Abstract
Water hyacinth (WH) has become a considerable concern for people across the globe due to its environmental and socio-economic hazards. Researchers are still trying to control this aquatic weed effectively without other environmental or economic losses. Research on WH focuses on converting this omnipresent excessive biomass into value-added products. The potential use of WH for phytoremediation and utilizing waste biomass in various industries, including agriculture, pharmaceuticals, and bioenergy, has piqued interest. The use of waste WH biomass as a feedstock for producing bioenergy and value-added chemicals has emerged as an eco-friendly step towards the circular economy concept. Here, we have discussed the extraction of bio-actives and cellulose as primary bioproducts, followed by a detailed discussion on different biomass conversion routes to obtain secondary bioproducts. The suggested multi-objective approach will lead to cost-effective and efficient utilization of waste WH biomass. Additionally, the present review includes a discussion of the SWOT analysis for WH biomass and the scope for future studies. An integrated biorefinery scheme is proposed for the holistic utilization of this feedstock in a cascading manner to promote the sustainable and zero-waste circular bio-economy concept.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eichhornia crassipes, commonly known as water hyacinth (WH), is an aquatic macrophyte belonging to the Pontederiaceae family. It originated in South America and spread worldwide in the late eighteenth century due to its highly invasive nature. WH was considered an ornamental plant due to its attractive flowers and foliage and was distributed widely. Once it escaped from cultivation, it became a severe pest, obstructing navigation and interfering with fisheries and other water activities. Because of the various social-environmental issues provoked, it became the world’s most notorious aquatic weed. Since then, research has been ongoing to develop strategies for its eventual application to humanity (Malik 2007; Ren and Zhang 2007; Zhang et al. 2010; Shu et al. 2014).
The climatic conditions in tropical and subtropical regions favor the growth of WH. Once infested, it proliferates, forming a dense mat over the water surface within a few weeks. It shows high productivity in summer and maintains its population from year to year despite its decrease in winter. It floats freely and propagates fast by asexual and sexual means; however, it commonly proliferates vegetatively through root stalks (Coetzee et al. 2017; Kitunda 2017). The rapidly growing WH blocks sunlight. Excessive growth increases the transpiration pull resulting in heavy and rapid water loss. The obstructed movement in heavily choked waterbody hampers the oxygen exchange, and decaying vegetation creates a foul smell. The decaying vegetation provides a breeding ground for mosquitoes and creates other health and hygiene-related issues. As a result, there is an overall imbalance between flora and fauna. Fishing, boating, swimming, irrigation, hydropower projects, and other activities are all impeded (Thamaga and Dube 2018; Dersseh et al. 2019).
Physical, chemical, and biological treatment methods attempted to control and eradicate this weed (Williams et al. 2005; Wilson et al. 2007; Greenfield et al. 2007; Tipping et al. 2014) are shown in Fig. 1. These methods require high costs and labor, but effective eradication is still impossible. WH repeatedly regrows, making all the efforts in vain (Williams et al. 2005; Kleinschroth et al. 2021). Moreover, excessive chemicals and biological agents adversely affect the associated biodiversity. Annual eradication of WH imposes an enormous and unnecessary burden on developing countries’ economies. As WH does not produce food or revenue, it is unappealing to face the costs of eradicating this pest.
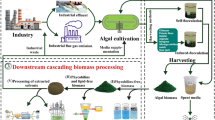
Reversing the perspective, WH is blessed with the potential for rapid growth, outranging other species. This exclusive property makes it a potential candidate to be utilized as a sustainable feedstock for various industries. This review aims to analyze the green potential of WH for its practical and sustainable utilization. The suggested multi-objective approach will be beneficial in developing cost-effective procedures compared to the single-objective methods proposed earlier. Most reports discuss WH for fuel and energy (Gunnarsson and Petersen 2007; Ganguly et al. 2012; Rezania et al. 2015; Bote et al. 2020a; Gaurav et al. 2020; Li et al. 2021), WH management and valorization (Yan et al. 2017; Sindhu et al. 2017; Guna et al. 2017), or contaminant removal from water bodies (Dhote and Dixit 2009; Mishra and Maiti 2017; Priya and Selvan 2017; Ting et al. 2018; Li et al. 2021; Madikizela 2021). Here, we have discussed the valuable bio-actives extracted from the WH biomass as primary bio-products, followed by the generation of secondary bio-products by biochemical, green synthesis, and thermochemical routes. To the best of our knowledge, a detailed discussion on WH extractives, including bio-actives, cellulose, and WH-based nano-particles, has been done for the first time. An extensive review of fuel, supercapacitor, catalyst, and other product generation from WH via thermochemical route has been done, which is lacking in the existing literature. Apart from this, here we have included the recent advancements in WH biomass applications in environmental and other sectors. Integrative and sustainable biomass utilization is the focus of the review, with the long-term goal of developing WH biorefinery. This review suggests a novel, systemic approach for utilizing WH biomass based on its compositional traits and gives an updated insight into its valorization. The sustainable utilization of WH biomass for phytoremediation, followed by its extraction and conversion to generate high-valued compounds, will enable the monetization of this weed. A systematic and integrative approach will benefit businesses, society, and the environment by promoting a circular economy for WH management (Fig. 2). We also discuss the benefits, shortcomings, and outlook of WH biorefinery via a SWOT analysis.
Primary bioproducts: phytochemicals extracted from WH
The aquatic macrophyte, WH, has existed in local environmental conditions since its introduction as an esthetic plant. The plant is highly stress-tolerant and has a high survival rate in harsh situations like water contaminated with heavy metals, dyes, and algae blooms. Its unwelcome, enormous, and recurring growth in water bodies prompted researchers to explore this weed for beneficial compounds. Several researchers have reported the composition of WH; a few of the reports are summarized in Table 1. Most reports discuss WH composition on a dry weight basis. WH is a simple biomass with a major portion of the mass composed of cellulose and hemicellulose, with a low amount of lignin. Therefore, WH can be a good source for harvesting cellulosic material. It is a good source of antioxidants, sterols, proteins, fatty acids, cellulose, vitamins, minerals, pigments, and other plant-based metabolites, as discussed in Table 2. However, the levels may vary depending on plant maturity and local environmental circumstances. Antioxidant additives, therapeutic factors, and structural biopolymers could be obtained from WH for applications in food, pharma, and other industries. Furthermore, after extracting inhibitory plant phenolics, residual structural biopolymers could be an ideal substrate for bioconversions to produce other valuable commodities. As discussed in Table 2, WH extractions provide more insights into its composition. The compositional review serves as an essential step to identify the range of possible applications for each by-product for consideration of WH as a potential biorefinery raw material.
Plant sterols
Ganguly et al. (1977) isolated sterols and confirmed the presence of β-sitosterol and stigmasterol from the dried pollen and pistils of WH. The existence of glycosides and phenolic compounds were also reported. Authors suggested the presence of sterols to protect the plant from desiccation, glycosides as a source of sugars, and phenolic compounds to repel approaching insects (Ganguly et al. 1977). GC–MS analysis of the lipophilic extract of WH was found to yield sterols of up to 1.12 wt% in roots and 4.45 wt% in flowers. Stigmasterol was present in WH with a yield of 4.44 g/kg of biomass, making WH a rich source of this compound (Silva et al. 2015).
Fileto-Perez et al. (2015) isolated fatty acids in WH through sequential extraction using solvents of increasing polarity in a soxhlet apparatus followed by derivatization with BF3. A total of 24 compounds were identified, which included 20 carboxylic acids, three steroids (including β-stigmasterol), and one terpenoid (squalene) (Fileto-Pérez et al. 2015). Supercritical CO2 was also used for sterol extraction. A maximum yield of total sterols (ƞTotal Sterol) was 0.35 wt%, the concentration of total sterols (CTotal Sterol) 38.3 wt%, and the concentration of stigmasterol (CStigm) 26.4 wt% was obtained at pressure 300 bar and 2.5 wt% ethanol as a co-solvent (Martins et al. 2016). De Melo et al. (2016) extended the work by utilizing yield response as a selectivity element in yield optimization using mathematical modeling. Total sterol yield increased from 0.64 to 1.88 wt%. Also, selectivity for stigmasterol increased at 40 °C compared to 60 °C (de Melo et al. 2016). The structures of the main phytosterols found in WH extracts are shown in Fig. 3(a)–(c).
Structures of some key phytoconstituents isolated from WH Biomass. Phytosterols: (a) β-sitosterol; (b) stigmasterol; (c) methyl cholesterol; medicinal applications (Martins et al. 2016). Phenolic compounds: (d) p-hydroxybenzoic acid, antioxidative and anti-inflammatory (Surendraraj et al. 2013); (e) shikimic acid, a precursor for antiviral oseltamivir phosphate (Ganorkar et al. 2022); Antioxidant: (f) glutathione (Bodo et al. 2004b); Antiprotozoal (DellaGreca et al. 2008; Costa et al. 2021): (g) phenylphenalene derivatives; Biopolymers: (h) cellulose; (i) hemicellulose; wide applications in food, pharma, biofuels, and environmental sectors (Istirokhatun et al. 2015; Tanpichai et al. 2021; Oyeoka et al. 2021)
Plant growth regulators
The plant hormone cytokinin was observed in WH extract (Nagar and Saha 1985). Results indicated the presence of zeatin (Z) and zeatin riboside (ZR) in both leaves and root extracts. The qualitative difference among the hormone found in different parts of the plant was attributed to the metabolic conversion of the hormone. Humic acid (HA) was isolated from the dried powder from different parts of the WH plant after successive extractions for lipids and uronic acids. The freeze-dried HA samples contained amino acids, monosaccharides, macroelements, and microelements and could be used for soil improvement (Ghabbour et al. 2004). Elgala et al. (2022) performed a randomized block experiment in which an aqueous extract of WH shoot was sprayed over tomato plants as a source of nutrients. The net yield of tomato plants increased by 1.84 and 1.63 times compared with the control (without foliar spray) and commercial synthetic chemical solution treatment, respectively (Elgala et al. 2022).
Antioxidants and therapeutic factors
WH aqueous extract has shown effective anti-parasitic activity against drug-resistant parasites. The extract was rich in steroids, flavonoids, alkaloids, tannins, and proteins with high antioxidant activity making it an effective and inexpensive anti-parasitic agent (Elagib 2020). A polar extract with a yield of 10 wt% for roots and 28.8 wt% for stalks from WH was rich in antioxidants and phenolics (Silva et al. 2015). Aboul-Enein et al. (2011) separated nine active fractions from WH, including alkaloid, propanoid, phthalate, and phenyl derivatives. Antibacterial, antifungal, antioxidant, and anticancer properties were evaluated, which showed promising medicinal potential (Aboul-Enein et al. 2011). WH ethanolic extracts rich in natural antioxidants increased the shelf life of unsaturated fish oils. HPLC analysis revealed the presence of phenolic compounds such as gallic, protocatechuic acid, gentisic acid, p-hydroxybenzoic acid, and others (Surendraraj et al. 2013). The structure of p-hydroxybenzoic acid is shown in Fig. 3(d). Two antioxidant peptides with a molecular weight of 442.3 and 278.2 Da were purified from WH leaf hydrolysates using gel filtration chromatography and RP-HPLC. They were identified as Phe-Phe-Glu and Leu-Phe, using MALDI-TOF–MS. The separated peptides could be used in food and pharma industries as natural antioxidants, as evident from free-radical scavenging assays (Zhang et al. 2018c).
Optimized process for fast and efficient extraction of glutathione using factorial 33 design reported 40 nmol equivalent glutathione (EG) per gram of dried plant (Bodo et al. 2004b). Freeze-dried samples exhibit the highest glutathione activity, while the EG value deteriorates rapidly when samples are heated above 60 °C. Two new phenylpropanoid derivatives with a potential role as phytoanticipins and phytoalexins were isolated from the ethyl acetate extract of WH. Compounds were characterized using 1H and 13C NMR after HPLC-based purification (DellaGreca et al. 2009). The study was extended further by Coasta et al. (2021), who extracted 19 phenylphenalenones (PhPs) from WH. Structures of four newly discovered PhPs were elucidated using 1H and 13C NMR. Furthermore, the two main PhPs, 2-hydroxy-8-(4-hydroxyphenyl)-phenalen-1-one (PPO1), and 2-hydroxy-8-(3,4-dihydroxyphenyl)-phenalen-1-one (PPO2) were tested for their antiprotozoal activity against Trypanosoma cruzi and cytotoxic activity against mammalian cells (NCTC-L929). Both PhPs showed moderate activity with EC50 value 38–67 μM (EC50 -50% effective concentration) against T. cruzi in comparison to standard drug benznidazole (EC50 value 16 μM) and no cytotoxicity against NCTC-L929 at the highest tested concentration of 200 μM (Costa et al. 2021). A general structure of PhP derivatives is shown in Fig. 3(g).
WH ethyl acetate extract was found to be effective against lead-induced toxicity. The test was performed on the albino rat, and the extract was effective in recovering the cellular damage caused by lead acetate (Ahmed et al. 2016). The heavy metal tolerance of WH was attributed to the presence of antioxidant enzymes: ascorbate peroxidase (APX), peroxidase (POX), superoxide dismutase (SOD), and catalase (CAT) (Malar et al. 2014). Ultrasound-assisted (UAE) along with conventional extraction was performed for different parts of WH to extract shikimic acid (SA) [Fig. 3(e)], a precursor for synthesizing the antiviral drug oseltamivir phosphate (Tamiflu®) (Ganorkar et al. 2022).
Allelopathic potential
WH was found effective for the purification of eutrophic water. Proteomic analysis revealed the synthesis of proteins associated with oxidation–reduction processes, nitrogen-phosphorus uptake, and metabolism in response to the stimulus. Synthesized proteins enhanced the nutrient uptake rate, hindering the growth of algae (Microcystis aeruginosa). The secretion of allelochemicals further synergized the effect (Li et al. 2015). Shanab et al. (2010) studied the allelopathic potential of WH’s methanolic extract. The crude extract was separated into five fractions. Each fraction demonstrated antibacterial, antifungal, and antialgal activity. Furthermore, the active components responsible for these activities were identified to be alkaloid and phthalate derivatives (Shanab et al. 2010). Similar phthalate-based therapeutic bio-actives have been isolated from WH, as discussed in “Antioxidants and therapeutic factors” section. (Aboul-Enein et al. 2011). However, special care should be taken while analyzing the data as xenobiotic compounds may be accumulated in the plant from the polluted water sites (Saeidnia and Abdollahi 2013; De Laet et al. 2019). The allelopathic potential of ethyl acetate fraction of purple-root WH (PRWH) was tested against blue-green algae (BGA). Eleven new phenylphenalene derivatives have been isolated and characterized. Seven have shown potential bioactivity against BGA when tested for Microcystis aeruginosa (Cheng et al. 2021). WH was found quite effective in controlling algal blooms (Qin et al. 2016). Fenced cultivation of WH in Dianchi lake, China was done to study its potential to purify algal blooms. The effect of water quality, algae distribution, and accumulation of nutrients like total nitrogen and phosphorus on effectiveness of WH was studied.
Pigments and other chemicals
A convenient and straightforward method to extract β-carotene from WH was patented by Panchanadikar et al. in 2005. The powdered plant material was extracted in an organic solvent, then enriched in acetone and filtered (Panchanadikar et al. 2005). Levelunic acid was synthesized through acid-catalyzed hydrolysis of WH at 150–175 °C. The yield of levelunic acid was 53% w/w of C6 sugars (Girisuta et al. 2008). The typical scheme for extracting phytometabolites is summarized in Fig. 4.
Cellulose
Cellulose nanofibers (CNFs) were extracted from WH by different methods [Fig. 3(h)–(i)]. The diameter of extracted CNFs was in the range of 10 − 30 nm. Alkaline treatment was sufficient to remove most of the lignin, making WH a sustainable source of cellulose (Thiripura Sundari and Ramesh 2012; Tanpichai et al. 2019). The size of nanofibrillated cellulose (NFC) fibrils decreases from 23 to 17 nm on high-speed homogenization; however, it increases the time by fourfold. The tensile strength increased almost threefold from 5.87 to 15.2 MPa, while the contact angle increased from 21.2 to 36°. These changes were attributed to a decrease in the porosity of the nanocellulose (NC) paper (Tanpichai et al. 2021). CNFs (5–50 nm) were simply prepared from WH biomass by subjecting extracted cellulose to ten defibrillation cycles. However, prolonged mechanical treatment resulted in higher water retention capacity (WRC) and specific surface area; a gradual decrease in crystallinity index, thermal degradation temperatures, and degree of polymerization were observed. A suspension of CNFs showed a steady increase in viscosity with the formation of a gel-like structure with shear-thinning behavior that was fitted better with a Herschel-Bulkley fluid model rather than a Bingham plastic model (Pakutsah and Aht-Ong 2020). WH cellulosic fibers can be extracted under milder conditions using a high-pressure homogenizer. CNFs with the highest water retention percentage (WR%) was obtained after five passes, while a decrease in crystallinity (CrI%) was observed as the number of passes increased from 1 to 5. TGA and DTG analysis revealed that CNFs could maintain thermal stability when used as reinforcements in bioplastics (Sun et al. 2020).
