Abstract
Although studies have already shown the effects of exposure to microplastics (MP) in different species, the effects over generations in these individuals remain poorly understood. Therefore, the present study aimed to evaluate the effect of polystyrene MP (spherical, 1 μm) on the responses of the free-living nematode Caenorhabditis elegans in a multigenerational approach over five subsequent generations. MP concentrations of both 5 and 50 μg/L induced a detoxification response, increasing glutathione S-transferase (GST) activity and inducing the generation of reactive oxygen species (ROS) and lipid peroxidation (TBARS). MP also demonstrated the ability to accumulate in the animal’s body during the 96 h of each generational exposure, and possibly, this constant interaction was the main reason for the decreased response in physiological parameters as in the exploratory behavior (body bending) of nematodes, and in the reproduction, being this last parameter most negatively affected during the five exposed generations, with a reduction of almost 50% in the last generation. These results emphasize the importance of multigenerational approaches, highlighting their advantage in the assessment of environmental contaminants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic disposal is a threat to ecosystems worldwide. Based on the last estimate of global plastic production, approximately 12 billion tons are predicted to accumulate in landfills and/or marine environments by the 2050s (Geyer et al. 2017). The accumulation of plastics in the environment can come from fixed or diffuse sources and is subject to the action of several physical, chemical, and biological factors (Ryan et al. 2009), particularly weathering (Chen et al. 2022). These factors can change the characteristics of plastics, such as size, since plastics can be fragmented into smaller particles, thus giving rise to microplastics (MP) in a secondary process of formation (Andrady 2011; Anik et al. 2021). The primary occurrence of MP in the environment is from the introduction of manufactured MP used in cosmetics and other products, such as cleaning (washing machine) and personal hygiene (toothpaste or gel) products (Andrady 2011; Lei et al. 2017). The most common MP in wastewater are polyethylene terephthalate (PET), polyethylene (PE), polypropylene (PP), and polystyrene (PS) (Zhang et al. 2020), which may exert different effects on organisms.
Reactive oxygen species (ROS) are naturally formed during cellular respiration; however, stressful situations such as MP exposure can induce an increase in these reactive species and change the redox homeostasis of cells (Sies and Jones 2020), altering the relation of antioxidants/pro-oxidants favoring the oxidative stress (OS) situation. Also, OS can influence behavioral, physiological, and morphological parameters related to aging disorders, such as decreased locomotion, health span, and lifespan (Qiu et al. 2020). Yu et al. (2020) observed that polystyrene microplastics (PS-MP) of 1 μm (100 μg/L) can induce OS, where high levels of ROS, lipofuscin accumulation, and altered expression of OS-related genes resulted in intestinal morphological disturbances. In addition, Lei et al. (2018a) observed that 2 days of 1 μm PS-MP exposure can enhance the expression of the gene gst-4 in the intestine of Caenorhabditis elegans and is responsible for decreased levels of Ca2+ in the intestinal tissue, which is related to high intestinal permeability, favoring MP accumulation. Schöpfer et al. (2020) observed a lower reproduction rate and reduced body length growth in C. elegans exposed to low-density polyethylene for 6 days.
Some studies have assessed MP effects in different generations, Chen et al. (2021) observed that 1 μm PS-MP (10 and 100 μg/L) parental exposure can induce transgenerational OS in C. elegans, with transference of MP to subsequent progeny and the persistence of the pro-oxidant conditions until the second non-exposed generation (augmented ROS production and lipofuscin accumulation).
Multigenerational studies are those where every subsequent generation is exposed (Josende et al. 2019a). Thus, multigenerational studies are used to highlight the non-observed effects of one generational exposure or when the tested concentrations are too low for the effects to be detected under acute or chronic approaches covering just one generation. Moreover, testing substances, drugs, and contaminants in realistic environmental concentrations is more feasible at different generations.
The nematode C. elegans is one of the most ideal model organisms for this purpose (Josende et al. 2019a). The adult C. elegans is approximately 1 mm in length, completely transparent, and mostly hermaphroditic (99.5%). C. elegans has a very rapid development cycle (72 h between embryonic/larval development to adult stage), short life expectancy (approximately 2–3 weeks), high reproductive rate (about 300 larvae/fertile adult), and fully sequenced genome (which is highly homologous with the human genome). Moreover, it is economically viable and requires simple maintenance compared with other biological models (Gonzales-Moragas et al. 2015).
Therefore, the aim of the present study was to evaluate the biochemical and physiological multigenerational effects of 1 μm spheric PS-MP at significant concentrations (5 and 50 μg/L) in five generations of a C. elegans population. We hypothesized that the PS-MP multigenerational exposure could directly induce deleterious oxidative effects in the C. elegans population, affecting biochemical biomarkers related to OS, and this oxidative scenario would also indirectly promote alterations in physiological biomarkers.
Materials and methods
Microplastic characterizations, suspension preparing, and experimental concentrations
Spherical polystyrene microparticles (PS-MP) (diameter, 1.1 μm; density 1.05 g/mL) were purchased from Sigma-Aldrich (LB11, lot number MKCK1497). Spherical fluorescent red polystyrene microparticles (FRPS-MP) (diameter, 1 μm; density, 1.05 g/mL), exclusively used for MP accumulation, were also purchased from Sigma-Aldrich (L2778, lot number MKCG4344). Physicochemical properties from PS-MP are given as follows: scanning electron microscopy (SEM) utilizing the JEOL JSM 6610 microscopy (Fig. S1 – Supplementary material); dynamic light scattering (DLS) indicated a hydrodynamic diameter of 1.84 μm ± 0.2 μm for PS-MP with a polydispersity index of 29.44% and 0.83 μm ± 0.28 μm for FRPS-MP with a polydispersity index of 12.78% (Litesizer™ 500). Potential Zeta registered was − 25.4 mV ± 0.43 mV for PS-MP and − 8.9 mV ± 0.34 m mV ± for FRPS-MP (Litesizer™ 500). The UV-vis showed fluorescent intensities in the range of 465–500 nm of excitation and 540–630 nm of emission. To avoid agglomeration and improve dispersion, the liquid PS-MP suspension used in the experiments was prepared with ultrapure water and sonicated (Eco-sonics Q3.0/40A, frequency 40 kHz) for 30 min before each generational exposure. The experimental concentrations for both PS-MP and FRPS-MP (5 and 50 μg/L) were based in pilot experiment where we assessed growth parameter in animals exposed to five different concentrations (1, 5, 10, 50, and 100 μg/L) of PS-MP (data not shown).
