Abstract
In the present work, bioethanol was produced by sugar fermentation obtained from water hyacinth using a novelty hybrid method composed of steam explosion and enzymatic hydrolysis, using hydrolytic enzymes produced by solid-state fermentation and water hyacinth as substrate. The highest activity, 42 U for xylanase and 2 U for cellulase per gram of dry matter, respectively, was obtained. Steam explosion pretreatment was performed at 190 ℃ for 1, 5, and 10 min, using water hyacinth sampled from the Maria Lizamba Lagoon, the Arroyo Hondo and the Amapa River. The highest amounts of reducing sugars of water hyacinth were obtained form the samples from the lagoon (5.4 g/50 g of dry matter) after 10 min of treatment. Steamed biomass was hydrolysed using the enzymes obtained by solid-state fermentation, obtained reducing sugars (maximum 15.5 g/L); the efficiency of enzymatic hydrolysis was 0.51 g of reducing sugars per gram of water hyacinth. Finally, reducing sugars were fermented using Saccharomyces cerevisiae for conversion to ethanol, with the highest ethanol concentration (7.13 g/L) and an ethanol yield of 0.23 g/g of dry matter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water hyacinth (WH) is an important aquatic plant high in hemicellulose and low in lignin content. It contains about 48% hemicellulose, 18% cellulose and 3% lignin, although the reported composition varies (Aswathy et al. 2010). The species is an important source of fermentable sugars for bioethanol production to substitute fossil fuels, and its residual biomass can also be converted into other value-added chemicals in a well-integrated biorefinery facility. Its further advantages are that it does not compete with food crops for arable land, proliferates in clear water and wastewater and is highly reproducible (Singh and Bishnoi 2013; Yan et al. 2015). However, the production of bioethanol from WH requires the recovery of fermentable sugars using pretreatments that destroy covalent bonds between hemicellulose and lignin but not between cellulose and hemicellulose within the complex physical mixture of lignocellulose (Sun et al. 2016). Hemicellulose and lignin are strongly intertwined and linked by covalent bonds that hinder saccharification, presenting a bottleneck for enzymatic hydrolysis or fermentation (Gütsch et al. 2012). Also, there are technical challenges in the pretreatment of biomass and in enzymatic saccharification, especially the sourcing of enzymes (Aswathy et al. 2010).
Regular ethanol production from lignocellulosic biomass is realised by three major steps: pretreatment to disrupt the recalcitrant structures and to facilitate polysaccharide accessibility due to the increase of the surface area, enhancing accessibility for enzymatic attacks in the hydrolysis step. Enzymatic hydrolysis is then performed to hydrolyse the polysaccharides into fermentable sugars, followed by their fermentation into bioethanol. Hydrothermal pretreatment as a steam explosion has been used as a promising method to enhance cellulose availability and hemicellulose recovery without requiring any chemicals; as an environmentally friendly method, it only uses compressed hot water as a solvent and maintains cellulose and hemicellulose availability for the enzymes, resulting in lower downstream detoxification costs compared to other pretreatment techniques (Batista et al. 2019; Pratto et al. 2020).
The steam explosion has been classified as a green and competitive technology as it only contains lignocellulosic feedstock and water, preventing corrosion problems and the formation of neutralisation sludge; this pretreatment has successfully been used in delignification and the removal of hemicellulose (Ibrahim et al. 2010; Oliveira et al. 2013; Martin-Sampedro et al. 2014a), facilitating hemicellulose removal and lignin transformation because of the increase in the surface area for cellulose hydrolysis (Singh et al. 2015). Hence, de-lignification can substantially improve biomass enzymatic saccharification. Pretreatment of WH via steam explosion is an effective delignification strategy (Das et al. 2015). Ferro et al. (2015) indicate that pretreatment enhances enzymatic accessibility of cellulose and increases the level of saccharification. In this context, we evaluated the steam explosion for subsequent enzymatic hydrolysis using hydrolytic enzymes produced by solid-state fermentation (SSF) for the recovery of fermentable sugars for bioethanol production, using Saccharomyces cerevisiae.