Nanocrystalline cellulose of mean particle size 93.0 nm was obtained from WH with the help of sonication. A slight decrease in the degradation temperature from 253 to 227 °C was observed while processing the raw fibers indicating its potential applicability (Packiam et al. 2021). WH fibers (WHFs) were investigated as green reinforcement material. Bio-epoxy composites augmented with NaOH and silane-treated WHFs were synthesized. Composites were found suitable for lightweight applications as indicated by the tensile strength, flexural, impact, hardness, thermal, dynamic, and surface morphology tests (Sumrith et al. 2020). In a recent study, epoxy composites were fabricated by reinforcing WH fibers. Different ratios of fiber to resin content were evaluated in which 35 wt% of fiber content shows improved composite properties for various applications (Dass and Chellamuthu 2022). Cellulosic WH fibers were reinforced with polyurethane at fiber loadings of 1–7 wt%, and the resulting composites were tested for oil adsorption. The maximum oil sorption capacity of 10–15 g/g was obtained with higher fiber loadings due to increased porosity at higher fiber concentrations (Sittinun et al. 2020). Microwave-assisted cellulose aerogels derivatized with polyvinyl alcohol (PVA) with a PVA/cellulose ratio of 4:3 showed an optimum capacity of 38.5 g of diesel adsorbed per gram of sorbent and 43.3 g/g with motor oil. Reusability studies indicated adsorption remained stable for up to 10 cycles. Moreover, the low thermal conductivity of aerogel (0.030 W/mK) also opens up potential applications as a thermal insulator (Nguyen et al. 2021).
Biodegradable films for food packaging applications were reported using WH CNFs. WH CNFs were reinforced in polyvinyl alcohol (PVA) and gelatin to synthesize composites. The effect of PVA, gelatin, and cellulose nanocrystal (CNC) concentration on tensile strength and elongation was optimized. The maximum tensile strength of 13.6 MPa and 80.7% elongation at break was obtained at the optimum value of 10 wt% for PVA, 5 wt% for gelatin, and 7 wt% for CNC. For a film with higher strength, a decrease in water absorption, water vapor permeability (WVP), and moisture uptake was observed (Oyeoka et al. 2021). WH cellulose was acetylated and used for membrane synthesis. The membranes were characterized for filtration of humic acids giving 65% rejection and a permeate flux of 460 Lm−2 h−1 at a transmembrane pressure of 0.5 atm (Istirokhatun et al. 2015).
Apart from the conventional applications, WH cellulose was also utilized to synthesize conductive aerogel for microelectronics, solar cells, and batteries. WH CNFs were blended with conducting polymers polypyrrole (PPy) and polyvinylpyrrolidone (PVP). The synthesis was optimized using Box-Behnken response surface methodology (RSM) by changing the ratios of CNF, PPy, and PVP. The electrical conductivity of the composite aerogels ranged from 0.1 to 6.23 S/cm, with an optimal value of 5.21 S/cm. (Ewulonu et al. 2020).
Slow-release fertilizers (SRF) were synthesized from WH cellulose to avoid fertilizer losses or the dose-dependent toxic effects of high concentrations of fertilizers. Polyacrylamide was grafted on the extracted cellulose, and the composite polymer was used as the carrier for the SRF. Different blends of polymer, nano-hydroxyapatite, and fertilizer were investigated along with nutrient release kinetics (Rop et al. 2018). Poly(ammonium) acrylate-co-acrylic acid-Sgrafted WH cellulose was explored further as a soil conditioner. The polymer hydrogel (PHG) was tested for its moisture-holding capacity and biodegradability (Rop et al. 2019). However, WH biomass could also act as a cross-linking agent for formulating SRFs (Silva et al. 2021).
The ability of WH cellulose as a biomedical nanocarrier for delivering the anticancer drug (methylene blue, MB) was assessed. The release of MB was found to follow first-order kinetics. A maximum release percentage value of 52% was obtained for MB at pH 7.4. Cell viability for the breast cancer cell line, MCF-7, decreased about seven times when the concentration of MB increased from 12.5 to 100 mg/mL. Simultaneously, only a moderate cytotoxic effect was observed for the normal cell line (WI-38) (Salahuddin et al. 2021b).
Miscellaneous
WH ash extract was used as a green medium for the palladium-catalyzed Suzuki reaction. EDX analysis reveals the presence of metals in WH ash, giving rise to the corresponding metal hydroxides imparting basicity to the medium (Sarmah et al. 2017). Acid-treated WH dried leaves were pyrolyzed to obtain carbon dots (CDs) which were then used to fabricate paper-based fluorescent sensors for on-site borax detection with a detection limit of 11.9 μM. The developed sensors were low-cost with high photostability (Prathumsuwan et al. 2019).
In 2018, Okwadha and Makomele reported a different but potentially significant application of utilizing WH extract as a plasticizer for producing self-compacting concrete. The presence of lignocellulose and saturated/unsaturated fatty acids in the extract was theorized to be responsible for the plasticizing effect (Okwadha and Makomele 2018). A recent study produced handmade paper by pulping and bleaching WH biomass using potassium hydroxide (KOH) and H2O2, respectively. The black liquor waste was used as a supplement for composting the kitchen waste. A significant and beneficial increase in bio-compost nitrogen and potassium content was observed (Islam et al. 2021). WH mulch could increase soil moisture by about 33% and control weed growth (Abdalla and Hafeez 1969). It exerts a selective allelopathic effect on weeds, decreases soil salinity, and improves soil texture (Anaya et al. 1987).
Apart from the above uses, WH biomass has been extensively explored as fish feed, ruminant fodder, and soil mulch. The possibility of incorporating WH in fish feed was studied for Labeo rohita fingerlings, Ctenopharyngodon idella fingerlings, and rainbow trout (Mahmood et al. 2018; Debnath et al. 2018; Rufchaei et al. 2020). The inclusion of some percentage of WH in the fish diet was found to improve fish immunity against pathogens, Lactococcus garvieae and Streptococcus iniae (Chang et al. 2013; Rufchaei et al. 2020). Various trials done on animals have suggested its possible application as cattle fodder (Agarwala 1988; Abdelhamid and Gabr 1991; de Vasconcelos et al. 2016). WH should not be offered as a sole feed for ruminants. A maximum of 50% replacement in the complete feed diet could be done without adverse health effects (Abdelhamid and Gabr 1991). Various pre-treatments for effective silage production from WH have been suggested to make the feed more palatable for animals (Bolenz et al. 1990).
Secondary bioproducts
Phytochemical-rich extract for green synthesis
WH is a rich source of various phytochemicals, which could be utilized for the green synthesis of nanoparticles without adding any extra reducing and capping agent. Synthesis of multiple nanoparticles based on WH metabolites has been reported, as summarized in Table 3.
Mochochoko et al. (2013) demonstrated the synthesis of silver nanoparticles (Ag-NPs) using WH cellulose as a reducing and capping agent. Effects of reaction time and solution pH on NPs synthesis were studied. Highly monodispersed, stable, and spherical particles with an average diameter of 2.68 ± 0.69 nm were produced under alkaline conditions (pH: 11) (Mochochoko et al. 2013). AgNPs are found effective against Staphylococcus aureus and Escherichia coli. The anticorrosion activity of AgNPs was tested by adding them to 1 M HCl solution in which pre-weighed aluminum coupons were submerged. A low corrosion current density was observed as the charge transfer resistance values increased with increasing AgNP concentration (Hublikar et al. 2021). Colorimetric studies indicated the excellent sensitivity of Ag-NPs prepared using WH extract for heavy metal ion detection, especially Hg2+ ions (Oluwafemi et al. 2019).
Rod-shaped iron oxide nanoparticles (FeNPs) were generated using WH leaf extract, and their antibacterial activity was analyzed against Staphylococcus aureus and Pseudomonas fluorescens. One hundred micrograms per milliliter FeNPs gives the highest zone of inhibition against studied microbes, while the lowest was observed at 25 µg/mL (Jagathesan and Rajiv 2018). Magnetic iron nanoparticles (FeNPs) of spherical shape with an average particle size of 13.5 ± 3.7 nm were synthesized from WH extract. The authors studied their role as a regulator in the fermentative hydrogen production from the lignocellulosic hydrolysate. Hydrogen production was increased by 23.5% with an optimum yield (YH2/S) of 83.2 ± 2.19 mL/g substrate on the addition of WH-magnetite-NP (20 mg/L) in the fermentation medium. This was due to increased hydrogenase activity, the critical enzyme for biochemical hydrogen production in the presence of WH-magnetite-NP (Zhang et al. 2021).
Roy et al. (2019) synthesized spherical copper nanoparticles (Cu-NPs) with 12–15-nm diameter using WH flower extract. These biogenic Cu-NPs instantly detected the presence of hazardous hydrogen peroxide (Roy et al. 2019). WH aqueous extract was used to produce spherical platinum nanoparticles (Pt-NPs) with an average diameter of 3.74 nm, while the hydrodynamic aggregate size was 73.3 nm (John Leo and Oluwafemi 2017). Synthesis of Cr2O3/ZnO photocatalysts was performed using WH aqueous extract. The maximum degradation efficiency of 85% was achieved for MB dye within 90 min in the presence of 0.08-CrZn catalyst, which was attributed to efficient electron/hole separation and high porosity of the synthesized heterocatalyst (Zelekew et al. 2021).
Biochemical conversion
Lignocellulosic materials, including plant dry matter or agro-wastes, are rich sources of biopolymers like cellulose, hemicellulose, and lignin, which could be converted into bioethanol and other valuable products by biochemical routes. In this context, a considerable amount of WH biomass with high cellulosic contents makes a sustainable raw substrate for fermentative productions. Table 4 summarizes the fermentative production of different valuable compounds using WH biomass as substrate.
Cellulolytic enzymes and bioethanol
Bioconversion of WH biomass into ethanol to be used as a motor fuel was carried out using yeast Pichia stipitis NRRL Y-7124. Dilute acid hydrolysis was performed with 1% (v/v) sulfuric acid, followed by heat treatment. Optimum fermentation conditions identified were an aeration rate of 0.02 vvm, a temperature of 30 °C, and pH 6.0, giving the highest ethanol yield (Yp/s) of 0.35 gp/gs with 76% of total sugar utilized (Nigam 2002). The potential of WH as promising biomass for ethanol production was compared using simultaneous saccharification and fermentation mode (SSF) and separated hydrolysis and fermentation mode (SHF). The higher ethanol concentration of 16.9 g/L and 0.17 g/g-dried biomass yield was produced with WH substrate using recombinant Escherichia coli (KO11) under SSF compared to water lettuce (Mishima et al. 2008).
Cellulase and xylanase were produced in SSF using WH substrate with a mixed culture of Trichoderma reesei and Aspergillus niger. A yield of 46.3 FPU/gds (gram dried substrate) for cellulase and 57.2 IU/gds for xylanase was observed when the mixed culture was used (Deshpande et al. 2008). Cellulase was produced using WH substrate by Aspergillus terreus. Afterward, the crude enzyme was used to hydrolyze alkali-treated WH biomass. The hydrolysate was then fermented by Kluveromyces marxianus, giving a maximum ethanol concentration of 8.4 g/L, which was further increased by 10% with the addition of commercial pectinase (Narra et al. 2017).
Carboxymethyl cellulase (CMCase) and protease were produced in WH using solid-state fermentation (SSF) by 12 different fungal strains. Ulocladium botrytis gave the best yield, with yeast extract as the best nitrogen source for CMCase and malt extract for protease production. Enzyme recovery of 40.3 and 56.3%; purification fold of 47.3 and 51.8; and specific activity of 852 and 1470 U/mg (unit per milligram) was reported for CMCase and protease, respectively (Abo-Elmagd and Housseiny 2012). Endoglucanase enzyme was produced by the bacterial strain Bacillus subtilis PF1 in SSF using WH. The enzymatic yield of 17 IU/gds for endoglucanase activity and 12 IU/gds for filter paper activity was observed within 30 h of fermentation. The addition of TiO2 nanoparticles (NPs) increased the thermal stability of enzymes (Khan et al. 2022). The ability of 100 fungal strains to produce hydrolytic enzymes using WH biomass was assessed. About five strains generated hydrolytic enzymes, among them, the strain Trichoderma harzianum, made the maximum yield. The highest enzyme yield of 149 ± 14.3 IU/gds for xylanase, 16.4 ± 0.6 IU/gds for cellulase, and 128 ± 14.8 IU/gds for β-d-xylopyranosidase was observed (Arana-Cuenca et al. 2019).
Pre-treatment of WH biomass with the dilute acid treatment (DAT) method and the novel method of using crude glycerol (CG) and ionic liquids (ILs) were compared. Authors found IL (1-butyl-3-methylimidazolium acetate) and CG treatments resulted in 3.3 and 1.9 times higher recovery of total reducing sugars, respectively, compared to DAT. However, CG pre-treatment gives a better ethanol yield than IL. Similar results were also observed for wheat straw (Guragain et al. 2011). Alkali-treated WH was used to produce crude enzymes, by which biomass was saccharified to produce ethanol. The ethanol concentrations of 4.3 g/L, 6.2 g/L, and 9.8 g/L were recorded by Saccharomyces cerevisiae, Scheffersomyces stipitis, and both cultures, respectively (Singh and Bishnoi 2013). A 1.78-fold higher bioethanol production was achieved when simultaneous saccharification and fermentation were performed on pre-treated WH biomass using thermotolerant Kluyveromyces marxianu. After pre-treatment, the level of reducing sugars was recorded as 224 mg/g dried biomass, giving an ethanol concentration of 7.34 g/L (Yan et al. 2015). Steam explosion pre-treatment and enzymatic saccharification of WH biomass were reported to produce bioethanol. SSF was performed on steam explosion pre-treated WH biomass to produce xylanase and cellulase with the activity of 42 U/g and 2 U/g of dry matter, respectively. The highest ethanol concentration of 7.13 g/L and yield of 0.23 g/gds was obtained when hydrolysate was fermented with Saccharomyces cerevisiae (Figueroa-Torres et al. 2020).
Dilute acid pre-treatment was found best, followed by enzymatic saccharification, which was then used to produce bioethanol. Using a co-culture of Saccharomyces cerevisiae and Zymomonas mobilis, a maximum concentration of 13.6 mg/mL of ethanol was attained (Das et al. 2016). Dilute acid followed by enzymatic hydrolysis was the most effective process of saccharifying WH biomass, resulting in 402.9 mg/g of reducing sugar at optimal conditions. The solid-state fermentation produced 1.29 g/L ethanol under optimum conditions of 38.9 °C for 82 h with 6 ml of yeast inoculum (Zhang et al. 2016). A high amount of reducing sugars, 431 mg/g, was obtained when combined dilute acid hydrolysis and microbial treatment were given to WH biomass. A maximum yield of 1.40 g/L bioethanol was obtained, making it a promising way of utilizing WH (Zhang et al. 2018b).
Biofuels
Several researchers have reported biogas generation by anaerobic fermentation of WH biomass. Typically, 60–70% methane was found in the generated biogas, with 15–25% CO2 and other gases. The considerable detail about the biogas is the absence of sulfur, which is advantageous for use as a motor fuel. A maximum value of 475 mL/g VS (volatile solids) for biogas and 214 mL/g VS for methane was recorded with a hydraulic retention time (HRT) of 45 days at 37 °C. Biogas thus produced contained 63.2% CH4 and 36.7% CO2, and the residual digest serves as an effective organic fertilizer for tomato cultivation (Keche et al. 2022). WH biomass was used holistically by utilizing WH shoot juice to produce biogas and the remaining fibers to produce biomass pellets. Biogas produced in this way consists of 68.7% CH4, 18.2% CO2, and 13.1% other gases with a specific methane yield of 237 L CH4/kg VS (Hudakorn and Sritrakul 2020).
A significant improvement in bio-methanation was observed by subjecting the WH biomass to weak acid hydrolysis and amending the process by adding 1% cattle dung biochar (BC) (Suthar et al. 2022). WH, cow manure, and sewage sludge were co-digested anaerobically at 37 °C to produce biogas. In 1 L batch, 812 mL of biogas was produced in 800 h with 65% methane, 14% carbon monoxide, and 21% other gases (Tasnim et al. 2017). A relatively higher yield of 505 L/kg of biomethane was obtained by anaerobic co-digestion of WH and dairy wastewater proportionally than the mono-digestion of WH or dairy wastewater alone. Also, a superior quality of bio-oil and biochar was obtained by utilizing the leftover residue (Arutselvy et al. 2021).