In vivo characterization/qualitative accumulation analysis of FRPS-MP
Prior to the experiment, we verified whether MP had the capacity to accumulate in exposed C. elegans. FRPS-MP suspensions (5 and 50 μg/L) were prepared, and 10 animals for each concentration in triplicate (n = 30 animals/group) were exposed to each concentration for 96 h (exposure time for one-generation assessment). Then, the animals were washed with S-Base liquid culture medium (3 g/L NaCl, 1 mL of 1 M CaCl2, 1 mL of 1 M MgSO4, 25 mL of 1 M K2HPO4 buffer, and 1 mL of 0.005 g/L cholesterol), centrifuged (1800 ×g, 5 min at 20 °C), and resuspended to prevent the MP from accumulating in the outer surface of animals. Next, FRPS-MP accumulation was confirmed by placing the animals in a 96 culture cells well-plate, and their bodies were inspected for fluorescent signals under an Olympus IX81 inverted epifluorescence microscope coupled with an Olympus DP72 CCD camera using a filter set of 510 nm for excitation and 550 nm for emission. The animals were anesthetized with 10 μL of NaN3 (20 μmol), and pictures were taken at 100, 200, and 400× magnification).
Caenorhabditis elegans
N2 Bristol C. elegans (wild strain) were cultivated in nematode growth medium (NGM) containing 17 g/L bacteriological agar, 3g/L NaCl, 2.5 g/L peptone, 25 mL of 0.5 M KH2PO4 buffer (pH 6), 1 mL of 1 M MgSO4, 1 mL of 1 M CaCl2, 1 mL of 0.005 g/L cholesterol (dissolved in ethanol), and 1 mL antibiotic/antimycotic solution (100×; Sigma- Aldrich). Petri dishes for nematode cultivation were filled with NGM to two-thirds of their volume and seeded with Escherichia coli bacteria (E. coli OP50; non-pathogenic strain) as a food source for the nematodes at an optical density of 1 (600 nm). Prior to the experiment, the animals in the culture plates were synchronized with caustic solution (0.5% NaClO and 0.8 M NaOH) to restrict the population to fertilized eggs (embryonic developmental stage). After this procedure, the obtained eggs were placed in Petri dishes containing NGM until hatching, after which E. coli OP50 was added. The Petri dishes were placed in an incubator at 20 °C for another 96 h until the experiment was started.
Multigenerational assay
On the day of exposure, the culture plates were washed with S-Base liquid medium to obtain the animals. The obtained nematode suspension was filtered (physical synchronization) through a 25-μm pore mesh to restrict the population to the L1 stage (this procedure was performed to avoid any possible effect caused by chemical synchronization). After synchronization, the animal population was estimated using the circular estimate method (CEM), designed in our laboratory, with high accuracy and precision (Josende et al., 2019b). Approximately 10,000 animals were placed in each Petri dish (100 mm in diameter, model K13-Kasvi) (three plates for each treatment) at the beginning of each generation in different experimental groups: control group (animals seeded with E. coli OP50 and S-Base liquid medium); MP5 group (animals seeded with E. coli OP50, S-Base liquid medium, and 5 μg/L of MP-PS), and MP50 group (animals seeded with E. coli OP50, S-Base liquid medium, and 50 μg/L of MP-PS). The experiments were performed in NGM with a thin layer of S-Base liquid medium (1.65 mL), which simulated the natural environment of the animals.
The multigenerational exposure was started immediately at F0 (first generation of exposed animals) and continued through new exposures in generations F1, F2, F3, and F4 for 96 h; five subsequent generations were tested in total at the end of the experiment. At each transition from a previous generation to the subsequent generation, the animal population from each Petri dish was collected in a 50-mL Falcon tube, and three samples of 250 μL of this nematode suspension were used for the reproduction analysis. The contents from the Falcon tube were filtered to separate the L1 larval stage from the remaining population. The L1 population from each plate was again estimated to begin the following generation with another 10,000 animals. The remaining population was collected and randomly separated into 25 animals from each plate for growth, behavioral, and PS-MP accumulation analysis (10, 5, and 10 animals, respectively). The remaining sample was aliquoted into five samples for biochemical analysis. The experiment was performed in triplicate.
Biochemical analysis
Sample preparation
Samples containing animals were homogenized by sonication (QSonica, model Q125; power, 125 W). The nematode suspension samples (S-Base liquid medium at 1:1 volume) were kept in an ice bath with the following sonication protocol: 50% amplitude, 40-s duration. After this process, the samples were centrifuged at 10,000 × g for 20 min at 4 °C. The protein content in each collected supernatant was quantified in a microplate reader at 600 nm using a commercial kit (Microprote Pyragollol; Bioclin). Finally, the samples were stored in an ultra-freezer at − 80 °C for further biochemical analysis, except for the quantification of ROS levels, which needs to be evaluated in fresh samples.