Methods
Microorganisms and inoculum
Trichoderma harzianum PBLA (Lopez-Ramirez et al. 2018) was used as inoculum for xylanase and cellulase production by SSF. Saccharomyces cerevisiae was used as inoculum in alcoholic fermentation. Both strains were provided by Plant Pilot of Solid-State Fermentation of the Autonomous Metropolitan University, Mexico. Trichoderma harzianum PBLA was grown in 250-mL Erlenmeyer flasks with potato dextrose agar (PDA) (BIOXON, Mexico) for seven days at 30 ℃. Generated spores were inoculated in Erlenmeyer flasks containing liquid YPD medium (20 g L− 1 of yeast extract, 20 g L− 1 of peptone, and 40 g L− 1 of glucose) and maintained at 30 ℃ for 24 h; mycelial growth was considered as inoculum in SSF.
Saccharomyces cerevisiae was propagated in culture medium with glucose (50 g L− 1), K2HPO4 (5 g L− 1), (NH4)2SO4 (2 g L− 1), MgSO4∙7H2O (0.4 g L− 1) and yeast extract (1 g L− 1) and was maintained at 30 ℃ for 36 h. Biomass was used as inoculum for ethanol production. Both strains were conserved in distilled water.
Sampling and collection of water hyacinth
The sampling area for WH (Eichornia crassipes) was defined on the Papaloapan Hydrological Basin reported by the National Commission for the Knowledge and Use of Biodiversity (CONABIO 2016). Samples were obtained from the Maria Lizamba Lagoon located at 18° 29′ N 70′’ and 96° 01′ 42′’ W, the Amapa River located at 18° 18′ 88′’ N and 96° 18′ 19′’ W, and the Arroyo Hondo River located at 18° 27′ 31′’ N and 96° 20′ 28′’ W.
Water hyacinth conditioning
Once sampled, WH was washed to remove impurities. Subsequently, leaves, stem, and root were separated, followed by drying in the sun for approximately 60 h at an average temperature of 35 ± 2 ℃. Finally, the dried biomass was cut into pieces of uniform size (1 cm) and stored until use as a substrate in SSF and steam explosion.
Solid-state fermentation
Water hyacinth (from the Maria Lizamba Lagoon) was used to support hydrolytic enzyme production (xylanase and cellulose) in packed bed columns (2.5 cm in diameter and 20 cm long) with oxygen supply by forced aeration. Prior to SSF, WH was impregnated (pretreated) with H2SO4 (0.25 M), homogenised and sterilised in an autoclave at 120 ℃ for 15 min. After sterilization, WH was impregnated with a culture medium containing the following macronutrients (g L− 1): glucose (50), KH2PO4 (5), NH4NO3 (5), Urea (2), MgSO4∙7H2O (0.42), CaCl2 (1), NaCl (5) and peptone (5), as well as 1 mL of micronutrients containing (g/100 mL): FeSO4∙7H20 (0.5), MnSO4∙7H2O (0.61), ZnSO4∙7H2O (0.1) and CoCl2∙H2O (0.036) (Mekala et al. 2008). The inoculum was adjusted to 2 × 107 spores/mL at 65% of initial moisture (quantified in a gravimetric balance Ohaus, Model MB45), and the initial pH value was adjusted at 5.5. Columns were packed with the inoculated mixture, oxygen was supplied with water-saturated air at a flow rate of 50 mL/min, and the packed columns were maintained at 30 ℃. The concentration of produced CO2 (respirometry) was measured online connecting the outflow air to a gas analyser (Ávila-Cisneros et al. 2014).
Enzymatic extract
The enzyme extracts consisted of the recuperated mixed fermented medium from SSF with distilled water in a ratio of 1:10 (w/v), vortexed for 1 min (Lopez-Ramirez et al. 2018) The extract was separated by centrifugation at 10,000 rpm for 15 min at 4 ℃. The supernatant was decanted and used as an enzyme source for xylanase and cellulase assays.
Xylanase and cellulase activity
Xylanase activity was determined by mixing 0.1 mL of the enzymatic extract with 0.9 mL of 0.25% (w/v) birchwood xylan solution in 0.1 M sodium citrate buffer pH 5.2. The mixture was incubated at 40 °C for 15 min. The enzymatic reaction was stopped by adding 1.5 mL of 3,5-dinitrosalicylic acid (DNS); reducing sugars were estimated by the DNS method (Miller 1959) using xylose as standard. Cellulase activity was determined by mixing 0.1 mL of the enzymatic extract with 0.9 mL of 0.25% (w/v) solution of carboxymethyl cellulose in 0.1 M sodium citrate buffer pH 5.2; the mixture was incubated at 40 ℃ for 30 min. The enzymatic reaction was stopped by the addition of 1.5 mL of DNS; reducing sugars were estimated by the DNS method (Miller et al. 1960) using glucose as standard.