A holistic and efficient approach for energy production using WH biomass was tested in single, two, and three-stage operations of dark fermentation, bio-methanation, and microbial fuel cells. Energy in the form of hydrogen, methane, and electricity was produced. About 60% energy recovery was obtained in an integrated three-stage process with an overall COD removal of 94% (Varanasi et al. 2018). Methane enrichment efficacy was assessed for biosurfactant (Iturin A), and sonic waves combined pre-treatment of WH biomass giving biomethane production of 69 L/kg COD at alkaline pH due to the higher cell lysis (Sethupathy et al. 2022). Biogas was produced by WH biomass and assessed for running an internal-combustion engine to generate electricity. Also, the residual waste was utilized for briquette formation (Bote et al. 2020b). The effect of wet air oxidation (WAO) and alkaline wet air oxidation (AWAO) pre-treatments on the structure of WH and its bio-methanation was studied. The highest bio-methanation of 310 ± 4.1 mg COD/g feed was obtained in the case of AWAO pre-treatment followed by WAO treatment and no-treatment. Alkaline conditions promote better cell disintegration and methanation (Castro and Agblevor 2020). The seasonal WH biomass was collected and tested for biofuel production. A variable lipid content of 6.79–10.5% was observed in WH, which produces biodiesel in the range of 3.22–6.36% via transesterification. The produced diesel had shown good stability and usability. Pigments and glycerol were obtained from the sediment of the transesterification process. Additionally, the extracted residue was subjected to mild acid hydrolysis followed by ethanol production (Shanab et al. 2018).
Biopolymers and organic acids
Cupriavidus necator bacteria was used to produce poly(3-hydroxybutyrate) (PHB) from WH hydrolysate. A maximum of 7 g/L of PHB and 12 g/L of dry cell weight was obtained in an optimized medium supplemented with (NH4)2SO4 (Radhika and Murugesan 2012). Production of biopolymer PHB using WH and Parthenium hysterophorus was compared. A relatively higher yield of 36.4 mg PHB/g raw biomass was obtained using WH hydrolysate compared to 17.6 mg PHB/g raw biomass for P. hysterophorus hydrolysate (Pradhan et al. 2017). In another approach, hydrolysis was initially performed by adding cellulase (40 FPU/g of dry WH) after the alkaline and mild acidic pre-treatment. The hydrolysate, with 523 mg/g reducing sugars, was fermented with Ralstonia eutropha (ATCC 17,699) to produce PHB. A maximum PHB of 73% with a titer of 7.3 g/L and yield of 0.429 g/g of reducing sugars was obtained when the medium was supplemented with corn steep liquor as a cheap nitrogen source (Saratale et al. 2020). Thermophilic Bacillus coagulans was used for lactate production at 55 °C and pH 5.5. The separate saccharification and fermentation method was more effective than the simultaneous saccharification and fermentation method, as indicated by the relatively higher L-lactate yield of 0.19 g/g of dried WH biomass with the former. This was theorized to be due to the denaturation of cellulases at higher temperature conditions (Akao et al. 2012).
Nutrient medium for microbes and mushroom cultivation
WH juices and dehydrated powder were found to be an efficient medium for culturing microbes such as Azotobacter chroococcum, Rhizobium leguminosarum, Bacillus megaterium, and Bacillus subtilis which are helpful in agriculture (Ahmed et al. 2018). Gulati (1987) investigated the replacement of mannitol with fungal (Trichoderma reesei) hydrolysates of various cellulosic biomasses for preparing the YEM medium. WH, pea husk, and molasses at proportions of 2:2:1 could be used as a substitute for mannitol. Interestingly, it gave higher rhizobacterial growth than the traditional yeast extract mannitol medium (Gulati 1987). Effective utilization of WH biomass as a texturizer for bio-mycopesticide (Isaria fumosorosea) production was studied. WH biomass increases the porosity of the medium during solid-state fermentation, thereby improving gaseous exchange and yields. Using 20% WH in a medium of parboiled rice gave 1.55 times higher biopesticidal conidia production than using rice alone as substrate without compromising its infectivity against Galleria mellonella larvae (Angel-Cuapio et al. 2015). WH has also been investigated for edible oyster mushrooms (Pleurotus ostreatus) production as a low-cost biomass (Murugesan et al. 1995; Nageswaran et al. 2003; Ejigu et al. 2022). The total yield was almost 20–45% higher than using paddy straw substrate. Mixing WH biomass with sawdust gave a 71% higher yield for the growth of P ostreatus than sawdust alone (Martínez-Nieto et al. 2014). Chen et al. (2010) reported a comparatively safer and more efficient approach of using WH to remove phosphorus and ammoniacal nitrogen from the pig farm biogas fluid sedimentation tank, followed by using the spent WH biomass for Pleurotus geesteranus cultivation substituting sawdust as substrate (Chen et al. 2010). This could be a unique way to treat wastewater and enrich the substrate with essential nutrients such as phosphorus and nitrogen. However, the results were also favorable when WH was procured directly from the infested canals which may be contaminated with heavy metals. The negligible presence of lead and cadmium in the fruiting bodies and spent WH substrate suggests the possible usage of WH as a cheaper substrate for mushroom cultivation. Authors suggested lower metal toxicity in mushrooms was due to their phytotoxical effects. They also suggested utilizing spent substrate as bovine fodder but after evaluating the presence of anti-nutritional factors in it (Hermoso-López Araiza et al. 2016).
Thermochemical conversion of WH
Pyrolysis, gasification, hydrothermal treatment, and combustion are some thermochemical conversion procedures used to convert WH biomass into various value-added products, as summarized in Table 5.
Hydrothermal carbonization
Hydrothermal carbonization (HTC) could be used as a green method for converting WH into a lower moisture material with enhanced carbon content. The kinetic studies for HTC of WH revealed an activation energy of 90 kJ/mol at a temperature range of 423–483 K, which was lower than the activation energy in pyrolysis treatment (Luo et al. 2011). The highest heating value (HHV) for hydrochar was 21 MJ/kg, relatively higher than WH (15 MJ/kg). This was due to the higher lignin content in the hydrochar. Lignin has higher thermal stability in comparison to cellulose and hemicellulose. Consequently, hyrdochar has a greater proportion of lignin compared to untreated WH. Furthermore, lignin has greater HHV than cellulose and hemicellulose, thus increasing the HHV value for hydrochar (Gao et al. 2013; Zhang et al. 2020). Another concern is that the WH hydrochar is more challenging to combust than hydrochar obtained from other plants such as wheat. This was because the extent of carbonization was greater for WH than other biomasses based on their compositional differences (Gao et al. 2016). HTC studies using RSM revealed temperature to be the most influential factor, in addition to the time and the biomass load for optimizing solid yield, carbon, nitrogen capture, and heating value (Román et al. 2020). Pre-treatment of WH biomass before carbonization by washing with water and acid decreased heavy oil yield, but a significant reduction in sulfur, nitrogen, and ash content was also observed (Yao et al. 2020).
Pyrolysis gas
The temperature was found to be the critical factor affecting pyrolytic products, such as bio-oil, biochar, and syngas, in fixed-bed reactor pyrolysis (Rahman 2018). A 42% increase in syngas yield was observed during the pyrolysis of WH biomass in the presence of FeCl3 (Trần et al. 2020). Optimization of the process revealed particle size of less than 200 μm affords the highest yield, which can further be increased by the addition of potassium chloride (KCl), calcium oxide (CaO), or magnesium oxide (MgO), with the highest yield obtained using KCl at 900 °C (Hu et al. 2015). WH pyrolysis in a fixed bed reactor with Ni catalyst resulted in higher hydrogen production (101.2 g/kg biomass) when the process was carried out in two stages with a temperature of 650–700 °C for stage 1 and about 800 °C for stage 2 (Liu et al. 2014).
Pyrolysis oil
The pyrolysis process comprises three stages: moisture removal, devolatilization, and residual breakdown; and for WH, the pyrolysis occurs between 250 and 550 °C. GC–MS analyses of pyrolytic WH bio-oil revealed the presence of 21 compounds, including phenols, alcohols, carboxylic acids, ketones, quinines, alkenes, alkanes, aldehydes, and aromatics. The pH was reported to be 2.93, lower than regular fuels. Bio-oil was considered an environmentally benign fuel because of its HHV of 28.4 MJ/kg and lack of sulfur (Wauton and Ogbeide 2018, 2019a).
The bioenergy potential of WH leaves was higher than that of roots or stems as per the pyrolysis studies performed (Huang et al. 2020). Copper catalysts produced a higher bio-oil yield (31%), while aluminum-based catalysts favored the production of gases and light hydrocarbons over bio-oil (Gulab et al. 2019). In optimizing WH pyrolysis for bio-oil synthesis, the optimum temperature, particle size, and flow rate were found to be 450 °C, 0.6 mm, and 100 cm3/min, respectively (Wauton and Ogbeide 2019b). A comparison study of two-stage pyrolysis of fresh, putrefied, and microbe-treated biomass with 25% (w/w biomass) clinker (silicate) catalyst indicated a higher yield of microbially or putrefied biomass (Hussain et al. 2017). Microwave-assisted fast pyrolysis was also reported with excellent results using a Ce-doped γ-Al2O3/ZrO2 mesoporous catalyst (Zhang et al. 2018a). However, when supplemented with 5 wt%, CaO accorded in higher sugars and phenols, while acid formation was lower (Liang et al. 2019).
It is well-known that WH grows in water bodies that may be contaminated with heavy metals; therefore, a practical approach is to study the pyrolytic fate of such contaminated biomass. Few schemes with heavy metal biosorption followed by pyrolysis were proposed. Lead (Pb) was first adsorbed on WH biomass, which was then pyrolyzed. Pb-contaminated biomass produced higher hydrogen concentration in pyrolysis gas with 56% higher bio-oil yield. The result was attributed to the stabilization of carbonyl and carboxyl groups by Pb2+ ions (Jiu et al. 2015). The leachability of Pb in Pb-contaminated WH biomass was lowered by pyrolysis in the presence of phosphates (Shi et al. 2017). Similarly, pyrolysis of chromium (Cr)-polluted WH resulted in an increased bio-oil production of up to 63.1% with an HHV of 26.7 MJ/kg. Furthermore, Cr was converted into a non-toxic amorphous state in the biochar, reducing its environmental harm (Lin et al. 2018).
Calcined WH biomass was assessed for its application as phosphate-rich fertilizer. At low calcination temperatures, the principal crystalline phase was CaCO3, while Ca(OH)2 and Ca-phosphates such as hydroxyapatite were formed at higher temperatures (650–900 °C). Also, no hazardous elements were detected in the ashes. Authors suggested its potential application as fertilizer (Ramirez et al. 2021). Carbonization of WH was done at 900 °C to obtain carbon fibers. The fibers were non-graphitic with a tensile strength of 600 MPa and axial modulus of 42 GPa, comparable to commercial carbon fiber (Soenjaya et al. 2015).
Supercapacitors
The first instance of WH-derived activated carbon supercapacitor electrodes was by Senthilkumar et al. (2012). The carbon was activated by ZnCl2; the activated carbon electrodes exhibited a high capacitance of 912 F/g in the presence of a KI electrolyte in a three-electrode configuration (Senthilkumar et al. 2012). Carbon microspheres created by subcritical hydrothermal carbonization of WH in the presence of dilute H2SO4 showed a capacitance of 185 F/g in a three-electrode configuration (Kurniawan et al. 2015). Supercapacitor electrodes synthesized from hierarchical porous activated carbon derived from WH showed a capacitance of 345 F/g in a three-electrode assembly at a current density of 0.5 A/g (Zheng et al. 2017). Hierarchical porous carbon was synthesized from WH leaves and employed as a supercapacitor electrode and lithium-ion battery electrode giving a capacitance of 256 F/g and lithium storage capacity of 590 mAh/g, which was much higher than commercial activated carbon and graphite (Mo et al. 2020). Lu et al. (2020) devised a novel technique for activating WH carbon using a combination of KOH and HNO3. This carbon was fabricated into a capacitor electrode which showed a capacitance of 374 F/g in a three-electrode configuration (Lu et al. 2020).
WH biomass enriched with nickel-nitrogen (Ni–N) was subjected to fast pyrolysis with KOH activation at 773 K, and Ni − N doped porous carbon material (WHPC@Ni) was prepared with a specific supercapacitance value of 552 F/g. WHPC@Ni showed a high stability of 97.5% even after 10,000 cycles. The enhanced capacitance was due to the formation of NiO.nanoparticles during the pyrolysis (Sima et al. 2019). Similarly, the capacitance of WH-carbonized biomass that was previously utilized for phytoremediation of Ni2+ exhibited a capacitance of 541 F/g in a three-electrode configuration (Shell et al. 2021). Polypyrrole coated on WH-polyester composite prepared by in situ polymerization showed high areal capacitance values of 104 mF/cm2 (Alzate et al. 2022). Saning et al. (2019) fabricated a magnetic carbon adsorbent and supercapacitor electrode by activating the hydrochar obtained from WH using KOH and Fe3+ ions. The electrodes showed a good capacitance of 100 F/g in a symmetric two-electrode configuration (Saning et al. 2019).
Oxygen-reduction reaction
Activated carbon derived from WH was evaluated as an oxygen reduction reaction (ORR) electrode and displayed an excellent onset potential of 0.98 V against the reversible hydrogen electrode (RHE) (Liu et al. 2015). Carbonization of WH using molten salts using ZnCl2 was carried out. The nitrogen-doped carbon achieved a high H2O2 production potential of 1.7 mmol/L at a current efficiency of 81%, which was used to degrade dimethyl phthalate through an electro-Fenton reaction (Liang et al. 2018). ORR electrode of nitrogen-doped graphite from WH containing iron (Fe) through carbonization at 700 °C in the presence of Fe(NO3)3 showed an E1/2 voltage of 0.797 V, which is equivalent to the performance of the Pt/C electrode (0.833 V) at Pt loading of 8 μg cm−2 (Yan et al. 2019). The efficiency of WH biochar as an oxygen reduction reaction (ORR) catalyst was investigated. Pyrolyzed biochar obtained at 900 °C shows a power density of 24.7 mW/m2 in an air-microbial fuel cell, which was higher than the conventional Pt/C catalyst making it an inexpensive, alluring alternative for this purpose (Allam et al. 2020). Activated carbon was prepared using WH leaves, shoot, and root samples via pyrolysis. Activated carbon derived from shoots showed the maximum ORR onset potential of 0.9 V, followed by roots and leaves (Morales et al. 2021).
Catalysis
WH-activated carbon (WHc) was prepared by pyrolyzing biomass at 700 ℃ for 2 h, followed by its utilization for synthesizing nickel oxide (NiO) doped, WHc/NiO nanocomposite for supercapacitor application. A high specific capacitance of 240 F/g was observed with 78.4% retention after 1000 cycles (Qiu et al. 2017). Apart from being a catalyst for energy applications, WH hydrochar catalyst was synthesized for catalyzing glucose to fructose isomerization reaction. Simple carbonization of biomass at 400 °C for 1 h formed the catalyst, which gives 31% fructose yield with 89% selectivity. The endogenous calcium salts eliminate the need for doping with expensive metals (Yang et al. 2022). A carbon-based catalyst was synthesized from WH leaves by giving hydrothermal treatment. The catalyst obtained at 220 ℃ contains the highest acid sites offering 97% fatty acid conversions and 60% furfural yield from xylose dehydration (Laohapornchaiphan et al. 2017). The degradation of 4-nonylphenol (4-NP) by AOP using WH biochar (WHBC) was studied. Seventy-seven percent degradation was achieved with 1.5 g/L of calcium peroxide-activated WHBC (Hung et al. 2022). More recently, degradation of reactive red 2 (RR2) dye has been reported using copper oxide-loaded activated carbon catalyst synthesized from WH roots prepared through the wet impregnation method. A 100% dye decolorization and 88.6% COD conversion were achieved at a catalyst dose of 6 g/L. However, in the presence of free radical scavengers, sodium bicarbonate and methanol, 42.9 and 59% of dye decolorization were achieved, respectively (Ayalkie Gizaw and Gabbiye Habtu 2022).
Environmental applications
WH compositional structural polymers confer the biomass surface with hydroxyl, carboxyl, and other functional groups. Hence, it acts as an efficient and economical adsorbent for multiple contaminants removal (Abdolali et al. 2014). Biosorption of pollutants such as dyes, heavy metals, and emerging pollutants using WH biomass is reported in the literature and is presented in Table 6.