Levels of ROS
The quantification of reactive oxygen species (ROS) accumulation levels was performed in a 96-well plate using a kinetic assay for 120 min in fresh samples of the homogenate obtained after each generation. 2,7′-Dichlorodihydrofluorescein (H2DCF-DA) was used as fluorophore because in the presence of ROS, this compound generates a detected fluorochrome using a fluorimeter with microplate reader (Victor 2, PerkinElmer) with wavelengths of 485 and 535 nm for excitation and emission, respectively (Viarengo et al. 1997 with adaptations by Josende et al. 2019a). The results were expressed in arbitrary units (AU).
Levels of TBARS
Lipid peroxide levels were determined by the reaction of malondialdehyde (MDA) with thiobarbituric acid (TBA, JT Baker) (Oakes and Van der Kraak 2003). Under acidic pH and high-temperature conditions, this reaction produces a chromogen that can be quantified by fluorimetry (FilterMax F5; Molecular Devices) using 520 and 580 nm waves for excitation and emission, respectively. We used tetramethoxypropane (TMP, Sigma-Aldrich) as a standard. The results were expressed as nmol/thiobarbituric acid reactive substances (TBARS)/mg protein.
CAT activity
To determine catalase (CAT) activity, decomposition of H2O2 (50 mM) was quantified at 240 nm for each sample (Beutler 1975). The results obtained were expressed as CAT units (1 unit corresponding to the hydrolysis of 1 μmol of H2O2/min and per mg/protein at 25 °C and pH 8.0).
GST activity
Glutathione S-transferase (GST) activity was determined by conjugating 1 mM reduced gluthatione GSH with 1 mM 1-chloro-2,4-dinitrobenzene (CDNB; Sigma-Aldrich) (Habig and Jakoby 1981). This conjugation generates a complex at 340 nm, which was detected using a spectrophotometer (ELx 800, BioTek), and the results were expressed as nmol/CDNB conjugated per min/mg of protein.
Physiological analysis
Growth
For the growth analysis, 10 animals were randomly collected every 96 h (new generation) and the growth of the animals was determined by measuring the body length. The measurements were performed using the free software ImageJ. Results were expressed as growth length (mm) (Josende et al. 2019a).
Behavior
At the end of each generation, five animals per plate were randomly selected for behavioral analysis. The animals were placed into a 96-well plate filled with 50 μL S-Base and filmed for 3 min with a smartphone (Motorola One Fusion; 48 mega pixels camera). For the analysis, the first minute was considered the recovery time before assessment and was, therefore, discarded. The following 60 s were converted to slow motion (Movavi software; 100 frames per second), and the number of body bends was counted. Each body bending was considered a complete alteration in the bending direction observed from the mid-body region. Data were expressed as the number of complete swimming moves per animal.
Reproduction
At the end of each generation, the plates were washed using 50 mL of S-Base liquid medium and three 250 μL aliquots of each plate were collected for reproduction assessment, which was estimated by CEM (Josende et al. 2019b). The results were expressed as the number of offspring per fertile adult, as described by Josende et al. (2019a).
Statistical analysis
The results (biochemical and physiological) obtained in each generation were first tested for normality and homoscedasticity and then analyzed by one-way ANOVA to compare statistical differences within each generation. Statistical relevance across the five generations was assessed using analysis of covariance (ANCOVA). For both cases, Newman–Keuls post-test was adopted. The level of significance was set at 5% (α = 0.05) (Zar 1984).
Results
The present study was designed to compare the outcomes of two PS-MP concentration (5 and 50 μg/L) groups and the control group for each generation, as well as the outcomes obtained for the five different generations exposed. Thus, each figure in this study provides the results reported for each analysis and is composed of graphics from each generation (graphics A, B, C, D, and E), plus a covariance analysis graphic (graphic F) comparing the five-generation effect for each group and a table showing the statistical significance obtained from covariance analysis.
Accumulation
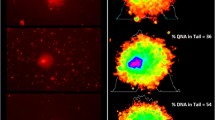
Figure 1B shows the animals from negative control group which does not present FRPS-MP into their bodies. Qualitative analysis of accumulation showed that the animals in both the MP groups were capable of ingesting FRPS-MP (Fig. 1D). FRPS-MP was distributed in three main sections of the intestinal lumen: foregut (Fig. 1D1), midgut (Fig. 1D2), and hindgut (Fig. 1D3). One hour after the animals recovered from anesthesia, the animals from both the MP groups were scanned again for FRPS-MP, and it was observed that FRPS-MP was eliminated from the intestinal content by individuals of both the MP groups (Fig. 1E).
In vivo FRPS-MP accumulation: C. elegans images taken with epifluorescent microscopy. A Animal from the Control group under bright field (100× magnification). B Animal from the Control group under fluorescent filter (100× magnification). C Animal from the PS-MP [50 μg/L] group under bright field (100× magnification). (D) Animal from the PS-MP [50 μg/L] group exhibiting fluorescent red polystyrene microplastics (FRPS-MP) inside its body under fluorescent filter (100× magnification); (D1) zoomed image of the foregut of D showing FRPS-MP agglomeration (400× magnification); (D2) zoomed image of the midgut of (D) showing FRPS-MP agglomeration (400× magnification); (D3) zoomed image of the hindgut of (D) showing FRPS-MP agglomeration (400× magnification); (E) animal from the PS-MP [5 μg/L] group after 1 h of the end of experiment showing no FRPS-MP fluorescence inside its body (the asterisk (*) indicates the fecal content with FRPS-MP) (200× magnification)
Biochemical responses
ROS
The MP5 group showed an increase in ROS content in the first and second generations (Fig. 2A and B), whereas the MP50 group showed significantly increased levels of ROS from the second to the fourth generation when compared with the control group (Fig. 2B, C, and D). In the last generation, all treatment groups exhibited similar ROS content levels compared with the control (Fig. 2E). Both treatments were significantly different from the control across the five generations (Fig. 2F).