One enzymatic unit (U) was defined as the amount of enzyme required to liberate 1 μmol of reducing sugars from the substrate per minute. Enzyme activities are reported as units per gram of dry matter (U/g dm). All assays were performed in duplicate.
Steam explosion
Pretreatment of WH was carried out using the emerging technology (steam explosion) at 190 ℃ with retention times of 1, 5, and 10 min. Batch processing was used for 50 g of WH samples.
Enzymatic hydrolysis of water hyacinth pretreated with steam explosion
Enzymatic saccharification was carried out by mixing 20 g of exploited WH and 200 mL of liquid generated from the steam explosion with the enzymatic extract (cellulase and xylanase), adjusting the enzymatic activity in the reaction at 36 U/g of pretreated WH. The enzymatic reaction was performed in 500-mL Erlenmeyer flasks with gentle agitation (125 rpm) in a water bath; the pH was adjusted to 5.2 with 0.1 M citrate buffer and maintained at 50 ℃ for 48 h. Samples were taken every 12 h, and the release of reducing sugars was monitored using the DNS method (Miller et al. 1960).
Ethanol production using water hyacinth hydrolysed by a combination of methods
Ethanol production was carried out using the product of WH pretreated by steam explosion combined with the enzymatic method as a single carbon source. Alcoholic fermentation was performed by adding the culture medium containing yeast extract 1 g L− 1, NH4SO4 2 g L− 1 and MgSO4∙7H2O; inoculum concentration was adjusted at 1 × 106 cellules/mL and maintained for 72 h at 30 ℃ with agitation at 125 rpm. After fermentation, the broth was centrifuged at 10,000 rpm for 15 min and the obtained supernatant was filtered through 0.45-μm filters and used for ethanol quantification.
Ethanol quantification
The ethanol content was analysed via the spectrometric method for ethanol quantification (Isarankura-Na-Ayudhya et al. 2007), using 0.1 M solution of potassium dichromate in 5 M sulphuric acid. The reaction was performed by mixing 300 μL of alcoholic samples with 3 mL of dichromate solution, maintained at room temperature for 30 min. Absorbance was measured at 590 nm, and ethanol concentration was determined using a standard curve of ethanol.
Results and discussion
Steam explosion
Results of the steam explosion of samples of WH obtained from the Maria Lizamba Lagoon, the Arroyo Hondo River and the Amapa River are shown in Fig. 1. Based on these results, the time of the steam explosion treatment is important, and maximum quantities of reducing sugars from WH sampled from all three sites were obtained after 10 min (maximum time assayed in this research) at 190 ℃. It should be noted that the highest level of reducing sugars (fermentable sugars) was obtained from WH sampled from the Maria Lizamba Lagoon (5.4 g/50 g of water hyacinth), in contrast to the results obtained for the Arroyo Hondo River (4.3 g/50 g of water hyacinth) and the Amapa River (3.4 g/50 g of water hyacinth). Tukey´s test showed a significant difference (p > 0.05) between reducing sugar levels, and for this reason, WH from the Maria Lizamba Lagoon was used for hydrolytic enzyme production by SSF.
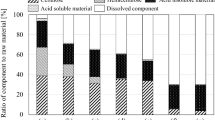
Steam explosion is classified as a green technology due to the reaction medium, which contains only lignocellulosic feedstock and water; this pretreatment has effectively been used in delignification and hemicellulose removal (Martin-Sampedro et al. 2014a, b; Ibrahim et al. 2011; Oliveira et al. 2013) through lignin transformation and increases the surface area for cellulose hydrolysis (Singh et al. 2015). In our study, WH was obviously hydrolysed and delignified after pretreatment by steam explosion, as can be seen in Fig. 1 (release of fermentable sugars) and Fig. 2a. Our results were similar to those found by Oliveira et al. (2013), who indicated that the steam explosion degraded lignin (Fig. 2b) or transformed/hydrolysed it by the explosion-produced rapid decompression and high temperatures to hemicelluloses (auto-hydrolysis) and free mono- and oligosaccharides. The sugar yields obtained in these work were lower than those obtained in other works, such a 19.7 g/100 g of dry olive leaves at 180 ℃ for 10 min (Romero-Garcia et al. 2016) and 46% of glucose after of two cycles of the steam explosion at 183 ℃ for 5 min for Eucalyptus globulus (Martin-Sampedro et al. 2014b). Similar results have been obtained for rice husk by Piñeros-Castro et al. (2011) at 190°℃ for 10 min. However, it is important to mention that the lignocellulosic raw materials pretreated with steam explosion were different from those obtained fromWH, since this is the first report.