Biosorption
Dyes
Removal of methylene blue dye using WH dried shoot treated with water, hydrochloric acid, nitric acid, sodium hydroxide, and sodium sulfite was studied. Water-washed WH showed an adsorption capacity of 427 mg/g due to the high specific surface area (El-Khaiary et al. 2009). Adsorption of Indosol dark-blue GL dye by WH dried roots showed a maximum adsorption capacity of 86 mg/g at pH 3. It was noted that the adsorption rate was very rapid for the initial 15 min, and equilibrium was attained after 4 h, which was independent of the initial dye concentration. Dye desorption was done by changing the pH of eluent from low to high (Khan et al. 2014). The removal of crystal violet, a mutagenic textile dye, was tested using WH dried root powder. A biosorption capacity of 323 mg/g was noted as per the Langmuir monolayer model (Kulkarni et al. 2017). WH oven-dried cellulose was investigated for crystal violet (CV) and congo red (CR) dye adsorption in an aqueous system. A maximum adsorption capacity of 182 mg/g for CV and 230 mg/g for CR was obtained. The process followed pseudo-second-order kinetics as indicated by higher R2 values (0.99, 0.97 for CV and CR, respectively), and the theoretical and experimental qe values were in agreement. The systems were fitted to Langmuir isotherm for CV and Freundlich isotherm for CR based on R2 values. However, deeper mechanistic insight into the reasons leading to a difference in the isotherms followed is lacking (Salahuddin et al. 2021a).
Heavy metal ions
Purification of heavy metals-contaminated water from mining and industrial sites using WH dried powder was suggested. WH showed a maximum adsorption capacity of 47 mg/g for the lead, followed by cadmium, copper, and zinc, respectively (Schneider et al. 1995). Adsorption of lead (Pb2+), cadmium (Cd2+), and zinc (Zn2+) ions on acid pre-treated WH dried powder was tested in binary and ternary systems. Langmuir model fitted well with the maximum adsorption capacity in the order of Pb2+ (26.3) > Cd2+ (12.6) > Zn2+ (12.6 mg/g). The multi-element effect on adsorption was also tested (Mahamadi and Nharingo 2010). The H3PO4-activated WH showed a maximum adsorption capacity of 119 mg/g for lead (Huang et al. 2014).
The effect of washing WH dried root powder with acid and alkali on the removal of chromium (VI) anions was studied. An adsorbate concentration of 5 mg/L gave an adsorption capacity of 1.28 mg/g. In comparison, at 10 mg/L, it was 0.828 mg/g, which was fitted to the Freundlich isotherm model, and the adsorption followed pseudo-second-order kinetics (Kumar and Chauhan 2019). Citric acid-treated WH was tested for heavy metal ion adsorption. Sorption capacities of 96.9 mg/g for chromium (Cr6+), 78.0 mg/g for copper (Cu2+), and 59.6 mg/g for nickel (Ni2+) ions were obtained (Qu et al. 2019). In one recent study, the adsorption of fluoride ions was tested on hydrous aluminum- and iron oxides-doped WH-alginate beads. The effect of pH, flow rate, bed depth, and other factors was studied. Hydrous aluminum oxide-doped WH shows the highest adsorption capacity of 4.43 mg/g (Murambasvina and Mahamadi 2020). Dried and pulverized WH roots were combined with sodium tripolyphosphate and were tested for adsorption of chromium, Cr (IV) from tannery wastewater. Langmuir adsorption capacity of 7.7 mg/g was obtained (Carreño-Sayago 2021).
WH biomass biochar has also been found effective for contaminants removal by adsorption. Cadmium (Cd) adsorption capacity of 70.3 mg/g was obtained (Zhang et al. 2015). WH biochar modified with ZnO nanoparticles showed a biosorption capacity of 43.48 mg/g for Cr(Vi) (Yu et al. 2018). WH biochar was investigated for the adsorption of trivalent chromium ions from the tannery wastewater. Chromium concentration in the water reduced from 3190.1 to 27.3 mg/L. The adsorption behavior was fitted to Freundlich isotherm and pseudo-first-order kinetics. The chloride, biochemical oxygen demand (BOD), and chemical oxygen demand (COD) were also reduced by 56%, 93.4%, and 92.6%, respectively (Hashem et al. 2020).
Emerging pollutants
Adsorption of antibiotic sulfachloropyridazine (SCP) was studied using WH root powder, and a maximum adsorption capacity of 227 mg/g was obtained. Adsorption followed acid–base interactions and was favored by acidic pH conditions (Liu et al. 2018). WH root powder was used as a low-cost adsorbent to remove 2,4-dichlorophenoxy acetic acid (2, 4-D), a common pesticide from an aqueous environment. A maximum monolayer adsorption capacity (qmax) of 40 mg/g was obtained using acid and ultrasound-treated biosorbent (Aswani and Pavan Kumar 2019). Magnetic WH-based biosorbent was investigated for its ability to adsorb ibuprofen and remove copper, zinc, nickel, and cobalt. The value of qmax (mg/g) was found to be 18.3 for Cu(II), 10.1 for Zn(II), 7.33 for Ni(II), and 1.02 mg/g for IBP. The selectivity was in order, Cu > Zn > Ni (Lima et al. 2020).
Phytoremediation
WH possesses an enormous capacity for bioaccumulating pollutants and could be utilized as a pollution bioindicator (De Laet et al. 2019). WH has been found to accumulate pollutants such as heavy metals, dyes, antibiotics, and several other contaminants. Pollutants, especially organic contaminants, increase the levels of ammoniacal nitrogen in domestic and industrial wastewater, giving rise to algal blooms and further deteriorating the water quality. Details on the application of WH for phytoremediation are shown in Table 7. WH plants could be utilized for the phytoremediation of lead. An increase in antioxidant enzymes such as superoxide dismutase, catalase, ascorbate peroxidase, and peroxidase in plant tissue was observed when exposed to a high lead concentration of 800 mg/L. These enzymes play a crucial role in increasing the tolerance against oxidative stress (Malar et al. 2014). Europium metal (Eu (III)), a non-radioactive surrogate for Americium (III), a radioactive waste, was tested for phytoremediation using WH plants grown in the greenhouse. The removal efficiency of 26% was observed for Eu (III), indicating its possible utilization for phytoremediation of radioactively polluted water (Kelley et al. 1999). Phytoremediation of paper and pulp industry wastewater using WH was studied. Regression modeling was done to investigate the effect of pH and initial metal ion concentration on the plant’s accumulation capacity. The studied model fits well and indicates efficient phytoremediation for heavy metals (Cd, Cu, Cr, Fe, Pb, Zn, Mn) (Kumar et al. 2020). The effectiveness of WH plants for removing heavy metals from the glass industry was tested for 40 days. Maximum removal of 91.3% for Cd, 93.6% for Cu, 92.8% for Fe, and 93.5% for Mn was observed (Singh et al. 2021).
The accumulation and biodegradation of a phosphorus insecticide, ethion, in WH plants were examined. The effect of plant-associated microbes on ethion removal was estimated by calculating the difference in the results obtained by non-sterile and sterile plants. The contribution of phytoaccumulation and phytodegradation was significantly higher (69%) than that of microbial degradation, which contributed only 12%. It suggested phytoaccumulation and phytodegradation were the primary mechanisms for ethion removal (Xia and Ma 2006). A 10-day-long randomized block experiment was performed to study the role of WH in removing chlorpyrifos, an organophosphate insecticide, from water. Removal was further increased in the presence of a root-associated bacterium identified as Acinetobacter sp. (Anudechakul et al. 2015). A study on the removal of antibiotic tetracyclines (TCs) and the effect of copper ions on the accumulation and translocation of TCs in WH plants concluded that combining a high concentration of copper and TCs could be more effective (Lu et al. 2014). WH was found to have excellent potential for the bioaccumulation of organophosphorus pesticides. It also effectively removed some of the organochlorine pesticides tested in an onsite study performed for irrigation canals of Mexico wetlands (Mercado-Borrayo et al. 2015). Removal of two herbicides (mesotrione and fomesafen) using WH plants was tested in the randomized block-designed experiments. About 70–92% removal was observed for mesotrione, and 22–34% for fomesafen was obtained after 14 days (Chen et al. 2019).
WH was found to remove 75.1% and 54.7% nitrates from underlay and sewage water, respectively, while for phosphates, a removal capacity of 78.9% in underlay water and 86.1% in sewage water was observed. GC–MS analysis of the hexane extract of WH revealed the presence of oleic acid (35.5%), an important compound. In addition, the mechanical properties of WH fibers were also studied. WH fiber’s tensile strength increases to 315 MPa when four strands are knitted together compared to the low tensile value of 14 MPa for a single WH fiber (Adelodun et al. 2020). Domestic wastewater was treated with WH plants for 30 days in continuous mode. A moderate removal capacity of 63.26 ± 10.47%, 61.96 ± 12.11%, and 51.91 ± 5.32% was observed for ammonium–nitrogen, phosphate–phosphorous, and chemical oxygen demand, respectively. Harvesting WH plants at a regular interval of 15–20 days was suggested for efficient performance. However, the authors emphasize the need to develop a more efficient harvesting method to remove selectively matured plants and leave baby plants in the system (Prasad et al. 2021).
Phytoremediation of dye-loaded wastewater was also studied using WH plants. Absorption and degradation of both cationic [rose bengal (RB), methylene blue (MB), crystal violet (CV), auramine O (AO), rhodamine B (RhB)], and anionic [xylenol orange (XO), phenol red (PR), cresol red (CR), ans methyl orange (MO)] dyes were studied by growing WH plants. WH plants can be a potential decolorizer with a color removal efficiency of 79 to 90.8% for cationic dyes and 33.3 to 62.8% for anionic dyes (Sharma et al. 2021). The river water near a dye industry was treated with WH, and the best results were observed within 7 days with an optimized WH biomass (20%). A removal efficiency of 46% for chromium and 43% for lead was observed. A significant decrease in pH, BOD, COD, and TDS values was also observed (Panneerselvam and Priya 2021).
Phytoremediation of oil spills using WH was studied in an experiment performed in Nigeria using 45 experimental units. An increase in total petroleum carbon in WH was detected, indicating its effectiveness for oil uptake. However, no significant increase in oil absorption was observed on stimulating plants with urea (Ndimele and Ndimele 2013). Phytoremediation of formaldehyde using WH was also tested. High removal efficiency for an initial formaldehyde concentration of 100–300 ppm was observed, which was further increased on stimulating plants with 0.5 ppm Eupatorium odoratum L. extract (Gong et al. 2018). The efficacy of WH for removing and degrading anionic surfactant, SDS, was studied. A significant increase in ascorbate peroxidase (APX) activity was observed in response to the stress generated by pollutants. The growth of WH was regulated by using Chromolaena odorata L. extract as a biostimulator (Gong et al. 2019).
Biorefinery integration for circular economy
Research significance
The incredible potential of biomass as a resource-generating sustainable material has recently come to light, and there is a good chance that it will grow its market share soon (Martínez-Ruano et al. 2018; Solarte-Toro et al. 2022). WH biomass is a fantastic feedstock for recovering nutrients and energy, in contrast to being a possible hazard to the ecosystem and environment. The current work investigates three alternative conversion methodologies, including biochemical conversion, thermochemical conversion, and green synthesis methodology, after directly extracting phytometabolites to manufacture valuable primary and secondary products from WH. The WH biomass can either be harvested directly from the infested waterbodies or after it has been used as a phytofilter to purify nutrient-laden wastewater (Fig. 2).
It is beneficial to remove all the solvent-soluble extractives, mainly different phytometabolites, including phenolic compounds, flavonoids, alkaloids, and others, to minimize contaminants during cellulose extraction. Eliminating these low molecular weight compounds is also advantageous while converting the biomass to valuable products by biochemical route, as these compounds tend to inhibit microbial growth during fermentation (Parawira and Tekere 2011; Jönsson et al. 2013). Indeed, these phytometabolite-rich extracts could be utilized for the green synthesis of nanoparticles, catalysts, or other similar commodities owing to their antioxidant potential and antimicrobial properties. Recently, the thermochemical conversion of biomass has been a hot topic among researchers as the increase in the demand for green energy has been observed. So forth, various types of thermochemical conversions have been studied for WH. However, hydrothermal conversion is suggested as a feasible process considering the high initial moisture content of the plant. It is a comparatively convenient energy efficient method and yields bio-oil, hydrochar, and other valuable products. More studies in this regard are needed.
Low-volume high-value bioproducts like phenolic compounds, flavonoids, alkaloids, and enzymes may increase the economic revenues of biomass many folds in comparison to the production of low-value bulk products such as biofuel and bioenergy alone. This is primarily due to the more significant financial and energy expenses associated with biofuel production brought on by high cultivation costs of biomass and poor value for biofuels (Escamilla-Alvarado et al. 2017). However, if procured directly from natural resources, the cultivation expense for WH might be avoided, which is an additional benefit. Hence, an inclusive approach of producing low-volume high-value and high-volume low-value products simultaneously via a suggested cascading framework could make biorefinery economically viable (Joglekar et al. 2019). Here we have provided new insights and an integrated strategy incorporating diverse sectors, which will undoubtedly increase the biorefinery’s economic feasibility.
Environmental implications
Based on WH’s potential for phytoremediation (Li et al. 2015; Qin et al. 2016; Ting et al. 2018; Singh et al. 2022), it is recommended to utilize or cultivate it as a phytofilter and use the harvested biomass as a biorefinery feedstock. Even though WH is widely distributed in nature (Kriticos and Brunel 2016; Thamaga and Dube 2018), this strategy can resolve the bottleneck of the steady supply of biomass in an economically sound approach. A comprehensive model for treating eutrophicated water and continual harvest of WH biomass for dry and rainy seasons has been proposed (Mahujchariyawong and Ikeda 2001). It is crucial to remember that a compositional variation is very likely based on the WH’s growing environment. The targeted final products and their uses should be the basis for deciding on the overall integration to harness the economic benefits.
Rapid industrialization in recent years has resulted in global warming due to the significant release of greenhouse gases (GHGs). One of the practical options for CO2 capture is the fixation of CO2 in biomass. Nowadays, microalgae are employed for this purpose by applying the carbon capture and utilization (CCU) approach (Cuéllar-Franca and Azapagic 2015). Being a photosynthetic plant, it can be argued that WH would capture carbon and be considered for the CCU model, which could lower the overall carbon emissions. In the end, the residual solid waste could be used as a biosorbent to treat wastewater, whether it was produced through direct extraction, conversion, or a mix of valorization techniques. This route provides a logical and efficient way to utilize the by-products generated during the processing steps. Many research studies support the concept of using spent biomass/biochar as a low-cost adsorbent to remove a wide variety of contaminants from wastewater (Mahamadi and Nharingo 2010; Mishra and Maiti 2017; Hung et al. 2022). The proposed holistic approach addresses all three dimensions, including social, economic, and environmental, to achieve sustainability goals (Moldavska and Welo 2019).
Circular bioeconomy
Developing nations harvest tonnes of WH annually (Sethupathy et al. 2022). The damaging ecological and socio-economic effects of WH biowaste could be minimized by implementing eco-friendly circular approaches, which effectively manage biomass waste through its beneficial processing (Tanveer et al. 2022). The idea of the biorefinery is concerned with the efficient and sustainable conversion of biomass into many industrial goods, such as chemicals, materials, and energy. It may be a promising solution for turning waste into value that eventually fits into a circular economy by promoting the concept of reducing, reusing, and recycling to ensure environmental viability. With an emphasis on reducing waste at every production stage, the integrated strategic framework suggested here would enable the well-organized exploitation of waste biomass and the generated by-products for creating value-added commodities. It offers innovative solutions to the current WH conundrum by aiming to engage with renewable resources while decreasing the reliance on fossil fuels, monetizing the waste biomass, and reducing GHG emissions (Kumar Sarangi et al. 2022; Moustakas and Loizidou 2022).
Table 8 discusses the proposed WH biorefinery’s SWOT (strength, weakness, opportunity, and threat) analysis, outlining its benefits and drawbacks in the current market scenario (Usmani et al. 2021). WH biomass is a good source of phytometabolites. Harnessing WH favors green engineering approaches of reduction, reuse, and recycling while promoting bioeconomy. Significant social and environmental benefits accrue due to the holistic utilization of WH biomass in biorefinery. Concerns against these approaches include seasonal variations in biomass growth and compositional variations, geographical limitations, high initial costs, absence of infrastructure, and mature technologies. Another threat to a potential WH biorefinery is the year-round availability of biomass and the logistics for collection from distributed sites. However, opportunities exist to bolster such efforts through policies that can assist in achieving UN Sustainable Development Goals.
Future perspectives
Utilizing lignocellulosic biomass as a sustainable feedstock to manufacture valuable products has gained attention over time. Several policies, environmental regulations, and protocols have been devised in many nations to reduce the ecological danger associated with waste biomass (Khoshnevisan et al. 2021). Although the public and private sectors have implemented many waste biomass valorization schemes, appropriate commercial success is yet to be gained. This might result from the absence of integrated management regulations, which must consider the advantages and limitations of the proposed biorefinery scheme. To develop a spatially explicit biorefinery model, it should incorporate features of economic viability along with ecological, social, and environmental impacts.