Reactive oxygen species levels (ROS): ROS levels measured over five generations. A First generation; B 2nd generation; C 3rd generation; D 4th generation; E 5th generation. F Covariance analysis of multigenerational study with table of statistical significance. Different letters indicate statistical differences among groups in comparison to control (α = 0.05) (n = 5)
Catalase
MP5 induced an increase in catalase activity in the control group only in the first generation (Fig. 3A). Covariance analysis showed no significant differences across the five generations (Fig. 3F).
Catalase (CAT) activity: CAT enzymatic levels measured over five generations. A First generation; B 2nd generation; C 3rd generation; D 4th generation; E 5th generation. F Covariance analysis of multigenerational study with table of statistical significance. Different letters indicate statistical differences among groups in comparison to control (α = 0.05) (n = 5)
GST
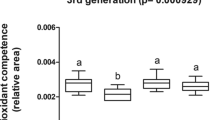
Both MP5 and MP50 groups demonstrated a similar trend for GST with an increased activity in the second, third, and fifth generations (Fig. 4B, C, and E) compared with the control group. Both treatments were significantly different from the control group across the five generations (Fig. 4F).
Glutathione S-transferase (GST) activity: GST activity levels measured over five generations. A First generation; B 2nd generation; C 3rd generation; D 4th generation; E 5th generation. F Covariance analysis of multigenerational study with table of statistical significance. Different letters indicate statistical differences among groups in comparison to control (α = 0.05) (n = 5)
TBARS
The MP5 group was able to induce lipid damage in the second, fourth, and fifth generation (Fig. 5B, D, and E), whereas the MP50 group induced lipid peroxidation in the first, second, third, and fifth generations compared with the control group (Fig. 5A, B, C, and E). Both treatments were significantly different from the control group across the five generations (Fig. 5F).
Thiobarbituric reactive substances (TBARS) levels: TBARS levels measured over five generations. A First generation; B 2nd generation; C 3rd generation; D 4th generation; E 5th generation. F Covariance analysis of multigenerational study with table of statistical significance. Different letters indicate statistical differences among groups in comparison to control (α = 0.05) (n = 5)
Physiological responses
Growth
The group treated with MP5 showed a decrease in growth in the first, third, and fifth generations (Fig. 6A, C, and E), whereas the group treated with MP50 had the same effect observed in the first and third generation (Fig. 6A and C) when compared with the control group. Both treatments were significantly different from the control group across the five generations (Fig. 6F).
Growth measurement: Growth of adult individuals measured over five generations. A First generation; B 2nd generation; C 3rd generation; D 4th generation; E 5th generation. F Covariance analysis of multigenerational study with table of statistical significance. Different letters indicate statistical differences among groups in comparison to control (α = 0.05) (n = 10)
Body bending
The group MP5 showed a decrease in behavior only in the first generation (Fig. 7A), whereas the group exposed to MP50 exhibited the same effect in the first, second, third, and fifth generation (Fig. 7A, B, C, and E) when compared with the control group. Only MP50 was significantly different from the control group throughout the five generations (Fig. 7F).
Body bending behavior assessment: locomotory behavior measured over five generations. A First generation; B 2nd generation; C 3rd generation; D 4th generation; E 5th generation. F Covariance analysis of multigenerational study with table of statistical significance. Different letters indicate statistical differences among groups in comparison to control (α = 0.05) (n = 5)
Reproduction
The group MP5 showed a decrease in reproduction in the second, fourth, and fifth generations (Fig. 8B, D, and E), whereas the group exposed to MP50 showed a decrease in reproduction in all the five generations (Fig. 8) when compared with the control group. Both treatments were significantly different from the control across the five generations (Fig. 8F).
Reproduction assay: reproduction assessed over five generations. A First generation; B 2nd generation; C 3rd generation; D 4th generation; E 5th generation. F Covariance analysis of multigenerational study with table of statistical significance. Different letters indicate statistical differences among groups in comparison to control (α = 0.05) (n = 5)
Discussion
Because of the limited information about multigenerational assessments related to MP exposure in C. elegans, other organisms were used for comparison based on their environmental importance, both as consumers and food sources, such as the water flea Moina macrocopa. However, some studies selected for comparison with our findings were performed using a much higher concentration condition (mg scale) or in just one environmental condition (aquatic or terrestrial). This may be a disadvantage, considering that our study was designed to simulate the C. elegans natural environment, which includes both aquatic and terrestrial environments.
MP accumulation
It has been proposed that the ingestion of MP depends on buccal cavity size (Fueser et al. 2019), and once ingested, spherical MP can be completely egested within 20–40 min from the intestine of C. elegans. In our study, we observed that after 96 h of continuous exposure to FRPS-MP, MP persisted inside the intestines of the animals in all the generations (Fig. 1B). To confirm the findings of Fueser et al. (2020), animals were transferred from their respective experimental arenas to new plates without MP. After 1 h in a non-contaminated arena, the animals were scanned for FRPS-MP fluorescence and no fluorescent signal was detected inside the animals; in one of the cases, the fecal content of the animal contained FRPS-MP (Fig. 1C).
This release of MP from the intestinal environment of the exposed animals can be explained by their spherical shape, which does not favor effective retention of the MP in the internal structures of the animal’s body. Thus, we infer that at least spherical MP accumulation is temporary (Fueser et al. 2020).