On the other hand, it is important to note that Piñeros-Castro et al. (2011) indicated that in the steam explosion process, the amounts of furfural and hydroxymethylfurfural increase at exposure times of more than 10 min. The presence of these compounds decreases both the specific growth rate and ethanol production in alcoholic fermentation (Taherzadeh et al. 1999); similar results have been observed by Sun et al. (2016). For this reason, no longer periods were assayed in the present work.
Hydrolytic enzyme production by SSF
The results of hydrolytic enzyme production in packed bed columns using T. harzianum PBLA as inoculum and WH from the María Lizamba Lagoon as substrate are shown in Figs. 3 and 4. The highest xylanase activity was obtained after 96 h (42 U/g of dry matter) of cultivation in SSF. However, after 72 h, a first activity peak occurred (40 U/g of dry matter). Maximum cellulase activity was observed after 68 h of cultivation (2 U/g of dry matter). These results indicate that the time required for maximum xylanolytic and cellulolytic activity was shorter than that for the production of hydrolytic enzymes by SSF, using the same support and fungus, reported by Arana-Cuenca et al. (2019); in their study, the highest xylanolytic and cellulolytic activity was observed after 108 h of cultivation, similar to the results reported by Lopez-Ramirez et al. (2018) (48 h) under similar conditions. The time needed to obtain the highest enzymatic activity in this work was shorter than that reported in other studies that used the same substrate in SSF. In a study be Deshpande et al. (2008), the time required to obtain the highest cellulase and xylanase activity using other supports and fungi was 9 days, while fir cellulase activity, Zhao et al. (2011) reported 7 days under optimized conditions. The activity levels found in the present study were lower for cellulase and xylanase activity produced via WH as a substrate in SSF. It should, however, be noted that only a few reports are available on cellulase and xylanase production using WH as a substrate in SSF (Lopez-Ramirez et al. 2018; Arana-Cuenca et al. 2019).
Maximum CO2 production occurred between 54 and 62 h in all analysed columns in SSF (Fig. 5). The respirometric results showed that xylanase and cellulase production started after reaching the maximum CO2 production. Respirometric analysis suggests that for the production of the hydrolytic enzyme by T. harzianum PBLA in SSF, it is necessary that the maximum rate of CO2 production has been reached; approximately after 12 h, maximum xylanase (first maximum, Fig. 3) and cellulase activity (Fig. 4) will be obtained, indicating that the SSF must be stopped. Respirometry analysis is a useful tool for the microbial study of physiology and metabolism (Lopez-Ramirez et al. 2018; Pliego-Sandoval et al. 2012; Méndez-González et al. 2020).
Enzymatic hydrolysis of water hyacinth pretreated with steam explosion
Lignocellulosic material, such as WH, may provide fermentable sugars for ethanol production. However, recalcitrant structures of WH biomass are difficult to convert. For that reason, it is necessary to use combinations of methods, as described by Singh et al. (2015), especially when enzymes are used for cellulose hydrolysis. In this case, pretreatment is required to make cellulose more accessible to the hydrolytic enzymes, facilitating its conversion to glucose (fermentable sugars). Martín-Sampedro et al. (2014b) indicated that the steam explosion facilitates enzymatic saccharification, and for this reason, we used enzymatic hydrolysis as a pretreatment method; additionally, hydrolytic enzymes (cellulases and xylanases) were produced via SSF using WH as a substrate because the specificity by substrates may be greatest.