Future study is still needed based on the local requirements accounting for the detailed economic, social, and environmental assessment through various simulation models (Aristizábal-Marulanda et al. 2021; Solarte-Toro et al. 2022). A regional financial analysis, considering the entire costs determined by raw material, utilities, labor, general maintenance, and administrative expenses, must be carried out before implementation (Giwa et al. 2018; Martínez-Ruano et al. 2018; Serna-Loaiza et al. 2018). There is still room for a life cycle assessment (LCA) for the WH biorefinery plan. LCA is a tool that helps in the process of identifying the steps that have a substantial environmental impact. These environmental hotspots could be addressed by process intensification (Joglekar et al. 2019). Process intensification methods may be used to extract phytometabolites or for biomass conversion procedures. Ultrasound, microwaves, supercritical or subcritical fluids, steam explosion, and other innovative technologies might be applied at the industrial scale. These novel technologies typically appear energy efficient achieving maximum yields in a shorter time (Nagula and Pandit 2016; Perino and Chemat 2019). Unfortunately, most of the current biomass conversion methods produce CO2 and methane, the greenhouse gases (GHG) that contribute to global warming (Hariz and Takriff 2017). Commercialization of the green potential of WH would directly cut down on GHG emissions through CO2 fixation, which is another crucial research topic. A sustainability index (SI) based on real-time data will help to support a strong, flourishing biomass processing sector (Joglekar et al. 2020). LCA, SI, and other models are not included in this study due to the lack of real-time data variables. The proposed integrated biorefinery model must yet undergo a region-specific techno-economic assessment for industrial scale-up to be implemented successfully.
Conclusion
WH eradication is a difficult task today, especially in poorer nations, due to the high expenses. The unique properties of this highly invasive plant make it a better alternative for developing sustainable bio-based products. WH acts as a good phytofilter, so its controlled growth will benefit the phytoremediation of water bodies, and the resulting biomass could be utilized for producing various primary and secondary bio-products. WH could become a viable resource for generating green plant-based products to fulfill the increasing demand for safe and eco-friendly products. Comprehensively, we have explored the potential for WH waste biomass valorization through direct extraction, its conversion into valued bioproducts, and its environmental implications to promote sustainability and a circular economy. WH has a high nutrient value. WH biomass is advantageous over other lignocellulosic waste due to its less complex nature, resulting in milder pre-treatment requirements. WH compositional analysis reveals the presence of fibrous polysaccharides and the absence of sticky substances, which ensures its rapid drying despite high initial moisture content. Phytoconstituents of WH could be investigated further in a specific- and application-based manner. Most of the research done to date involves using conventional solvents and methods. Process intensification is needed to make the processing more economical and efficient. The overall multi-objectives framework should be followed by emphasizing the concept of reduction, reuse, and recycling to attain the goals of sustainability and circular economy. A careful techno-economic analysis based on the local parameters is desirable to harness the maximum benefits. We have explored the cutting-edge future WH biorefinery opportunities extending the conventional methods as a commercially sustainable response to several issues confronting us today to manage WH growth and promote a circular economy efficiently.
Data availability
Not applicable.
References
Abdalla AA, Hafeez ATA (1969) Some aspects of utilization of water hyacinth (Eichhornia crassipes). PANS Pest Artic News Summ 15:204–207. https://doi.org/10.1080/04345546909415116
Abdelhamid AM, Gabr AA (1991) Evaluation of water hyacinth as a feed for ruminants. Arch Für Tierernaehrung 41:745–756. https://doi.org/10.1080/17450399109428519
Abdolali A, Guo WS, Ngo HH, Chen SS, Nguyen NC, Tung KL (2014) Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Bioresour Technol 160:57–66. https://doi.org/10.1016/j.biortech.2013.12.037
Abo-Elmagd HI, Housseiny MM (2012) Purification and characterization of carboxymethyl cellulase and protease by Ulocladium botrytis Preuss ATCC 18042 using water hyacinth as a substrate under solid state fermentation. Ann Microbiol 62:1547–1556. https://doi.org/10.1007/s13213-011-0409-0
Aboul-Enein AM, Al-Abd AM, Shalaby E, Abul-Ela F, Nasr-Allah AA, Mahmoud AM, El-Shemy HA (2011) Eichhornia crassipes (Mart) solms. Plant Signal Behav 6:834–836. https://doi.org/10.4161/psb.6.6.15166
Adelodun AA, Hassan UO, Nwachuckwu VO (2020) Environmental, mechanical, and biochemical benefits of water hyacinth (Eichhornia crassipes). Environ Sci Pollut Res 27:30210–30221. https://doi.org/10.1007/s11356-020-09221-1
Agarwala ON (1988) Water hyacinth (Eichhornia crassipes) silage as cattle feed. Biol Wastes 24:71–73. https://doi.org/10.1016/0269-7483(88)90028-6
Ahmed AMA, Khan SJ, Mojumder N, Sharmin F, Rahman A, Bakar MA, Chowdhury JMKH, Azadi MA (2016) Water hyacinth (Eichhornia crassipes fractions potentially normalize the lead (Pb) poisoning and enhance in vitro thrombolysis. Orient Pharm Exp Med 16:321–331. https://doi.org/10.1007/s13596-016-0243-9
Ahmed RH, Badawi HM, Ali AS, Fayez M (2018) Growth performance of rhizobacteria on water hyacinth (Eichhornia crassipes) juices and dehydrated powder. Egypt J Aquat Res 44:1–7. https://doi.org/10.1016/j.ejar.2018.01.002
Akao S, Maeda K, Nakatani S, Hosoi Y, Nagare H, Maeda M, Fujiwara T (2012) Comparison of simultaneous and separate processes: saccharification and thermophilic L-lactate fermentation of catch crop and aquatic plant biomass. Environ Technol 33:1523–1529. https://doi.org/10.1080/09593330.2012.669412
Allam F, Elnouby M, El-Khatib KM, El-Badan DE, Sabry SA (2020) Water hyacinth (Eichhornia crassipes) biochar as an alternative cathode electrocatalyst in an air-cathode single chamber microbial fuel cell. Int J Hydrog Energy 45:5911–5927. https://doi.org/10.1016/j.ijhydene.2019.09.164
Alzate DJG, Peñafiel FCR, Binag CA (2022) Polypyrrole on pineapple (Ananas comosus) and water hyacinth (Eichhornia crassipes) polyester blended textiles as promising electrode materials for supercapacitor applications. Mater Chem Phys 279:125774. https://doi.org/10.1016/j.matchemphys.2022.125774
Anaya AL, Ramos L, Hernandez JG, Cruz R (1987) Allelopathy in Mexico. In: Waller GR (ed) Allelochemicals: role in agriculture and forestry. American Chemical Society, Washington, DC, pp 89–101
Angel-Cuapio A, Figueroa-Montero A, Favela-Torres E, Viniegra-González G, Perraud-Gaime I, Loera O (2015) Critical values of porosity in rice cultures of isaria fumosorosea by adding water hyacinth: effect on conidial yields and quality. Appl Biochem Biotechnol 177:446–457. https://doi.org/10.1007/s12010-015-1754-4
Anudechakul C, Vangnai AS, Ariyakanon N (2015) Removal of chlorpyrifos by water hyacinth (Eichhornia crassipes) and the role of a plant-associated bacterium. Int J Phytoremediation 17:678–685. https://doi.org/10.1080/15226514.2014.964838
Arana-Cuenca A, Tovar-Jiménez X, Favela-Torres E, Perraud-Gaime I, González-Becerra AE, Martínez A, Moss-Acosta CL, Mercado-Flores Y, Téllez-Jurado A (2019) Use of water hyacinth as a substrate for the production of filamentous fungal hydrolytic enzymes in solid-state fermentation. 3 Biotech 9:21. https://doi.org/10.1007/s13205-018-1529-z
Aristizábal-Marulanda V, Solarte-Toro JC, Cardona Alzate CA (2021) Study of biorefineries based on experimental data: production of bioethanol, biogas, syngas, and electricity using coffee-cut stems as raw material. Environ Sci Pollut Res 28:24590–24604. https://doi.org/10.1007/s11356-020-09804-y
Arutselvy B, Rajeswari G, Jacob S (2021) Sequential valorization strategies for dairy wastewater and water hyacinth to produce fuel and fertilizer. J Food Process Eng 44:e13585. https://doi.org/10.1111/jfpe.13585
Aswani MT, Pavan Kumar MV (2019) A novel water hyacinth based biosorbent for 2,4-dichlorophenoxyacetic acid (2,4-D) removal from aqueous solution. Desalin Water Treat 165:163–176. https://doi.org/10.5004/dwt.2019.24581
Ayalkie Gizaw B, Gabbiye Habtu N (2022) Catalytic wet air oxidation of azo dye (reactive red 2) over copper oxide loaded activated carbon catalyst. J Water Process Eng 48:102797. https://doi.org/10.1016/j.jwpe.2022.102797
Bodo R, Ahmanache K, Hausler R, Azzouz A (2004a) Optimized extraction of total proteic mass from water hyacinth dry leaves. J Environ Eng Sci 3:529–536. https://doi.org/10.1139/s04-023
Bodo R, Azzouz A, Hausler R (2004b) Antioxidative activity of water hyacinth components. Plant Sci 166:893–899. https://doi.org/10.1016/j.plantsci.2003.12.001
Bolenz S, Omran H, Gierschner K (1990) Treatments of water hyacinth tissue to obtain useful products. Biol Wastes 33:263–274. https://doi.org/10.1016/0269-7483(90)90130-K
Bote MA, Naik VR, Jagadeeshgouda KB (2020a) Review on water hyacinth weed as a potential bio fuel crop to meet collective energy needs. Mater Sci Energy Technol 3:397–406. https://doi.org/10.1016/j.mset.2020.02.003
Bote MA, Naik VR, Jagdeeshgouda KB (2020b) Production of biogas with aquatic weed water hyacinth and development of briquette making machine. Mater Sci Energy Technol 3:64–71. https://doi.org/10.1016/j.mset.2019.09.001
Carreño-Sayago UF (2021) Development of microspheres using water hyacinth (Eichhornia crassipes) for treatment of contaminated water with Cr(VI). Environ Dev Sustain 23:4735–4746. https://doi.org/10.1007/s10668-020-00776-0
Castro YA, Agblevor FA (2020) Effect of wet air oxidation on the composition and biomethanation of water hyacinth. Biomass Convers Biorefinery 1–12. https://doi.org/10.1007/s13399-020-00825-8
Chang C-C, Tan H-C, Cheng W (2013) Effects of dietary administration of water hyacinth (Eichhornia crassipes) extracts on the immune responses and disease resistance of giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol 35:92–100. https://doi.org/10.1016/j.fsi.2013.04.008
Chen X, Jiang Z, Chen X, Lei J, Weng B, Huang Q (2010) Use of biogas fluid-soaked water hyacinth for cultivating Pleurotus geesteranus. Bioresour Technol 101:2397–2400. https://doi.org/10.1016/j.biortech.2009.11.045
Chen Z, Huang L, Song S, Zhang Y, Li Y, Tan H, Li X (2019) Enhanced disappearance of mesotrione and fomesafen by water hyacinth (Eichhornia crassipes) in water. Int J Phytoremediation 21:583–589. https://doi.org/10.1080/15226514.2018.1540543
Cheng G-G, Liu Y-P, Gu J, Qian S-Y, Yang H-J, Na Z-Y, Luo X-D (2021) Phytochemicals and allelopathy of induced water hyacinth against Microcystis aeruginosa. J Nat Prod 84:1772–1779. https://doi.org/10.1021/acs.jnatprod.1c00075
Coetzee JA, Hill MP, Ruiz-Téllez T, Starfinger U, Brunel S (2017) Monographs on invasive plants in Europe No 2: Eichhornia crassipes (Mart.) Solms. Bot Lett 164:303–326. https://doi.org/10.1080/23818107.2017.1381041
Costa MF, Luiz MM, de Souza LC, Tempone AG, Lago JHG, Nascimento IR (2021) Phenylnaphthalic anhydrides from water hyacinth (Pontederia crassipes Mart.). Phytochem Lett 46:1–5. https://doi.org/10.1016/j.phytol.2021.09.003
Cuéllar-Franca RM, Azapagic A (2015) Carbon capture, storage and utilisation technologies: a critical analysis and comparison of their life cycle environmental impacts. J CO2 Util 9:82–102. https://doi.org/10.1016/j.jcou.2014.12.001
Das A, Ghosh P, Paul T, Ghosh U, Pati BR, Mondal KC (2016) Production of bioethanol as useful biofuel through the bioconversion of water hyacinth (Eichhornia crassipes). 3 Biotech 6:70. https://doi.org/10.1007/s13205-016-0385-y
Dass A, Chellamuthu S (2022) Physico chemical and mechanical properties of natural cellulosic water hyacinth fiber and its composites. J Nat Fibers 19:11413–11423. https://doi.org/10.1080/15440478.2022.2025979
De Laet C, Matringe T, Petit E, Grison C (2019) Eichhornia crassipes: a powerful bio-indicator for water pollution by emerging pollutants. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-43769-4
de Melo MMR, Silva RP, Silvestre AJD, Silva CM (2016) Valorization of water hyacinth through supercritical CO2 extraction of stigmasterol. Ind Crops Prod 80:177–185. https://doi.org/10.1016/j.indcrop.2015.11.036
de Vasconcelos GA, Véras RML, de Lima SJ, Cardoso DB, de Castro SP, de Morais NNG, Souza AC (2016) Effect of water hyacinth (Eichhornia crassipes) hay inclusion in the diets of sheep. Trop Anim Health Prod 48:539–544. https://doi.org/10.1007/s11250-015-0988-z
Debnath D, Yengkokpam S, Bhattacharjya BK, Biswas P, Prakash C, Kohli MPS, Sharma AP (2018) Effect of dietary incorporation of dry-powdered water hyacinth (Eichhornia crassipes) meal on growth and digestibility of Labeo rohita fingerlings. Proc Zool Soc 71:74–82. https://doi.org/10.1007/s12595-016-0187-6
DellaGreca M, Previtera L, Zarrelli A (2009) Structures of new phenylphenalene-related compounds from Eichhornia crassipes (water hyacinth). Tetrahedron 65:8206–8208. https://doi.org/10.1016/j.tet.2009.07.069
DellaGreca M, Previtera L, Zarrelli A (2008) Revised structures of phenylphenalene derivatives from Eichhornia crassipes. Tetrahedron Lett 49:3268–3272. https://doi.org/10.1016/j.tetlet.2008.03.072
Dersseh MG, Melesse AM, Tilahun SA, Abate M, Dagnew DC (2019) Chapter 19 - Water hyacinth: review of its impacts on hydrology and ecosystem services—lessons for management of Lake Tana. In: Melesse AM, Abtew W, Senay G (eds) Extreme hydrology and climate variability. Elsevier, pp 237–251
Deshpande SK, Bhotmange MG, Chakrabarti T, Shastri PN (2008) Production of cellulase and xylanase by Trichoderma reesei (QM 9414 mutant), Aspergillus niger and mixed culture by solid state fermentation (SSF) of water hyacinth (Eichhornia crassipes). Indian J Chem Technol 15:449–456
Dhote S, Dixit S (2009) Water quality improvement through macrophytes—a review. Environ Monit Assess 152:149–153. https://doi.org/10.1007/s10661-008-0303-9
Ejigu N, Sitotaw B, Girmay S, Assaye H (2022) Evaluation of oyster mushroom (Pleurotus ostreatus) production using water hyacinth (Eichhornia crassipes) biomass supplemented with agricultural wastes. Int J Food Sci 2022:9289043. https://doi.org/10.1155/2022/9289043
Elagib SM (2020) Antiparasitic activity of Eichhornia crassipes leaves extract. Biocatal Agric Biotechnol 24:101556. https://doi.org/10.1016/j.bcab.2020.101556
Elgala AM, Abd-Elrahman SH, Saudy HS, Nossier MI (2022) Exploiting Eichhornia crassipes shoots extract as a natural source of nutrients for producing healthy tomato plants. Gesunde Pflanz. https://doi.org/10.1007/s10343-022-00622-5