Another important point is the mechanism by which MP accumulate in organisms. In a study by Mueller et al. (2020b), it was found that ingested MP were capable of inducing mechanical damage in the intestine of exposed C. elegans. Mechanical damage to the intestine is considered the main reason for MP bioaccumulation because MP agglomeration can rely on a deficit in the intestinal barrier, which is crucial for MP toxicity (Mueller et al. 2020a). Therefore, it is suggested that the integrity of the intestinal barrier is mainly responsible for preventing MP translocation to the pseudocoelom, thus preventing MP from entering other tissues and possibly favoring MP accumulation and deleterious effects in exposed animals. In addition, contradictory reports are present in the existing literature. Many authors have proposed that one of the main conditions for MP accumulation is their irregular shape, mostly related to environmental MP as fiber MP (i.e., clothes) and aged MP (i.e., degraded plastics formed through the secondary process) (Browne et al. 2007; Ryan et al. 2009; Andrady 2011; Chen et al. 2022); however, many other studies have reported that spherical MP are the most harmful MP (Yu et al. 2020; Shang et al. 2020; Schwarzer et al. 2022).
Biochemical effects
In this study, we observed that different MP concentrations caused an imbalance in the redox state and the first two generations of the MP5 group exhibited higher ROS concentrations compared with that of other groups (Fig. 2A and B). The MP50 group induced ROS production in the second, third, and fourth generations (Fig. 2B, C, and D). Microparticles can induce toxicological effects due to their physical characteristics such as size, shape, and concentration (Liu et al. 2021; Schwarzer et al. 2022). The spherical PS-MP induced a pro-oxidative situation in different generations, and despite the ROS levels of the MP50 being not as high as observed in the group MP5, they lasted longer (from the second to the fourth generation) (Fig. 2), which poses a threat to the maintenance of cellular homeostasis. In a study evaluating different sizes of spherical PS-MP (0.1, 1.0, and 5.0 μm), Lei et al. (2018a) observed that MP of 1.0 μm were more effective in disrupting the redox state than the other sizes in C. elegans. A similar result was also observed by Yu et al. (2020), who observed that 100 μg/L of 1 μm spherical PS-MP induced higher levels of ROS compared to lower concentrations (0.1, 1, and 10 μg/L). However, Chen et al. (2021) observed that C. elegans exposed to 1 μm spherical PS-MP at concentrations ranging from 10 to 100 μg/L showed an increase in ROS levels, and when the subsequent generations were not exposed after parental generation exposure (F0), this effect remained until the F2 generation (second unexposed generation), emphasizing an MP transgenerational exposure effect. In the present study, in the fifth generation, the levels of ROS returned to levels similar to those in the control group (Fig. 2E); however, the GST activity remained high in the groups exposed to both MP concentrations (Fig. 4), indicating that while the ROS levels decreased, the organisms continued to attempt detoxification, probably to restore cellular homeostasis.
In fact, the covariance analysis showed a strong correlation among the generational effects (Fig. 2F), indicating that redox imbalance induced by ROS can persist in future generations; in order to survive, animals may have to increase their antioxidant capacity by allocating energy from other physiological processes.
To cope with redox imbalance, aerobic organisms possess an enzymatic ADS composed of a variety of enzymes, including catalase, which is responsible for detoxifying H2O2, one of the most common ROS (Sies and Jones 2020). Although H2O2 plays a crucial role in multiple signaling pathways, its accumulation in cellular environments can be harmful. In this study, only the group exposed to MP5 in the first generation showed an increase in CAT activity, indicating that H2O2 was abundantly produced in subsequent generations (Fig. 3A), whereas CAT activity remained unchanged in subsequent generations.
Perhaps, from the first generation, there was a predominance of other ROS, such as superoxide anion radicals or hydroxyl radicals, since ROS levels in the group MP5 remained elevated up to the second generation, whereas the MP50 group maintained increased ROS levels from the second to the fourth generation (Fig. 2). Although there is a lack of data on CAT activity in multigenerational studies with C. elegans, Chen et al. (2021) showed that transgenerational exposure of 1 μm spherical PS-MP (100 μg/L concentration) can modulate the expression of the ctl-1 gene (catalase gene), which was positively expressed in the parental generation (F0) (2–3-fold) and F1 (1–2-fold), but not expressed in the generations F3 and F4, reinforcing the idea that first generations are more susceptible to H2O2 than subsequent generations.
Another enzymatic activity assessed in this study was that of GST, a class of enzymes responsible for detoxifying several environmental contaminants, damage products, and ROS (Sies and Jones 2020). In the present study, in the second, third, and fifth generations, both MP groups showed positive modulation in relation to the control group, suggesting that there was some type of oxidative insult in the organism and, therefore, the GST activity increased (Fig. 4). In a multigenerational study conducted with M. macrocopa, Kim and Rhee (2021) exposed the organism for 14 days in each generation (three-generation) assessment and observed that GST activity was increased in animals after exposure for concentrations ranging from 1 to 500 μg/L of MP.
The covariance analysis was not statistically different between the MP groups but significantly different from the control over generations, showing that MP exposure recruits detoxification capacity independently of concentration (Fig. 4F).
The second, fourth, and final generations exhibited lipid peroxidation (Fig. 5). Similarly, the MP50 group showed an increase through the assessed generations with an emphasis on the third generation when higher levels of lipid peroxidation were reached (Fig. 5C). Lipid peroxidation can be related to a series of biological disorders such as deficits in lipid storage and intestinal barrier permeability (Fueser et al. 2022; Kim and Rhee 2021; Yu et al. 2020; Lei et al. 2018b; Qu et al. 2018). Yu et al. (2020) observed that 1 μm PS-MP induced intestinal injury in C. elegans exposed to concentrations varying from 10 to 100 μg/L, and that these concentrations were closely related to other altered parameters such as body bending (also assessed in the present study). More recently, Fueser et al. (2022) observed that PS-MP of a wide range of sizes were capable of disturbing feeding by reducing food consumption, and this reduction was followed by a decrease in growth and ability to reproduce.
In general, this study showed that exposure to MP can cause lipid peroxidation throughout generations. In fact, covariance analysis showed that both MP concentrations were capable of interfering with the oxidized lipid content of exposed organisms. Lipid peroxidation can interfere with several biochemical and physiological processes once membrane permeability changes, affecting cellular processes associated with these membranes; in addition, the management of these oxidized molecules represents a high energy cost to cells that can compromise physiological processes such as growth and reproduction (Fueser et al. 2022).
