshan Mine, China. Environ Sci Pollut Res 29, 16332–16345 (2022). https://doi.org/10.1007/s11356-021-16807-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16807-w