El-Khaiary MI, Gad FA, Mahmoud MS, Samy HE-D (2009) Adsorption of methylene blue from aqueous solution by chemically treated water hyacinth. Toxicol Environ Chem 91(1079):1094. https://doi.org/10.1080/02772240802541379
Escamilla-Alvarado C, Poggi-Varaldo HM, Ponce-Noyola MT (2017) Bioenergy and bioproducts from municipal organic waste as alternative to landfilling: a comparative life cycle assessment with prospective application to Mexico. Environ Sci Pollut Res 24:25602–25617. https://doi.org/10.1007/s11356-016-6939-z
Ewulonu CM, Chukwuneke JL, Nwuzor IC, Achebe CH (2020) Fabrication of cellulose nanofiber/polypyrrole/polyvinylpyrrolidone aerogels with box-Behnken design for optimal electrical conductivity. Carbohydr Polym 235:116028. https://doi.org/10.1016/j.carbpol.2020.116028
Figueroa-Torres LA, Lizardi-Jiménez MA, López-Ramírez N, Varela-Santos EC, Hernández-Rosas F, Favela-Torres E, Hernández-Martínez R (2020) Saccharification of water hyacinth biomass by a combination of steam explosion with enzymatic technologies for bioethanol production. 3 Biotech 10:432. https://doi.org/10.1007/s13205-020-02426-8
Fileto-Pérez HA, Rutiaga-Quiñones OM, Sytsma MD, Lorne IM, Luo W, Pankow JF, Rutiaga-Quiñones JG (2015) GC/MS analysis of some extractives from Eichhornia crassipes. BioResources 10:7353–7360
Ganguly A, Chatterjee PK, Dey A (2012) Studies on ethanol production from water hyacinth—a review. Renew Sustain Energy Rev 16:966–972. https://doi.org/10.1016/j.rser.2011.09.018
Ganguly P, Chanda S, Barua AK, Choudhury MK (1977) Role of steroids and amino acids in pollen germination of Eichhornia crassipes Solms. Grana 16:41–43. https://doi.org/10.1080/00173134.1977.11864638
Ganorkar PV, Jadeja GC, Desai MA (2022) Extraction of shikimic acid from water hyacinth (Eichhornia crassipes) using sonication: an approach towards waste valorization. J Environ Manage 305:114419. https://doi.org/10.1016/j.jenvman.2021.114419
Gao Y, Wang X, Wang J, Li X, Cheng J, Yang H, Chen H (2013) Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy 58:376–383. https://doi.org/10.1016/j.energy.2013.06.023
Gao Y, Yu B, Wu K, Yuan Q, Wang X, Chen H (2016) Physicochemical, pyrolytic, and combustion characteristics of hydrochar obtained by hydrothermal carbonization of biomass. BioResources 11:4113–4133
Gaurav GK, Mehmood T, Cheng L, Klemeš JJ, Shrivastava DK (2020) Water hyacinth as a biomass: a review. J Clean Prod 277:122214. https://doi.org/10.1016/j.jclepro.2020.122214
Ghabbour EA, Davies G, Lam Y-Y, Vozzella ME (2004) Metal binding by humic acids isolated from water hyacinth plants (Eichhornia crassipes [Mart.] Solm-Laubach: Pontedericeae) in the Nile Delta, Egypt. Environ Pollut 131:445–451. https://doi.org/10.1016/j.envpol.2004.02.013
Girisuta B, Danon B, Manurung R, Janssen LPBM, Heeres HJ (2008) Experimental and kinetic modelling studies on the acid-catalysed hydrolysis of the water hyacinth plant to levulinic acid. Bioresour Technol 99:8367–8375. https://doi.org/10.1016/j.biortech.2008.02.045
Giwa A, Adeyemi I, Dindi A, Lopez CG-B, Lopresto CG, Curcio S, Chakraborty S (2018) Techno-economic assessment of the sustainability of an integrated biorefinery from microalgae and Jatropha: a review and case study. Renew Sustain Energy Rev 88:239–257. https://doi.org/10.1016/j.rser.2018.02.032
Gong Y, Chen J, Pu R (2019) The enhanced removal and phytodegradation of sodium dodecyl sulfate (SDS) in wastewater using controllable water hyacinth. Int J Phytoremediation 21:1080–1089. https://doi.org/10.1080/15226514.2019.1606779
Gong Y, Zhou X, Ma X, Chen J (2018) Sustainable removal of formaldehyde using controllable water hyacinth. J Clean Prod 181:1–7. https://doi.org/10.1016/j.jclepro.2018.01.220
Greenfield BK, Siemering GS, Andrews JC, Rajan M, Andrews SP, Spencer DF (2007) Mechanical shredding of water hyacinth (Eichhornia crassipes): effects on water quality in the Sacramento-San Joaquin River Delta, California. Estuaries Coasts 30:627–640. https://doi.org/10.1007/BF02841960
Gulab H, Hussain K, Malik S, Hussain M (2019) Effect of process conditions on bio-oil composition and production from catalytic pyrolysis of water hyacinth biomass. Waste Biomass Valor 10:2595–2609. https://doi.org/10.1007/s12649-018-0238-5
Gulati SL (1987) Sugars produced from cellulosic wastes as possible substrates for growth of Rhizobium inocula. Biol Wastes 21:301–305. https://doi.org/10.1016/0269-7483(87)90075-9
Guna V, Ilangovan M, Anantha Prasad MG, Reddy N (2017) Water hyacinth: a unique source for sustainable materials and products. ACS Sustain Chem Eng 5:4478–4490. https://doi.org/10.1021/acssuschemeng.7b00051
Gunnarsson CC, Petersen CM (2007) Water hyacinths as a resource in agriculture and energy production: a literature review. Waste Manag 27:117–129. https://doi.org/10.1016/j.wasman.2005.12.011
Guragain YN, De Coninck J, Husson F, Durand A, Rakshit SK (2011) Comparison of some new pretreatment methods for second generation bioethanol production from wheat straw and water hyacinth. Bioresour Technol 102:4416–4424. https://doi.org/10.1016/j.biortech.2010.11.125
Hariz HB, Takriff MS (2017) Palm oil mill effluent treatment and CO2 sequestration by using microalgae—sustainable strategies for environmental protection. Environ Sci Pollut Res 24:20209–20240. https://doi.org/10.1007/s11356-017-9742-6
Hashem MA, Hasan M, Momen MA, Payel S, Nur-A-Tomal MS (2020) Water hyacinth biochar for trivalent chromium adsorption from tannery wastewater. Environ Sustain Indic 5:100022. https://doi.org/10.1016/j.indic.2020.100022
Hermoso-López Araiza JP, Quecholac-Piña X, Beltrán-Villavicencio M et al (2016) Integral valorization of the water hyacinth from the canals of Xochimilco: production of edible mushrooms and forage. Waste Biomass Valor 7:1203–1210. https://doi.org/10.1007/s12649-016-9526-0
Hu Z, Ma X, Li L (2015) Optimal conditions for the catalytic and non-catalytic pyrolysis of water hyacinth. Energy Convers Manag 94:337–344. https://doi.org/10.1016/j.enconman.2015.01.087
Huang H, Liu J, Liu H, Evrendilek F, Buyukada M (2020) Pyrolysis of water hyacinth biomass parts: bioenergy, gas emissions, and by-products using TG-FTIR and Py-GC/MS analyses. Energy Convers Manag 207:112552. https://doi.org/10.1016/j.enconman.2020.112552
Huang Y, Li S, Chen J, Zhang X, Chen Y (2014) Adsorption of Pb(II) on mesoporous activated carbons fabricated from water hyacinth using H3PO4 activation: adsorption capacity, kinetic and isotherm studies. Appl Surf Sci 293:160–168. https://doi.org/10.1016/j.apsusc.2013.12.123
Hublikar LV, Ganachari SV, Raghavendra N, Patil VB, Banapurmath NR (2021) Green synthesis silver nanoparticles via Eichhornia Crassipes leaves extract and their applications. Curr Res Green Sustain Chem 4:100212. https://doi.org/10.1016/j.crgsc.2021.100212
Hudakorn T, Sritrakul N (2020) Biogas and biomass pellet production from water hyacinth. Energy Rep 6:532–538. https://doi.org/10.1016/j.egyr.2019.11.115
Hung C-M, Chen C-W, Huang C-P, Dong C-D (2022) Degradation of 4-nonylphenol in marine sediments using calcium peroxide activated by water hyacinth (Eichhornia crassipes)-derived biochar. Environ Res 211:113076. https://doi.org/10.1016/j.envres.2022.113076
Hussain Z, Bashir N, Khan MI, Hussain K, Sulaiman SA, Naz MY, Ibrahim KA, AbdEl-Salam NM (2017) Production of highly upgraded bio-oils through two-step catalytic pyrolysis of water hyacinth. Energy Fuels 31:12100–12107. https://doi.org/10.1021/acs.energyfuels.7b01252
Hussain Z, Khan KM, Khan A, Ullah S, Karim A, Perveen S (2013) The conversion of biomass into liquid hydrocarbon fuel by two step pyrolysis using cement as catalyst. J Anal Appl Pyrolysis 101:90–95. https://doi.org/10.1016/j.jaap.2013.02.007
Islam MN, Rahman F, Faruk MO, Das AK, Adhikary N, Debrot AO, Ahsan MN (2021) Water hyacinth (Eichhornia crassipes (Mart.) Solms.) as an alternative raw material for the production of bio-compost and handmade paper. J Environ Manage 294:113036. https://doi.org/10.1016/j.jenvman.2021.113036
Istirokhatun T, Rokhati N, Rachmawaty R, Meriyani M, Priyanto S, Susanto H (2015) Cellulose isolation from tropical water hyacinth for membrane preparation. Procedia Environ Sci 23:274–281. https://doi.org/10.1016/j.proenv.2015.01.041
Jagathesan G, Rajiv P (2018) Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity. Biocatal Agric Biotechnol 13:90–94. https://doi.org/10.1016/j.bcab.2017.11.014
Jiu B-B, Li B-X, Yu Q-J (2015) Effects of Pb on pyrolysis behavior of water hyacinth. J Anal Appl Pyrolysis 112:270–275. https://doi.org/10.1016/j.jaap.2015.01.015
Joglekar SN, Darwai V, Mandavgane SA, Kulkarni BD (2020) A methodology of evaluating sustainability index of a biomass processing enterprise: a case study of native cow dung–urine biorefinery. Environ Sci Pollut Res 27:27435–27448. https://doi.org/10.1007/s11356-019-06309-1
Joglekar SN, Pathak PD, Mandavgane SA, Kulkarni BD (2019) Process of fruit peel waste biorefinery: a case study of citrus waste biorefinery, its environmental impacts and recommendations. Environ Sci Pollut Res 26:34713–34722. https://doi.org/10.1007/s11356-019-04196-0
John Leo A, Oluwafemi OS (2017) Plant-mediated synthesis of platinum nanoparticles using water hyacinth as an efficient biomatrix source – an eco-friendly development. Mater Lett 196:141–144. https://doi.org/10.1016/j.matlet.2017.03.047
Jönsson LJ, Alriksson B, Nilvebrant N-O (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16. https://doi.org/10.1186/1754-6834-6-16
Keche DD, Fetanu ZM, Babiso WZ, Wachemo AC (2022) Anaerobic digestion of urea pretreated water hyacinth removed from Lake Abaya; bio-methane potential, system stability, and substance conversion. RSC Adv 12:8548–8558. https://doi.org/10.1039/D2RA00303A
Kelley C, Mielke RE, Dimaquibo D, Curtis AJ, DeWitt JG (1999) Adsorption of Eu(III) onto roots of water hyacinth. Environ Sci Technol 33:1439–1443. https://doi.org/10.1021/es9807789
Khan M, Singh T, Pal DB, Khan S, Ahmad S, Jandrajupalli SB, Haque S, Singh R, Srivastava N (2022) Enhanced production of bacterial hydrolytic endoglucanase enzyme using waste leaves of water hyacinth and its thermal stability under the influence of TiO2 nanoparticles. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02421-4
Khan MdMR, Mukhlish MZB, Mazumder MSI, Ferdous K, Prasad DMR, Hassan Z (2014) Uptake of Indosol Dark-blue GL dye from aqueous solution by water hyacinth roots powder: adsorption and desorption study. Int J Environ Sci Technol 11:1027–1034. https://doi.org/10.1007/s13762-013-0363-4
Khoshnevisan B, Duan N, Tsapekos P, Awasthi MK, Liu Z, Mohammadi A, Angelidaki I, Tsang DCW, Zhang Z, Pan J, Ma L, Aghbashlo M, Tabatabaei M, Liu H (2021) A critical review on livestock manure biorefinery technologies: sustainability, challenges, and future perspectives. Renew Sustain Energy Rev 135:110033. https://doi.org/10.1016/j.rser.2020.110033
Kitunda JM (2017) A history of the water hyacinth in Africa: the flower of life and death from 1800 to the present. Lexington Books, Lanham
Kleinschroth F, Winton RS, Calamita E, Niggemann F, Botter M, Wehrli B, Ghazoul J (2021) Living with floating vegetation invasions. Ambio 50:125–137. https://doi.org/10.1007/s13280-020-01360-6
Kriticos DJ, Brunel S (2016) Assessing and managing the current and future pest risk from water hyacinth, (Eichhornia crassipes), an invasive aquatic plant threatening the environment and water security. Plos One 11:e0120054. https://doi.org/10.1371/journal.pone.0120054
Kulkarni MR, Revanth T, Acharya A, Bhat P (2017) Removal of crystal violet dye from aqueous solution using water hyacinth: equilibrium, kinetics and thermodynamics study. Resour-Effic Technol 3:71–77. https://doi.org/10.1016/j.reffit.2017.01.009
Kumar P, Chauhan MS (2019) Adsorption of chromium (VI) from the synthetic aqueous solution using chemically modified dried water hyacinth roots. J Environ Chem Eng 7:103218. https://doi.org/10.1016/j.jece.2019.103218
Kumar Sarangi P, Subudhi S, Bhatia L, Saha K, Mudgil D, Prasad Shadangi K, Srivastava RK, Pattnaik B, Arya RK (2022) Utilization of agricultural waste biomass and recycling toward circular bioeconomy. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-20669-1
Kumar V, Singh J, Kumar P (2020) Regression models for removal of heavy metals by water hyacinth (Eichhornia crassipes) from wastewater of pulp and paper processing industry. Environ Sustain 3:35–44. https://doi.org/10.1007/s42398-019-00093-x
Kurniawan F, Wongso M, Ayucitra A, Soetaredjo FE, Angkawijaya AE, Ju Y-H, Ismadji S (2015) Carbon microsphere from water hyacinth for supercapacitor electrode. J Taiwan Inst Chem Eng 47:197–201. https://doi.org/10.1016/j.jtice.2014.10.002
Laohapornchaiphan J, Smith CB, Smith SM (2017) One-step preparation of carbon-based solid acid catalyst from water hyacinth leaves for esterification of oleic acid and dehydration of xylose. Chem – Asian J 12:3178–3186. https://doi.org/10.1002/asia.201701369
Li F, He X, Srishti A, Song S, Tan HTW, Sweeney DJ, Ghosh S, Wang C-H (2021) Water hyacinth for energy and environmental applications: a review. Bioresour Technol 327:124809. https://doi.org/10.1016/j.biortech.2021.124809
Li X, Xi H, Sun X, Yang Yunqiang Yang S, Zhou Y, Zhou X, Yongping Y (2015) Comparative proteomics exploring the molecular mechanism of eutrophic water purification using water hyacinth (Eichhornia crassipes). Environ Sci Pollut Res 22:8643–8658. https://doi.org/10.1007/s11356-014-4020-3
Liang J, Tang D, Huang L, Chen Y, Ren W, Sun J (2018) High oxygen reduction reaction performance nitrogen-doped biochar cathode: a strategy for comprehensive utilizing nitrogen and carbon in water hyacinth. Bioresour Technol 267:524–531. https://doi.org/10.1016/j.biortech.2018.07.085
Liang J, Yu Z, Chen L, Fang S, Ma X (2019) Microwave pretreatment power and duration time effects on the catalytic pyrolysis behaviors and kinetics of water hyacinth. Bioresour Technol 286:121369. https://doi.org/10.1016/j.biortech.2019.121369
Lima JRA, de Farias DL, Menezes THS, Oliveira RVM, Silva IAA, da Costa CG, Romão LPC (2020) Potential of a magnetic hybrid material produced using water hyacinth (Eichhornia crassipes) for removal of inorganic and organic pollutants from aqueous media. J Environ Chem Eng 8:104100. https://doi.org/10.1016/j.jece.2020.104100
Lin H-J, Rong C-X, Jiu B-B, Li B-X, Yu Q-J, Gan L-H, Zhang Z-Y (2018) Effects of chromium on pyrolysis characteristic of water hyacinth (Eichornia crassipes). Renew Energy 115:676–684. https://doi.org/10.1016/j.renene.2017.08.045
Liu L, Hu S, Shen G, Farooq U, Zhang W, Lin S, Lin K (2018) Adsorption dynamics and mechanism of aqueous sulfachloropyridazine and analogues using the root powder of recyclable long-root Eichhornia crassipes. Chemosphere 196:409–417. https://doi.org/10.1016/j.chemosphere.2018.01.003
Liu S, Zhu J, Chen M, Xin W, Yang Z, Kong L (2014) Hydrogen production via catalytic pyrolysis of biomass in a two-stage fixed bed reactor system. Int J Hydrog Energy 39:13128–13135. https://doi.org/10.1016/j.ijhydene.2014.06.158