Physiological effects
Because growth can be related to the capacity of organism development, it can be used as a parameter to compare the effects of different experimental conditions (Gonzales-Moragas et al. 2015). In the present study, we observed that C. elegans exposed to PS-MP were susceptible to both concentrations in an intermittent manner.
This response pattern could be related to an attempt by the organism to deal with environmental stressors, thus presenting a similar modulatory response already observed in this study for GST activity and lipid peroxidation (Figs. 4 and 5, respectively). In terms of growth, wild-type C. elegans, approximately 1 mm in length, is considered an adult, and despite the differences observed in this parameter, all animals sampled, in all treatments, as well as in all generations, remained above 1 mm long (Fig. 6).
Thus, PS-MP contamination does not appear to represent a biological concern for C. elegans, although other studies have reported different results. In a study performed by Yu et al. (2020), C. elegans exposed to 1, 10, and 100 μg/L of 1 μm spherical PS-MP were 20% shorter than the control group, and Lei et al. (2018b) reported that C. elegans exposed to 1 and 5 μm spherical PS-MP also presented a decrease in body length when exposed to higher concentration condition (5 mg/m2). Finally, our study showed that both concentrations of MP were statistically different from the control group at the end of the experiment (Fig. 6F). In this sense, decreases in body length can be a reflex from abnormal development that can be linked with behavioral and reproductive alterations, as the following assessed parameters suggest.
Body bending is an exploratory behavior that reflects the functional state of motor neurons and is of extreme importance to feeding behavior and reproduction (Qu et al. 2019). In our study, we observed that MP5 caused a decrease in locomotion in the first generation (Fig. 7A), whereas MP50 caused decreases in this parameter, ranging from 20 to 40% in all generations, except the fourth (Fig. 7).
If animals are not capable of performing regular exploratory movements, they face an additional challenge, as they are less effective in the search for food. Qu et al. (2019) assessed the effects of prolonged exposure (from L1 to adulthood) of 0.1 μm spheric polystyrene in four generations, ranging from 1 to 1000 μg/L, and found that concentrations similar to those in our study (10 and 100 μg/L) were capable of reducing the rate of body bending. Using spherical polystyrene with the same characteristics as in the previous study, Qiu et al. (2020) reported that concentrations of 1 and 10 μg/L also reduced the body bend rate when compared to the control group. Taking into consideration these findings and complementing them with our results (Fig. 7), it is reasonable to assume that independent of the size, PS-MP causes a decrease in the rate of the body bend locomotor behavior in a concentration-dependent manner. Additionally, in an eight-generation transgenerational study, Liu et al. (2021) observed that parental generation C. elegans exposed to 0.1, 1, 10, and 100 μg/L of 100 nm PS-NP were capable to recover from parental exposure effects in a progressive pattern (0.1 μg/L in the F1; 1 μg/L in the F2; 10 μg/L in the F3; 100 μg/L in the F4), which reinforces our inference about the locomotory response effect, validated through the covariance analysis, where we observed that only MP50 was different from the control group (Fig. 7F). Ultimately, body bending is a process that requires a considerable amount of energy for proper locomotion; thus, animals that present some deficit in this parameter can be more susceptible to toxic effects elicited in biochemical analysis (see the “Biochemical effects” section).
From an ecological perspective, reproduction is by far the most important parameter assessed in multigenerational studies because it is directly related to the maintenance of populations (Josende et al. 2019a). In the present study, we observed an overall decrease in the reproductive parameters at both the PS-MP concentrations (Fig. 8); however, for MP5, the reproductive values obtained from the first and third generations were similar to those of the control group (Fig. 8A and C). In the MP50 group, a progressive decrease was recorded throughout the generations, with the lowest reproductive value (approximately 47.5% in relation to the control group) obtained for the final generation (Fig. 8E).
In a study by Schöpfer et al. (2020), approximately 23% decrease in the number of offspring from parental generation of C. elegans exposed to 10 mg/L of low-density polyethylene was observed. In another study, Mueller et al. (2020b) observed that exposures from 0.1 to 10 μm polystyrene beads could induce toxic effects in C. elegans, with a 50% inhibition of reproduction independent of the bead size. Both percentages of inhibition induced by PS-MP are concerning (47.5% in our study and 50% in Mueller et al. 2020a), because larger decreases in reproduction can pose a serious threat to population maintenance as well as for species survival if there is a long-term inhibitory event.
Mueller et al. (2020b) performed a 21-day multigenerational study for C. elegans, where instead of exposing each generation, they exposed one population for 21 days continuously. In contrast to our study, the approach used by Mueller’s study evaluates population dynamics instead of reproduction capacity, and for this reason, both studies cannot be satisfactorily compared, but at least, both agree in their conclusions about the fact that PS-MP exposures showed a risk for the continuity of the species.
Höss et al. (2022) studied the effects of 1 μm PS-MP at high concentrations (0.1, 1, and 10 mg/ml) and concluded that food availability is the main cause of lower reproduction when associated with MP contamination. In our study, we inactivated the bacteria used as food; thus, bacterial metabolism, as well as the variability of bacterial culture growth, was avoided. However, if an MP is accompanied by other environmental contaminants, it is plausible that the interaction between the contaminant and the bacterial content can interfere with bacterial culture growth, thus affecting the nutritive conditions that can be crucial for the ecological safety of benthic ecosystems in MP contamination.