Enzymatic hydrolysis of WH from the María Lizamba Lagoon pretreated by steam explosion showed that this process improved enzymatic saccharification, as can be seen in the enzymatic kinetics shown in Fig. 6. The results indicate that the maximum reducing sugars were obtained after 48 h of enzymatic reaction (15.5 g/L); however, after 24 h, a level of 14.5 g/L of reducing sugars was obtained. The efficiency of enzymatic hydrolysis was 0.46 and 0.51 g of reducing sugars per gram of WH (dry matter) after 24 and 48 h of the enzymatic reaction, respectively. The enzymatic efficiency obtained in this work is highly important; according to Zhang et al. (2016), the use of enzymes in the saccharification of water hyacinth is larger for ethanol production; however, when the hydrolytic enzymes were produced by SSF from WH (present work), the costs of ethanol production were reduced. Also, the efficiency of saccharification obtained in the present research (0.46 and 0.51) is higher than that obtained when WH was saccharified using acid pretreatment followed by enzymatic hydrolysis using commercial enzymes (0.40 g of reducing sugars per g of WH), even when the enzymatic reaction conditions were optimised (Zhang et al. 2016). The result obtained after 48 h of enzymatic hydrolyses corresponds to the theoretical maximum of reducing sugars from WH (0.51 g/g) reported by Xia et al. (2013). We conclude that the combination of steam explosion pretreatment and enzymatic hydrolysis with WH-produced enzymes is adequate to obtain higher amounts of fermentable sugars. The yields after 24 h of enzymatic reaction were similar to those obtained by Satyanagalakshmi et al. (2011), who reported 0.48 g/g after 24 h of incubation. However, it is important to mention that in the cited works, commercial enzymes for WH hydrolysis were used, and the results obtained by these authors after 48 and 72 h were similar to those obtained after 24 h in the present research. In contrast to previous findings, in our study, the efficiency of enzymatic reaction was superior after 48 h increasing reducing sugars in 5% compared to the results obtained after 24 h of the enzymatic reaction. The yields of reducing sugars were lower in our study than in the studies by Aswathy et al. (2010) (0.73 g/g) and Sukumaran et al. (2009) (0.71 g/g), who used enzymes produced by SSF, and in Das et al. (2015) (0.567 g/g), who used commercial enzymes. The amount of fermentable sugars was higher than that reported in other works, probably because enzyme production using WH as support by SSF increases the specificity for the same substrate. This is the first report that used enzymes produced by SSF, using the substrate for saccharification.
Comparing the results on fermentable sugars obtained from WH saccharified by steam explosion followed by enzymatic hydrolysis with those found for other lignocellulosic material, such as sugar cane bagasse (Bunterngsook et al. 2018; with 0.79 g/g), our results were lower. However, it should be noted that we used recombinant enzymes; however, our yield was higher than that reported for saccharified wheat bran, where a yield of reducing sugars of 0.19 g/g was obtained (Jiang and Guo 2016). It is important to mention that the yield of fermentable sugars depends on the recalcitrant strength of the substrate to be saccharified and on the amount of fermentable sugars they contain.
On the other hand, as mentioned by Singh et al. (2015) and Martín-Sampedro et al. (2014b), the steam explosion improved enzymatic saccharification. After the steam explosion, cellulose was more accessible to the enzymes. We used WH as the source of cellulases and xylanases for SSF to reduce ethanol production costs. This is the first report on this combination of pretreatments for saccharification, namely the steam explosion followed by enzymatic hydrolysis.
Ethanol production
To verify the effect of pretreatments on ethanol production, reducing sugars obtained after enzymatic hydrolysis of WH were used as carbon sources for alcoholic fermentation by Saccharomyces cerevisiae. We obtained the highest ethanol concentration (7.13 g/L) after 72 h of fermentation, with an ethanol yield of 0.23 g/g of dry matter (WH) or 0.46 g/g of reducing sugars with 80% of reducing sugars consumed. The kinetics of ethanol production from WH biomass saccharified with SSF-produced enzymes can be seen in Fig. 7. The ethanol yield was higher than that reported by Ma et al. (2010) (0.192 g/g of dry matter), who used reducing sugars obtained from WH pretreated with acid after enzymatic hydrolysis. Likewise, the volumetric ethanol yield in our study was higher those of previous studies, e.g. 3.49 g/L (Ganguly et al. 2013), 4.25 g/L, (Aswathy et al. 2010), 9.8 g/L (Singh et al. 2013), while it was lower than several previously found values (10.1 g/L (Mishima et al. 2008), 10.44 g/L (Das et al. 2016), 13.6 g/L (Ganguly et al. 2013)) and comparable with the 0.46 g/g of reducing sugar reported by Kumar et al. (2009), who used different pretreatments for WH saccharification. Ethanol production from cellulosic materials greatly depends on the disruption of the lignocellulosic structure; different pretreatments including acid, alkali, microwave, liquid hot water and compound pretreatments have been used for this purpose. For ethanol production using WH as a substrate, an effective method is an acid pretreatment (Zhang et al. 2016). However, the ethanol content obtained in the present work (7.13 g/L), when steam explosion followed by enzymatic hydrolysis was used, was higher than that obtained (1.289 g/L) when acid hydrolysis followed by enzymatic hydrolysis was used (Zhang et al. 2016). Our results indicate that combined pretreatment may improve bioethanol production from WH. It is, however, important to indicate that this is the first report on the use of steam explosion and enzymatic saccharification (using enzymes produced in the same substrate). The yield of ethanol may be increased if S. cerevisiae is replaced by microorganisms that use hexoses and pentoses since the former can only use hexoses (Das et al. 2015; Ganguly et al. 2013).