Liu X, Zhou Y, Zhou W, Li L, Huang S, Chen S (2015) Biomass-derived nitrogen self-doped porous carbon as effective metal-free catalysts for oxygen reduction reaction. Nanoscale 7:6136–6142. https://doi.org/10.1039/C5NR00013K
Lu Q, Zhou S, Zhang Y, Chen M, Li B, Wei H, Zhang D, Zhang J, Liu Q (2020) Nanoporous carbon derived from green material by an ordered activation method and its high capacitance for energy storage. Nanomaterials 10:1058. https://doi.org/10.3390/nano10061058
Lu X, Gao Y, Luo J, Yan S, Rengel Z, Zhang Z (2014) Interaction of veterinary antibiotic tetracyclines and copper on their fates in water and water hyacinth (Eichhornia crassipes). J Hazard Mater 280:389–398. https://doi.org/10.1016/j.jhazmat.2014.08.022
Luo G, James Strong P, Wang H, Ni W, Shi W (2011) Kinetics of the pyrolytic and hydrothermal decomposition of water hyacinth. Bioresour Technol 102:6990–6994. https://doi.org/10.1016/j.biortech.2011.04.048
Madikizela LM (2021) Removal of organic pollutants in water using water hyacinth (Eichhornia crassipes). J Environ Manage 295:113153. https://doi.org/10.1016/j.jenvman.2021.113153
Mahamadi C, Nharingo T (2010) Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresour Technol 101:859–864. https://doi.org/10.1016/j.biortech.2009.08.097
Mahmood S, Khan N, Iqbal KJ, Ashraf M, Khalique A (2018) Evaluation of water hyacinth (Eichhornia crassipes) supplemented diets on the growth, digestibility and histology of grass carp (Ctenopharyngodon idella) fingerlings. J Appl Anim Res 46:24–28. https://doi.org/10.1080/09712119.2016.1256291
Mahujchariyawong J, Ikeda S (2001) Modelling of environmental phytoremediation in eutrophic river — the case of water hyacinth harvest in Tha-chin River, Thailand. Ecol Model 142:121–134. https://doi.org/10.1016/S0304-3800(01)00283-6
Malar S, Shivendra Vikram S, Favas PJC, Perumal V (2014) Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot Stud 55:54. https://doi.org/10.1186/s40529-014-0054-6
Malik A (2007) Environmental challenge vis a vis opportunity: the case of water hyacinth. Environ Int 33:122–138. https://doi.org/10.1016/j.envint.2006.08.004
Martínez-Nieto P, García-Gómez G, Mora-Ortiz L, Robles-Camargo G (2014) Polluting macrophytes Colombian lake Fúquene used as substrate by edible fungus Pleurotus ostreatus. World J Microbiol Biotechnol 30:225–236. https://doi.org/10.1007/s11274-013-1443-9
Martínez-Ruano JA, Caballero-Galván AS, Restrepo-Serna DL, Cardona CA (2018) Techno-economic and environmental assessment of biogas production from banana peel (Musa paradisiaca) in a biorefinery concept. Environ Sci Pollut Res 25:35971–35980. https://doi.org/10.1007/s11356-018-1848-y
Martins PF, de Melo MMR, Sarmento P, Silva CM (2016) Supercritical fluid extraction of sterols from Eichhornia crassipes biomass using pure and modified carbon dioxide. Enhancement of stigmasterol yield and extract concentration. J Supercrit Fluids 107:441–449. https://doi.org/10.1016/j.supflu.2015.09.027
Mercado-Borrayo BM, Cram Heydrich S, Pérez IR, Hernández Quiroz M, De León Hill CP (2015) Organophosphorus and organochlorine pesticides bioaccumulation by Eichhornia crassipes in irrigation canals in an urban agricultural system. Int J Phytoremediation 17:701–708. https://doi.org/10.1080/15226514.2014.964841
Mishima D, Kuniki M, Sei K, Soda S, Ike M, Fujita M (2008) Ethanol production from candidate energy crops: water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Bioresour Technol 99:2495–2500. https://doi.org/10.1016/j.biortech.2007.04.056
Mishra S, Maiti A (2017) The efficiency of Eichhornia crassipes in the removal of organic and inorganic pollutants from wastewater: a review. Environ Sci Pollut Res 24:7921–7937. https://doi.org/10.1007/s11356-016-8357-7
Mo C, Zhang J, Zhang G (2020) Hierarchical porous carbon with three dimensional nanonetwork from water hyacinth leaves for energy storage. J Energy Storage 32:101848. https://doi.org/10.1016/j.est.2020.101848
Mochochoko T, Oluwafemi OS, Jumbam DN, Songca SP (2013) Green synthesis of silver nanoparticles using cellulose extracted from an aquatic weed; water hyacinth. Carbohydr Polym 98:290–294. https://doi.org/10.1016/j.carbpol.2013.05.038
Moldavska A, Welo T (2019) A holistic approach to corporate sustainability assessment: incorporating sustainable development goals into sustainable manufacturing performance evaluation. J Manuf Syst 50:53–68. https://doi.org/10.1016/j.jmsy.2018.11.004
Morales SL, Baas-López JM, Barbosa R, Pacheco D, Escobar B (2021) Activated carbon from water hyacinth as electrocatalyst for oxygen reduction reaction in an alkaline fuel cell. Int J Hydrog Energy 46:25995–26004. https://doi.org/10.1016/j.ijhydene.2021.04.094
Moustakas K, Loizidou M (2022) Effective waste management with emphasis on circular economy. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-24670-6
Murambasvina G, Mahamadi C (2020) Effective fluoride adsorption using water hyacinth beads doped with hydrous oxides of aluminium and iron. Groundw Sustain Dev 10:100302. https://doi.org/10.1016/j.gsd.2019.100302
Murugesan AG, Vijayalakshmi GS, Sukumaran N, Mariappan C (1995) Utilization of water hyacinth for oyster mushroom cultivation. Bioresour Technol 51:97–98. https://doi.org/10.1016/0960-8524(95)00063-K
Nagar PK, Saha S (1985) Distribution of cytokinin-like activity in different plant parts of the water hyacinth, Eichhornia crassipes. Physiol Plant 64:328–332. https://doi.org/10.1111/j.1399-3054.1985.tb03348.x
Nageswaran M, Gopalakrishnan A, Ganesan M, Vedhamurthy A (2003) Evaluation of water hyacinth and paddy straw waste for culture of oyster mushrooms. J Aquat Plant Manage 41:122–123. http://aquaticcommons.org/id/eprint/1795. Accessed 13 Sept 2022
Nagula KN, Pandit AB (2016) Process intensification of delignification and enzymatic hydrolysis of delignified cellulosic biomass using various process intensification techniques including cavitation. Bioresour Technol 213:162–168. https://doi.org/10.1016/j.biortech.2016.03.152
Narra M, Divecha J, Shah D, Balasubramanian V, Vyas B, Harijan M, Macwan K (2017) Cellulase production, simultaneous saccharification and fermentation in a single vessel: a new approach for production of bio-ethanol from mild alkali pre-treated water hyacinth. J Environ Chem Eng 5:2176–2181. https://doi.org/10.1016/j.jece.2017.04.043
Ndimele PE, Ndimele CC (2013) Comparative effects of biostimulation and phytoremediation on crude oil degradation and absorption by water hyacinth (Eichhornia crassipes [Mart.] Solms). Int J Environ Stud 70:241–258. https://doi.org/10.1080/00207233.2013.771503
Nguyen TTV, Tri N, Tran BA, Dao Duy T, Nguyen ST, Nguyen T-A, Phan AN, Mai Thanh P, Huynh HKP (2021) Synthesis, characteristics, oil adsorption, and thermal insulation performance of cellulosic aerogel derived from water hyacinth. ACS Omega 6:26130–26139. https://doi.org/10.1021/acsomega.1c03137
Nigam JN (2002) Bioconversion of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to motor fuel ethanol by xylose–fermenting yeast. J Biotechnol 97:107–116. https://doi.org/10.1016/S0168-1656(02)00013-5
Okwadha GDO, Makomele DM (2018) Evaluation of water hyacinth extract as an admixture in concrete production. J Build Eng 16:129–133. https://doi.org/10.1016/j.jobe.2018.01.002
Oluwafemi OS, Anyik JL, Zikalala NE, Sakho EHM (2019) Biosynthesis of silver nanoparticles from water hyacinth plant leaves extract for colourimetric sensing of heavy metals. Nano-Struct Nano-Objects 20:100387. https://doi.org/10.1016/j.nanoso.2019.100387
Oyeoka HC, Ewulonu CM, Nwuzor IC, Obele CM, Nwabanne JT (2021) Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J Bioresour Bioprod 6:168–185. https://doi.org/10.1016/j.jobab.2021.02.009
Packiam KK, Murugesan B, Kaliyannan Sundaramoorthy PM, Srinivasan H, Dhanasekaran K (2021) Extraction, purification and characterization of nanocrystalline cellulose from Eichhornia crassipes (Mart.) Solms: A Common Aquatic Weed Water Hyacinth. J Nat Fibers 19:7424–435. https://doi.org/10.1080/15440478.2021.1946886
Pakutsah K, Aht-Ong D (2020) Facile isolation of cellulose nanofibers from water hyacinth using water-based mechanical defibrillation: insights into morphological, physical, and rheological properties. Int J Biol Macromol 145:64–76. https://doi.org/10.1016/j.ijbiomac.2019.12.172
Panchanadikar V, Joshi S, Babu S, Bhide S (2005) Beta-carotene enriched extract from water hyacinth eichhornia crassipes. US Patent Application: US20050214389A1
Panneerselvam B, Priya KS (2021) Phytoremediation potential of water hyacinth in heavy metal removal in chromium and lead contaminated water. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1901896
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31:20–31. https://doi.org/10.3109/07388551003757816
Peng H, Wang Y, Tan TL, Chen Z (2020) Exploring the phytoremediation potential of water hyacinth by FTIR spectroscopy and ICP-OES for treatment of heavy metal contaminated water. Int J Phytoremediation 22:939–951. https://doi.org/10.1080/15226514.2020.1774499
Perino S, Chemat F (2019) Green process intensification techniques for bio-refinery. Curr Opin Food Sci 25:8–13. https://doi.org/10.1016/j.cofs.2018.12.004
Pradhan S, Borah AJ, Poddar MK, Dikshit PK, Rohidas L, Moholkar VS (2017) Microbial production, ultrasound-assisted extraction and characterization of biopolymer polyhydroxybutyrate (PHB) from terrestrial (P. hysterophorus) and aquatic (E. crassipes) invasive weeds. Bioresour Technol 242:304–310. https://doi.org/10.1016/j.biortech.2017.03.117
Prasad R, Sharma D, Yadav KD, Ibrahim H (2021) Preliminary study on greywater treatment using water hyacinth. Appl Water Sci 11:88. https://doi.org/10.1007/s13201-021-01422-4
Prathumsuwan T, Jaiyong P, In I, Paoprasert P (2019) Label-free carbon dots from water hyacinth leaves as a highly fluorescent probe for selective and sensitive detection of borax. Sens Actuators B Chem 299:126936. https://doi.org/10.1016/j.snb.2019.126936
Priya ES, Selvan PS (2017) Water hyacinth (Eichhornia crassipes) – an efficient and economic adsorbent for textile effluent treatment – a review. Arab J Chem 10:S3548–S3558. https://doi.org/10.1016/j.arabjc.2014.03.002
Qin H, Zhang Z, Liu H, Li D, Wen X, Zhang Y, Wang Y, Yan S (2016) Fenced cultivation of water hyacinth for cyanobacterial bloom control. Environ Sci Pollut Res 23:17742–17752. https://doi.org/10.1007/s11356-016-6799-6
Qiu Z, Huang T, Zhao C, Luo J, Hu Z (2017) Water hyacinth-derived activated carbon/NiO nanocomposite as a facile electrode material for high performance supercapacitor. Micro Nano Lett 12:231–235. https://doi.org/10.1049/mnl.2016.0526
Qu W, He D, Guo Y, TangY SJ, Zhou L, Zhu R, Song R-J (2019) Modified water hyacinth functionalized with citric acid as an effective and inexpensive adsorbent for heavy metal-ion removal. Ind Eng Chem Res 58:18508–18518. https://doi.org/10.1021/acs.iecr.9b03401
Radhika D, Murugesan AG (2012) Bioproduction, statistical optimization and characterization of microbial plastic (poly 3-hydroxy butyrate) employing various hydrolysates of water hyacinth (Eichhornia crassipes) as sole carbon source. Bioresour Technol 121:83–92. https://doi.org/10.1016/j.biortech.2012.06.107
Rahman MA (2018) Pyrolysis of water hyacinth in a fixed bed reactor: parametric effects on product distribution, characterization and syngas evolutionary behavior. Waste Manag 80:310–318. https://doi.org/10.1016/j.wasman.2018.09.028
Ramirez A, Pérez S, Flórez E, Acelas N (2021) Utilization of water hyacinth (Eichhornia crassipes) rejects as phosphate-rich fertilizer. J Environ Chem Eng 9:104776. https://doi.org/10.1016/j.jece.2020.104776
Ren MX, Zhang QG (2007) Clonal diversity and structure of the invasive aquatic plant Eichhornia crassipes in China. Aquat Bot 87:242–246. https://doi.org/10.1016/j.aquabot.2007.06.002
Rezania S, Ponraj M, Din MFM, Songip AR, Sairan FM, Chelliapan S (2015) The diverse applications of water hyacinth with main focus on sustainable energy and production for new era: an overview. Renew Sustain Energy Rev 41:943–954. https://doi.org/10.1016/j.rser.2014.09.006
Román S, Ledesma B, Álvarez A, Coronella C, Qaramaleki SV (2020) Suitability of hydrothermal carbonization to convert water hyacinth to added-value products. Renew Energy 146:1649–1658. https://doi.org/10.1016/j.renene.2019.07.157
Rop K, Karuku GN, Mbui D, Michira I, Njomo N (2018) Formulation of slow release NPK fertilizer (cellulose-graft-poly(acrylamide)/nano-hydroxyapatite/soluble fertilizer) composite and evaluating its N mineralization potential. Ann Agric Sci 63:163–172. https://doi.org/10.1016/j.aoas.2018.11.001
Rop K, Mbui D, Njomo N, Karuku GN, Michira I, Ajayi RF (2019) Biodegradable water hyacinth cellulose-graft-poly(ammonium acrylate-co-acrylic acid) polymer hydrogel for potential agricultural application. Heliyon 5:e01416. https://doi.org/10.1016/j.heliyon.2019.e01416
Roy K, Ghosh CK, Sarkar CK (2019) Rapid detection of hazardous H2O2 by biogenic copper nanoparticles synthesized using Eichhornia crassipes extract. Microsyst Technol 25:1699–1703. https://doi.org/10.1007/s00542-017-3480-z
Rufchaei R, Mirvaghefi A, Hoseinifar SH, Valipour A, Nedaei S (2020) Effects of dietary administration of water hyacinth (Eichhornia crassipes) leaves extracts on innate immune parameters, antioxidant defence and disease resistance in rainbow trout (Oncorhynchus mykiss). Aquaculture 515:734533. https://doi.org/10.1016/j.aquaculture.2019.734533
Saeidnia S, Abdollahi M (2013) Are medicinal plants polluted with phthalates? DARU J Pharm Sci 21:43. https://doi.org/10.1186/2008-2231-21-43
Salahuddin N, Abdelwahab MA, Akelah A, Elnagar M (2021a) Adsorption of Congo red and crystal violet dyes onto cellulose extracted from Egyptian water hyacinth. Nat Hazards 105:1375–1394. https://doi.org/10.1007/s11069-020-04358-1
Salahuddin N, Akelah A, Elnagar M, Abdelwahab MA (2021b) Antibacterial and cytotoxicity of methylene blue loaded-cellulose nanocarrier on breast cancer cell line. Carbohydr Polym Technol Appl 2:100138. https://doi.org/10.1016/j.carpta.2021.100138
Saleh HM (2014) Stability of cemented dried water hyacinth used for biosorption of radionuclides under various circumstances. J Nucl Mater 446:124–133. https://doi.org/10.1016/j.jnucmat.2013.11.038
Saning A, Herou S, Dechtrirat D, Ieosakulrat C, Pakawatpanurut P, Kaowphong S, Thanachayanont C, Titirici M-M, Chuenchom L (2019) Green and sustainable zero-waste conversion of water hyacinth (Eichhornia crassipes) into superior magnetic carbon composite adsorbents and supercapacitor electrodes. RSC Adv 9:24248–24258. https://doi.org/10.1039/C9RA03873F
Saratale RG, Cho S-K, Ghodake GS, Shin H-S, Saratale GD, Park Y, Lee H-S, Bharagava RN, Kim D-S (2020) Utilization of noxious weed water hyacinth biomass as a potential feedstock for biopolymers production: a novel approach. Polymers 12:1704. https://doi.org/10.3390/polym12081704