In this study, we observed a significant decrease in reproduction, mainly in the MP50 group, even when compared with the MP5 group, as evidenced by covariance analysis (Fig. 8F). Overall, when comparing the pattern response of other variables measured in this study, we assumed that the reproductive parameters were strongly affected by the ROS levels (Fig. 2) and lipid peroxidation content (Fig. 5). As ROS induced alterations in the metabolism of exposed animals, favoring the accumulation of lipid damage, and indirectly by the body bending behavior (Fig. 7), exposed animals apparently needed to deal with the extra MP content inside their intestine (Fig. 1), which could interfere with the normal alimentary capacity, thus leading to a general deficit in acquiring nutrients from the environment.
Conclusion
The threat of MP is a serious concern for the present as well as the future, since MP is ubiquitous in the environment, passing from the emission sources to the fate compartments and crossing the abiotic/biotic interface. Herein, we used a multigenerational study focusing on the effects of MP on organisms from an environmental perspective, evaluating biomarkers that could help to better understand the challenges faced by organisms in an MP contamination scenario. We observed that despite the concentration, 1 μm PS-MP was able to induce oxidative responses in C. elegans by generating high levels of ROS, which consequently led to lipid peroxidation damages. Furthermore, GST enzyme was positively modulated, demonstrating that antioxidant defenses can be constantly recruited to deal with oxidative stressors. In addition, physiological parameters were altered, especially reproduction, which is the most important indicator that prolonged exposures can be harmful for the existence and survival of species in their natural environments, as well as for ecological balance. Multigenerational studies are challenging to perform; however, the conceptualization of this type of approach relies on the search for more realistic assessments, demonstrating great potential to help organizations, policymakers, and governors in building new environmental safety guidelines.
Data Availability
Not applicable.
References
Andrady AL (2011) Microplastics in the marine environment. Marine Pollution Bulletin 62:1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Anik AH, Hossain S, Alam M, Sultan MB, Hasnine MT, Rahman MM (2021) Microplastics pollution: a comprehensive review on the sources, fates, effects, and potential remediation. Environmental Nanotechnology, Monitoring and Management 16:100530. https://doi.org/10.1016/j.enmm.2021.100530
Beutler E, Beutler B, Matsumoto F (1975) Glutathione peroxidase activity of inorganic selenium and seleno-dl-cysteine. Experientia 31:769–770. https://doi.org/10.1007/BF01938453
Browne MA, Galloway T, Thompson T (2007) Microplastic – an emerging contaminant of potential concern? Integrated Environmental Assessment and Management 3:559–561. https://doi.org/10.1002/ieam.5630030412
Chen H, Hua X, Li H, Wang C, Dang Y, Ding P, Yu Y (2021) Transgenerational neurotoxicity of polystyrene microplastics induced by oxidative stress in Caenorhabditis elegans. Chemosphere. 972:129642. https://doi.org/10.1016/j.chemosphere.2021.129642
Chen H, Yang Y, Wang C, Hua X, Li H, Xie D, Xiang M, Yu Y (2022) Reproductive toxicity of UV-photodegraded polystyrene microplastics induced by DNA damage-dependent cell apoptosis in Caenorhabditis elegans. Science of the Total Environment 811:152350. https://doi.org/10.1016/j.scitotenv.2021.152350
Fueser H, Mueller MT, Weiss L, Höss S, Traunspurger W (2019) Ingestion of microplastics by nematodes depends on feeding strategy and buccal cavity size. Environmental Pollution 255:113227. https://doi.org/10.1016/j.envpol.2019.113227
Fueser H, Mueller MT, Traunspurger W (2020) Rapid ingestion and egestion of spherical microplastics by bacteria-feeding nematodes. Chemosphere. 261:128162. https://doi.org/10.1016/j.chemosphere.2020.128162
Fueser H, Pilger C, Kong G, Huser T, Traunspurger W (2022) Polystyrene microbeads influence lipid storage distribution in C. elegans as revealed by coherent anti-Stokes Raman scattering (CARS) microscopy. Environmental Pollution 294:118662. https://doi.org/10.1016/j.envpol.2021.118662
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Science advances 3:e1700782. https://doi.org/10.1126/sciadv.1700782
Gonzalez-Moragas L, Roig A, Laromaine A (2015) C. elegans as a tool for in vivo nanoparticle assessment. Advances in Colloid and Interface Science 219:10–26. https://doi.org/10.1016/j.cis.2015.02.001
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-Transferases, in: Methods in enzymology, detoxication and drug metabolism: conjugation and related systems, vol 77. Academic Press, pp 398–405. https://doi.org/10.1016/S0076-6879(81)77053-8
Höss S, Rauchschwalbe MT, Fueser H, Traunspurger W (2022) Food availability is crucial for effects of 1-μm polystyrene beads on the nematode Caenorhabditis elegans in freshwater sediments. Chemosphere 298:134101. https://doi.org/10.1016/j.chemosphere.2022.134101
Josende ME, Nunes SM, Müller L, dos Santos Francisco W, Gelesky MA, Monserrat JM, Ventura-Lima J (2019a) Multigenerational effects of ecotoxicological interaction between arsenic and silver nanoparticles. Science of the Total Environment 696:133947. https://doi.org/10.1016/j.scitotenv.2019.133947
Josende ME, Nunes SM, Müller L, Cravo MF, Monserrat JM, Ventura-Lima J (2019b) Circular estimative method (CEM) – a simple method to estimative Caenorhabditis elegans culture densities in liquid medium. Biological procedures online 21:1. https://doi.org/10.1186/s12575-018-0089-2
Kim J, Rhee JS (2021) Biochemical and physiological responses of the water flea Moina macrocopa to microplastics: a multigenerational study. Molecular & Cellular Toxicology 17:523–532. https://doi.org/10.1007/s13273-021-00162-5