Comparing our results with those obtained using other lignocellulosic materials showed that the yields of ethanol obtained in the present research (23%) are higher, even when the same methods of saccharification were used (steam explosion followed by enzyme hydrolysis). However, it should be noted that the enzymes used in previous studies were commercial enzymes. For example, in reports on ethanol production from bagasse of cane, cane leaves and sugarcane straw, ethanol yields were 16.2, 20.3 and 5.7%, respectively, as reported by Mokomele et al. (2018) and Pratto (2020).
In general, the processes for obtaining ethanol from lignocellulosic biomass present technical–economic problems such as energy costs and the costs of the enzymes used, as well as enzyme specificity: in this work, the cost of hydrolytic enzymes was kept low by producing them on the substrate to be hydrolysed, thus solving not only the cost problem, also obtaining highly specific enzymes (Madadi et al. 2017). Regarding the operating costs generated by the steam explosion, as commented by Pratto et al. (2020), the projected operating costs of an economic analysis in an ethanol production process from lignocellulosic biomass are reduced by up to 20%, even using steam explosion. This is because it is a process carried out for only a few seconds to a few minutes, requiring low amounts of energy (Tu et al. 2017; Kumari and Singh 2018; Kucharska et al. 2018).
Conclusions
The combination of pretreatments proposed in the present investigation allowed better recovery of reducing sugars from WH than other proposed pretreatments. Also, the steam explosion allows higher efficiency of enzymatic hydrolysis by making the substrates more accessible for enzymes. Likewise, the recovered reducing sugars have a higher ethanol conversion rate than reported previously for water hyacinth. In this sense, SSF is an excellent process for hydrolytic enzyme production, reducing the costs of ethanol production.
References
Arana-Cuenca A, Tovar-Jiménez X, Favela-Torres E, Perraud-Gaime I, González-Becerra AE, Martínez A, Téllez-Jurado A (2019) Use of water hyacinth as a substrate for the production of filamentous fungal hydrolytic enzymes in solid-state fermentation. 3 Biotech. https://doi.org/10.1007/s13205-018-1529-z
Aswathy US, Sukumaran RK, Devi GL, Rajasree KP, Singhania RR, Pandey A (2010) Bio-ethanol from water hyacinth biomass: an evaluation of enzymatic saccharification strategy. Biores Technol 101(3):925–930. https://doi.org/10.1016/j.biortech.2009.08.019
Ávila-Cisneros N, Velasco-Lozano S, Huerta-Ochoa S, Córdova-López J, Gimeno M, Favela-Torres E (2014) Production of thermostable lipase by Thermomyces lanuginosus on solid-state fermentation: selective hydrolysis of sardine oil. Appl Biochem Biotechnol 174(5):1859–1872. https://doi.org/10.1007/s12010-014-1159-9
CONABIO, 2016. Sistema de Información sobre Especies Invasoras. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Disponible en: https://www.biodiversidad.gob.mx/especies/Invasoras/invasoras.html Accessed 01 February 2020
Batista G, Souza RB, Pratto B, dos Santos-Rocha MS, Cruz AJ (2019) Effect of severity factor on the hydrothermal pretreatment of sugarcane straw. Biores Technol 275:321–327. https://doi.org/10.1016/j.biortech.2018.12.073
Bunterngsook B, Laothanachareon T, Chotirotsukon C, Inoue H, Fujii T, Hoshino T, Kraikul N (2018) Development of tailor-made synergistic cellulolytic enzyme system for saccharification of steam exploded sugarcane bagasse. J Biosci Bioeng 125(4):390–396. https://doi.org/10.1016/j.jbiosc.2017.11.001