Sarmah M, Dewan A, Thakur AJ, Bora U (2017) Extraction of base from Eichhornia crassipes and its implication in palladium-catalyzed Suzuki cross-coupling reaction. ChemistrySelect 2:7091–7095. https://doi.org/10.1002/slct.201701057
Schneider IAH, Rubio J, Misra M, Smith RW (1995) Eichhornia crassipes as biosorbent for heavy metal ions. Miner Eng 8:979–988. https://doi.org/10.1016/0892-6875(95)00061-T
Senthilkumar ST, Selvan RK, Lee YS, Melo JS (2012) Electric double layer capacitor and its improved specific capacitance using redox additive electrolyte. J Mater Chem A 1:1086–1095. https://doi.org/10.1039/C2TA00210H
Serna-Loaiza S, Martínez A, Pisarenko Y, Cardona-Alzate CA (2018) Integral use of plants and their residues: the case of cocoyam (Xanthosoma sagittifolium) conversion through biorefineries at small scale. Environ Sci Pollut Res 25:35949–35959. https://doi.org/10.1007/s11356-018-2313-7
Sethupathy A, Sobana Piriya P, Ranjith Kumar R, Shanthi M, Rangabhashiyam S, Arun C, Vasanth Ragavan K (2022) Assessment of methane enrichment efficacy of pre-disintegrated water hyacinth biomass using sonic wave assisted biosurfactant. Fuel 316:123375. https://doi.org/10.1016/j.fuel.2022.123375
Shanab SMM, Hanafy EA, Shalaby EA (2018) Water hyacinth as non-edible source for biofuel production. Waste Biomass Valor 9:255–264. https://doi.org/10.1007/s12649-016-9816-6
Shanab SMM, Shalaby EA, Lightfoot DA, El-Shemy HA (2010) Allelopathic effects of water hyacinth [Eichhornia crassipes]. Plos One 5:e13200. https://doi.org/10.1371/journal.pone.0013200
Sharma R, Saini H, Paul DR, Chaudhary S, Nehra SP (2021) Removal of organic dyes from wastewater using Eichhornia crassipes: a potential phytoremediation option. Environ Sci Pollut Res 28:7116–7122. https://doi.org/10.1007/s11356-020-10940-8
Shell KM, Vohra SY, Rodene DD, Gupta RB (2021) Phytoremediation of nickel via water hyacinth for biocarbon-derived supercapacitor applications. Energy Technol 9:2100130. https://doi.org/10.1002/ente.202100130
Shi L, Wang L, Zhang T, Li J, Huang X, Cai J, Lü J, Wang Y (2017) Reducing the bioavailability and leaching potential of lead in contaminated water hyacinth biomass by phosphate-assisted pyrolysis. Bioresour Technol 241:908–914. https://doi.org/10.1016/j.biortech.2017.06.025
Shu X, Zhang Q, Wang W (2014) Effects of temperature and light intensity on growth and physiology in purple root water hyacinth and common water hyacinth (Eichhornia crassipes). Environ Sci Pollut Res 21:12979–12988. https://doi.org/10.1007/s11356-014-3246-4
Silva IAA, de Macedo OFL, Cunha GC, Oliveira RVM, Romão LPC (2021) Using water hyacinth (Eichhornia crassipes) biomass and humic substances to produce urea-based multi-coated slow release fertilizer. Cellulose 28:3691–3701. https://doi.org/10.1007/s10570-021-03741-w
Silva RP, de Melo MMR, Silvestre AJD, Silva CM (2015) Polar and lipophilic extracts characterization of roots, stalks, leaves and flowers of water hyacinth (Eichhornia crassipes), and insights for its future valorization. Ind Crops Prod 76:1033–1038. https://doi.org/10.1016/j.indcrop.2015.07.055
Sima X-F, Jiang S-F, Shen X-C, Jiang H (2019) Harvesting biomass-based Ni–N doped carbonaceous materials with high capacitance by fast pyrolysis of Ni enriched spent wetland biomass. Ind Eng Chem Res 58:13868–13878. https://doi.org/10.1021/acs.iecr.9b02126
Sindhu R, Binod P, Pandey A, Madhavan A, Alphonsa JA, Vivek N, Gnansounou E, Castro E, Faraco V (2017) Water hyacinth a potential source for value addition: an overview. Bioresour Technol 230:152–162. https://doi.org/10.1016/j.biortech.2017.01.035
Singh A, Bishnoi NR (2013) Comparative study of various pretreatment techniques for ethanol production from water hyacinth. Ind Crops Prod 44:283–289. https://doi.org/10.1016/j.indcrop.2012.11.026
Singh J, Kumar P, Eid EM, Taher MA, El-Morsy MHE, Osman HEM, Al-Bakre DA, Kumar V (2022) Phytoremediation of nitrogen and phosphorus pollutants from glass industry effluent by using water hyacinth (Eichhornia crassipes (Mart.) Solms): application of RSM and ANN techniques for experimental optimization. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-23601-9
Singh J, Kumar V, Kumar P, Kumar P (2021) Kinetics and prediction modeling of heavy metal phytoremediation from glass industry effluent by water hyacinth (Eichhornia crassipes). Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03433-9
Singh R, Balagurumurthy B, Prakash A, Bhaskar T (2015) Catalytic hydrothermal liquefaction of water hyacinth. Bioresour Technol 178:157–165. https://doi.org/10.1016/j.biortech.2014.08.119
Sittinun A, Pisitsak P, Ummartyotin S (2020) Improving the oil sorption capability of porous polyurethane composites by the incorporation of cellulose fibers extracted from water hyacinth. Compos Commun. https://doi.org/10.1016/j.coco.2020.04.017
Soenjaya SA, Handoyo N, Edi Soetaredjo F, Angkawijaya AE, Ju Y-H, Ismadji S (2015) Preparation of carbon fiber from water hyacinth liquid tar. Int J Ind Chem 6:1–7. https://doi.org/10.1007/s40090-014-0026-4
Solarte-Toro JC, Ortiz-Sanchez M, Cardona Alzate CA (2022) Environmental life cycle assessment (E-LCA) and social impact assessment (SIA) of small-scale biorefineries implemented in rural zones: the avocado (Persea Americana var. Americana) case in Colombia. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-20857-z
Sumrith N, Techawinyutham L, Sanjay MR, Dangtungee R, Siengchin S (2020) Characterization of alkaline and silane treated fibers of ‘Water Hyacinth Plants’ and reinforcement of ‘Water Hyacinth Fibers’ with bioepoxy to develop fully biobased sustainable ecofriendly composites. J Polym Environ 28:2749–2760. https://doi.org/10.1007/s10924-020-01810-y
Sun D, Onyianta AJ, O’Rourke D, Perrin G, Popescu C-M, Saw LH, Cai Z, Dorris M (2020) A process for deriving high quality cellulose nanofibrils from water hyacinth invasive species. Cellulose 27:3727–3740. https://doi.org/10.1007/s10570-020-03038-4
Surendraraj A, Farvin KHS, Anandan R (2013) Antioxidant potential of water hyacinth (Eichornia crassipes): in vitro antioxidant activity and phenolic composition. J Aquat Food Prod Technol 22:11–26. https://doi.org/10.1080/10498850.2011.621582
Suthar S, Sharma B, Kumar K, Rajesh Banu J, Tyagi VK (2022) Enhanced biogas production in dilute acid-thermal pretreatment and cattle dung biochar mediated biomethanation of water hyacinth. Fuel 307:121897. https://doi.org/10.1016/j.fuel.2021.121897
Tanpichai S, Biswas SK, Witayakran S, Yano H (2019) Water hyacinth: a sustainable lignin-poor cellulose source for the production of cellulose nanofibers. ACS Sustain Chem Eng 7:18884–18893. https://doi.org/10.1021/acssuschemeng.9b04095
Tanpichai S, Mekcham S, Kongwittaya C, Kiwijaroun W, Thongdonsun K, Thongdeelerd C, Boonmahitthisud A (2021) Extraction of nanofibrillated cellulose from water hyacinth using a high speed homogenizer. J Nat Fibers 19:5676–5696. https://doi.org/10.1080/15440478.2021.1889432
Tanveer M, Khan SAR, Umar M, Yu Z, Sajid MJ, Haq IU (2022) Waste management and green technology: future trends in circular economy leading towards environmental sustainability. Environ Sci Pollut Res 29:80161–80178. https://doi.org/10.1007/s11356-022-23238-8
Tasnim F, Iqbal SA, Chowdhury AR (2017) Biogas production from anaerobic co-digestion of cow manure with kitchen waste and water hyacinth. Renew Energy 109:434–439. https://doi.org/10.1016/j.renene.2017.03.044
Thamaga KH, Dube T (2018) Remote sensing of invasive water hyacinth (Eichhornia crassipes): a review on applications and challenges. Remote Sens Appl Soc Environ 10:36–46. https://doi.org/10.1016/j.rsase.2018.02.005
Thiripura Sundari M, Ramesh A (2012) Isolation and characterization of cellulose nanofibers from the aquatic weed water hyacinth—Eichhornia crassipes. Carbohydr Polym 87:1701–1705. https://doi.org/10.1016/j.carbpol.2011.09.076
Ting WHT, Tan IAW, Salleh SF, Wahab NA (2018) Application of water hyacinth (Eichhornia crassipes) for phytoremediation of ammoniacal nitrogen: a review. J Water Process Eng 22:239–249. https://doi.org/10.1016/j.jwpe.2018.02.011
Tipping PW, Martin MR, Pokorny EN, Nimmo KR, Fitzgerald DL, Dray FA, Center TD (2014) Current levels of suppression of waterhyacinth in Florida USA by classical biological control agents. Biol Control 71:65–69. https://doi.org/10.1016/j.biocontrol.2014.01.008
Trần TK, Kim N, Leu H-J, Pham M, Luong N, Vo H (2020) The production of hydrogen gas from modified water hyacinth (Eichhornia Crassipes) biomass through pyrolysis process. Int J Hydrog Energy 46:13976–13984. https://doi.org/10.1016/j.ijhydene.2020.08.225
Usmani Z, Sharma M, Awasthi AK, Lukk T, Tuohy MG, Gong L, Nguyen-Tri P, Goddard AD, Bill RM, Nayak SC, Gupta VK (2021) Lignocellulosic biorefineries: the current state of challenges and strategies for efficient commercialization. Renew Sustain Energy Rev 148:111258. https://doi.org/10.1016/j.rser.2021.111258
Varanasi JL, Kumari S, Das D (2018) Improvement of energy recovery from water hyacinth by using integrated system. Int J Hydrog Energy 43:1303–1318. https://doi.org/10.1016/j.ijhydene.2017.11.110
Wauton I, Ogbeide SE (2019a) Determination of the activation energy of water hyacinth ( Eichhornia crassipes ) pyrolysis. Int J Green Energy 16:1571–1576. https://doi.org/10.1080/15435075.2019.1677236
Wauton I, Ogbeide SE (2018) Characterization of pyrolytic bio-oil from water hyacinth (Eichhornia crassipes) pyrolysis in a fixed bed reactor. Biofuels 12:899–904. https://doi.org/10.1080/17597269.2018.1558838
Wauton I, Ogbeide SE (2019b) Investigation of the production of pyrolytic bio-oil from water hyacinth (Eichhornia crassipes) in a fixed bed reactor using pyrolysis process. Biofuels 13:189–195. https://doi.org/10.1080/17597269.2019.1660061
Williams AE, Duthie HC, Hecky RE (2005) Water hyacinth in Lake Victoria: why did it vanish so quickly and will it return? Aquat Bot 81:300–314. https://doi.org/10.1016/j.aquabot.2005.01.003
Wilson JRU, Ajuonu O, Center TD, Hill MP, Julien MH, Katagira FF, Neuenschwander P, Njoka SW, Ogwang J, Reeder RH, Van T (2007) The decline of water hyacinth on Lake Victoria was due to biological control by Neochetina spp. Aquat Bot 87:90–93. https://doi.org/10.1016/j.aquabot.2006.06.006
Xia H, Ma X (2006) Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresour Technol 97:1050–1054. https://doi.org/10.1016/j.biortech.2005.04.039
Yan J, Wei Z, Wang Q, He M, Li S, Irbis C (2015) Bioethanol production from sodium hydroxide/hydrogen peroxide-pretreated water hyacinth via simultaneous saccharification and fermentation with a newly isolated thermotolerant Kluyveromyces marxianu strain. Bioresour Technol 193:103–109. https://doi.org/10.1016/j.biortech.2015.06.069
Yan S-H, Song W, Guo J-Y (2017) Advances in management and utilization of invasive water hyacinth (Eichhornia crassipes) in aquatic ecosystems – a review. Crit Rev Biotechnol 37:218–228. https://doi.org/10.3109/07388551.2015.1132406
Yan Z, Dai C, Zhang M, Lv X, Zhao X, Xie J (2019) Nitrogen doped porous carbon with iron promotion for oxygen reduction reaction in alkaline and acidic media. Int J Hydrog Energy 44:4090–4101. https://doi.org/10.1016/j.ijhydene.2018.12.180
Yang L, Shuang E, Liu J, Sheng K, Zhang X (2022) Endogenous calcium enriched hydrochar catalyst derived from water hyacinth for glucose isomerization. Sci Total Environ 807:150660. https://doi.org/10.1016/j.scitotenv.2021.150660
Yao Z, Ma X, Xiao Z (2020) The effect of two pretreatment levels on the pyrolysis characteristics of water hyacinth. Renew Energy 151:514–527. https://doi.org/10.1016/j.renene.2019.11.046
Yu J, Jiang C, Guan Q, Ning P, Gu J, Chen Q, Zhang J, Miao R (2018) Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 195:632–640. https://doi.org/10.1016/j.chemosphere.2017.12.128
Zelekew OA, Fufa PA, Sabir FK, Duma AD (2021) Water hyacinth plant extract mediated green synthesis of Cr2O3/ZnO composite photocatalyst for the degradation of organic dye. Heliyon 7:e07652. https://doi.org/10.1016/j.heliyon.2021.e07652
Zhang B, Zhong Z, Li T, Xue Z, Ruan R (2018a) Bio-oil production from sequential two-step microwave-assisted catalytic fast pyrolysis of water hyacinth using Ce-doped γ-Al2O3/ZrO2 composite mesoporous catalyst. J Anal Appl Pyrolysis 132:143–150. https://doi.org/10.1016/j.jaap.2018.03.006
Zhang C, Ma X, Chen X, Tian Y, Zhou Y, Lu X, Huang T (2020) Conversion of water hyacinth to value-added fuel via hydrothermal carbonization. Energy 197:117193. https://doi.org/10.1016/j.energy.2020.117193
Zhang F, Wang X, Yin D, Peng B, Tan C, Liu Y, Tan X, Wu S (2015) Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J Environ Manage 153:68–73. https://doi.org/10.1016/j.jenvman.2015.01.043
Zhang Q, Wei Y, Han H, Weng C (2018b) Enhancing bioethanol production from water hyacinth by new combined pretreatment methods. Bioresour Technol 251:358–363. https://doi.org/10.1016/j.biortech.2017.12.085
Zhang Q, Weng C, Huang H, Achal V, Wang D (2016) Optimization of bioethanol production using whole plant of water hyacinth as substrate in simultaneous saccharification and fermentation process. Front Microbiol 6:1411. https://doi.org/10.3389/fmicb.2015.01411
Zhang Q, Zhang Y, Li Y, Ding P, Xu S, Cao J (2021) Green synthesis of magnetite nanoparticle and its regulatory effect on fermentative hydrogen production from lignocellulosic hydrolysate by Klebsiella sp. Int J Hydrog Energy 46:20413–20424. https://doi.org/10.1016/j.ijhydene.2021.03.142
Zhang Y, Shen Y, Zhang H, Wang L, Zhang H, Qian H, Qi X (2018c) Isolation, purification and identification of two antioxidant peptides from water hyacinth leaf protein hydrolysates (WHLPH). Eur Food Res Technol 244:83–96. https://doi.org/10.1007/s00217-017-2941-z
Zhang Y-Y, Zhang D-Y, Barrett SCH (2010) Genetic uniformity characterizes the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Mol Ecol 19:1774–1786. https://doi.org/10.1111/j.1365-294X.2010.04609.x
Zheng K, Li Y, Zhu M, Yu X, Zhang M, Shi L, Cheng J (2017) The porous carbon derived from water hyacinth with well-designed hierarchical structure for supercapacitors. J Power Sources 366:270–277. https://doi.org/10.1016/j.jpowsour.2017.09.034
Acknowledgements
Authors are thankful to Dr. K. I. Sagrolikar for assistance with English language editing. We are thankful to all the teaching and non-teaching staff and friends for their support and motivation.
Funding
This work was supported financially by the Institute of Chemical Technology, Marathwada Campus, Jalna, Maharashtra, India.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Data collection and analysis were performed by Shruti Bajpai and Dr. Parag Nemade. The first draft of the manuscript was written by Shruti Bajpai, and it was further edited and written by Dr. Parag Nemade. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bajpai, S., Nemade, P.R. An integrated biorefinery approach for the valorization of water hyacinth towards circular bioeconomy: a review. Environ Sci Pollut Res 30, 39494–39536 (2023). https://doi.org/10.1007/s11356-023-25830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25830-y