Lei K, Qiao F, Liu Q, Wei Z, Qi H, Cui S, Yue X, Deng Y, An L (2017) Microplastics releasing from personal care and cosmetics products in China. Marine pollution bulletin 123:122–126. https://doi.org/10.1016/j.marpolbul.2017.09.016
Lei L, Liu M, Song Y, Lu S, Hu J, Cao C, Xie B, Shi H, He D (2018a) Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environmental science. Nano 5:2009–2020. https://doi.org/10.1039/c8en00412a
Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D (2018b) Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Science of the Total Environment 619-620:1–8. https://doi.org/10.1016/j.scitotenv.2017.11.103
Liu H, Tian L, Wang S, Wang D (2021) Size-dependent transgenerational toxicity induced by nanoplastics in nematode Caenorhabditis elegans. Science of the Total Environment 790:148217. https://doi.org/10.1016/j.scitotenv.2021.148217
Mueller MT, Fueser H, Trac LN, Mayer P, Traunspurger W, Höss S (2020b) Surface-related toxicity of polystyrene beads to nematodes and the role of food availability. Environmental Science & Technology 54:1790–1798. https://doi.org/10.1021/acs.est.9b06583
Mueller MT, Fueser H, Höss S, Traunspurger W (2020a) Species-specific effects of long-term microplastic exposure on the population growth of nematodes, with a focus on microplastic ingestion. Ecological indicators 118:106698. https://doi.org/10.1016/j.ecolind.2020.106698
Oakes KD, Van Der Kraak GJ (2003) Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquatic Toxicology 63:447–463. https://doi.org/10.1016/s0166-445x(02)00204-7
Qiu Y, Luo L, Yang Y, Kong Y, Li Y, Dayong W (2020) Potential toxicity of nanopolystyrene on lifespan and aging process of nematode Canorhabditis elegans. Science of the Total Environment 705:135918. https://doi.org/10.1016/j.scitotenv.2019.135918
Qu M, Xu K, Li Y, Wong G, Wang D (2018) Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Science of the Total Environment 643:119–126. https://doi.org/10.1016/j.scitotenv.2018.06.173
Qu M, Luo L, Yang Y, Kong Y, Wang D (2019) Nanopolystyrene-induced microRNAs response in Caenorhabditis elegans after long-term and lose-dose exposure. Science of the Total Environment 697:134131. https://doi.org/10.1016/j.scitotenv.2019.134131
Ryan PG, Moore CJ, van Franeker JA, Moloney CL (2009) Monitoring the abundance of plastic debris in the marine environment. Philosophical Transactions of the Royal Society B: Biological Sciences 364:1999–2012. https://doi.org/10.1098/rstb.2008.0207
Schöpfer L, Menzel R, Schnepf U, Ruess L, Marthan S, Brümmer F, Pagel H, Kandeler E (2020) Microplastics effects on reproduction and body length of the soil-dwelling nematode Caenorhabditis elegans. Frontiers of Environmental Science & Engineering 8:41. https://doi.org/10.3389/fenvs.2020.00041
Schwarzer M, Brehm J, Vollmer M, Jasinski J, Xu C, Zaiuddin S, Fröhlich T, Schott M, Greiner A, Scheibel T, Laforsch C (2022) Shape, size and polymer dependent effects of microplastics on Daphnia magna. Journal of Hazardous Materials 426:128136. https://doi.org/10.1016/j.jhazmat.2021.128136
Shang X, Lu J, Feng C, Ying Y, He Y, Fang S, Lin Y, Dahlgren R, Ju J (2020) Microplastic (1 and 5μm) exposure disturbs lifespan and intestine function in the nematode Caenorhabditis elegans. Science of the Total Environment 705:135837. https://doi.org/10.1016/j.scitotenv.2019.135837
Sies H, Jones DP (2020) Reactive oxygen species (ROS) as pleiotropic physiological signaling agents. Nature reviews. Molecular cell biology 21:363–383. https://doi.org/10.1038/s41580-020-0230-3
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Marine environmental research 44:69–84. https://doi.org/10.1016/S0141-1136(96)00103-1
Yu Y, Chen H, Hua X, Dang Y, Han Y, Yu Z, Chen X, Ding P, Li H (2020) Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans. Science of the Total Environment 726:138679. https://doi.org/10.1016/j.scitotenv.2020.138679
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice-Hall, Englewood Cliffs, N.J., p 718
Zhang X, Chen J, Li J (2020) The removal of microplastics in the wastewater treatment process and their potential impact anaerobic digestion due to polluants association. Chemosphere. 251:126360. https://doi.org/10.1016/j.chemosphere.2020.126360
Acknowledgements
The authors thank Marcos Gelesky, Felipe Kessler, and Tito R.S.C. Junior. for their support in MP characterization.
Funding
Juliane Ventura-Lima received research productivity fellowship from the Brazilian agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), process number PQ 313707/2020-0. Larissa Müller and Gabriela Soares received fellowship of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Marcelo Estrella Josende, recipient of post-doctoral fellowship CAPES Program, Fundação de amparo à pesquisa do estado do Rio Grande do Sul (process number 21/2551-0001968-9), financed this study.
Author information
Authors and Affiliations
Contributions
Gabriela Corrêa Soares: conceptualization, formal analysis, investigation, methodology, project administration, validation, writing original draft; Marcelo Estrella Josende: conceptualization, formal analysis, investigation, methodology, validation, writing original draft; Larissa Müller: formal analysis, investigation, methodology; Juliane Ventura-Lima: conceptualization, formal analysis, investigation, methodology, project administration, validation, writing original draft, funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

Fig. S1
Representative SEM images of MPs (microbeads, size 1.1 μm, polystyrene from Sigma-Aldrich). (PNG 9361 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soares, G.C., Müller, L., Josende, M.E. et al. Biochemical and physiological effects of multigenerational exposure to spheric polystyrene microplastics in Caenorhabditis elegans. Environ Sci Pollut Res 30, 69307–69320 (2023). https://doi.org/10.1007/s11356-023-27162-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27162-3