Das A, Ghosh P, Paul T, Ghosh U, Pat BR, Mondal KC (2016) Production of bioethanol as useful biofuel through the bioconversion of water hyacinth (Eichhornia crassipes). 3 Biotech 6(1):70. https://doi.org/10.1007/s13205-016-0385-y
Das S, Bhattacharya A, Haldar S, Ganguly A, Gu S, Ting YP, Chatterjee PK (2015) Optimization of enzymatic saccharification of water hyacinth biomass for bio-ethanol: comparison between artificial neural network and response surface methodology. SM&T 3:17–28. https://doi.org/10.1016/j.susmat.2015.01.001
Deshpande SK, Bhotmange MG, Chakrabarti T, Shastri PN (2008) Production of cellulase and xylanase by Trichoderma reesei (QM 9414 mutant), Aspergillus niger and mixed culture by solid state fermentation (SSF) of water hyacinth (Eichhornia crassipes). Ind J Chem Technol 15:449–456. https://hdl.handle.net/123456789/2855
Ferro MD, Fernandes MC, Paulino AF, Prozil SO, Gravitis J, Evtuguin DV, Xavier AM (2015) Bioethanol production from steam explosion pretreated and alkali extracted Cistus ladanifer (rockrose). Biochem Eng J 104:98–105. https://doi.org/10.1016/j.bej.2015.04.009
Ganguly A, Das S, Bhattacharya A, Dey A, Chatterjee PK (2013) Enzymatic hydrolysis of water hyacinth biomass for the production of ethanol: optimization of driving parameters. Indian J Exp Biol 51:556–566. https://nopr.niscair.res.in/handle/123456789/19383
Gütsch JS, Nousiainen T, Sixta H (2012) Comparative evaluation of autohydrolysis and acid-catalyzed hydrolysis of Eucalyptus globulus wood. Bioresour Technol 109:77–85. https://doi.org/10.1016/j.biortech.2012.01.018
Ibrahim MM, Agblevor FA, El-Zawawy WK (2010) Isolation and characterization of cellulose and lignin from steam-exploded lignocellulosic biomass. BioResources 5(1):397–418
Ibrahim MM, El-Zawawy WK, Abdel-Fattah YR, Soliman NA, Agblevor FA (2011) Comparison of alkaline pulping with steam explosion for glucose production from rice straw. Carbohydr Polym 83(2):720–726. https://doi.org/10.1016/j.carbpol.2010.08.046
Isarankura-Na-Ayudhya C, Kongpanpee T, Prabkate P, Prachayasittikul V, Tantimongcolwat T (2007) Appropriate technology for the bioconversion of water hyacinth (Eichhornia crassipes) to liquid ethanol. EXCLI J 6:167–176. https://doi.org/10.17877/DE290R-344
Jiang ST, Guo N (2016) The steam explosion pretreatment and enzymatic hydrolysis of wheat bran. Energ Sources Part A 38(2):295–299. https://doi.org/10.1080/15567036.2012.744118
Kucharska K, Rybarczyk P, Hołowacz I, Łukajtis R, Glinka M, Kamiński M (2018) Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 23(11):2937. https://doi.org/10.3390/molecules23112937
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renewand Susta Energ Rev 90:877–891. https://doi.org/10.1016/j.rser.2018.03.111
Kumar A, Singh LK, Ghosh S (2009) Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to ethanol by Pichia stipitis. Biores Technol 100(13):3293–3297. https://doi.org/10.1016/j.biortech.2009.02.023
Lopez-Ramirez N, Volke-Sepulveda T, Gaime-Perraud I, Saucedo-Castañeda G, Favela-Torres E (2018) Effect of stirring on growth and cellulolytic enzymes production by Trichoderma harzianum in a novel bench-scale solid-state fermentation bioreactor. Bioresour Technol 265:291–298. https://doi.org/10.1016/j.biortech.2018.06.015
Ma F, Yang N, Xu C, Yu H, Wu J, Zhang X (2010) Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water hyacinth. Biores Technol 101(24):9600–9604. https://doi.org/10.1016/j.biortech.2010.07.084
Madadi M, Tu Y, Abbas A (2017) Recent status on enzymatic saccharification of lignocellulosic biomass for bioethanol production. Electron J Biol 13(2):135–143
Martin-Sampedro R, Eugenio ME, Moreno JA, Revilla E, Villar JC (2014a) Integration of a kraft pulping mill into a forest biorefinery: Pre-extraction of hemicellulose by steam explosion versus steam treatment. Bioresour Technol 153:236–244. https://doi.org/10.1016/j.biortech.2013.11.088
Martin-Sampedro R, Revilla E, Villar J, Eugenio M (2014b) Enhancement of enzymatic saccharification of Eucalyptus globulus: steam explosion versus steam treatment. Biores technol 167:186–191. https://doi.org/10.1016/j.biortech.2014.06.027
Mekala NK, Singhania RR, Sukumaran RK, Pandey A (2008) Cellulase production under solid-state fermentation by Trichoderma reesei RUT C30: statistical optimization of process parameters. Appl Biochem Biotechnol 151(2–3):122–131. https://doi.org/10.1007/s12010-008-8156-9
Méndez-González F, Loera O, Saucedo-Castañeda G, Favela-Torres E (2020) Forced aeration promotes high production and productivity of infective conidia from Metarhizium robertsii in solid-state fermentation. Biochem Eng J. https://doi.org/10.1016/j.bej.2020.107492
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Miller GL, Blum R, Glennon WE, Burton AL (1960) Measurement of carboxymethylcellulase activity. Anal Biochem 1:127–132. https://doi.org/10.1016/0003-2697(60)90004-X
Mishima D, Kuniki M, Sei K, Soda S, Ike M, Fujita M (2008) Ethanol production from candidate energy crops: water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Biores Technol 99(7):2495–2500. https://doi.org/10.1016/j.biortech.2007.04.056
Mokomele T, da Costa SL, Balan V, Van Rensburg E, Dale BE, Görgens JF (2018) Ethanol production potential from AFEX™ and steam-exploded sugarcane residues for sugarcane biorefineries. Biotechnol Biofuels 11(1):127. https://doi.org/10.1186/s13068-018-1130-z
Oliveira FM, Pinheiro IO, Souto-Maior AM, Martin C, Gonçalves AR, Rocha GJ (2013) Industrial-scale steam explosion pretreatment of sugarcane straw for enzymatic hydrolysis of cellulose for production of second generation ethanol and value-added products. Bioresour Technol 130:168–173. https://doi.org/10.1016/j.biortech.2012.12.030
Piñeros-Castro Y, Velasco GA, Proaños J, Cortes W, Ballesteros I (2011) Production of fermentable sugarsby enzymatic hydrolysis of steam-exploded rice husks. Rev Ion 24 (2):23–28. https://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-100X2011000200004
Pliego-Sandoval J, Amaya-Delgado L, Mateos-Díaz JC, Rodríguez J, Cordova J, Alba A, Herrera-López EJ (2012) Multiplex gas sampler for monitoring respirometry in column-type bioreactors used in solid-state fermentation. Biotechnol Equip 26(3):3031–3038. https://doi.org/10.5504/BBEQ.2012.0013
Pratto B, dos Santos-Rocha MSR, Longati AA, de Sousa JR, Cruz AJG (2020) Experimental optimization and techno-economic analysis of bioethanol production by simultaneous saccharification and fermentation process using sugarcane straw. Biores Technol 297:122494. https://doi.org/10.1016/j.biortech.2019.122494
Romero-García JM, Lama-Muñoz A, Rodríguez-Gutiérrez G, Moya M, Ruiz E, Fernández-Bolaños J, Castro E (2016) Obtaining sugars and natural antioxidants from olive leaves by steam-explosion. Food Chem 210:457–465. https://doi.org/10.1016/j.foodchem.2016.05.003
Satyanagalakshmi K, Sindhu R, Binod P, Janu KU, Sukumaran RK, Pandey A (2011) Bioethanol production from acid pretreated water hyacinth by separate hydrolysis and fermentation. J Sci Ind Res 70:156–161 https://nopr.niscair.res.in/handle/123456789/10978
Singh A, Bishnoi NR (2013) Comparative study of various pretreatment techniques for ethanol production from water hyacinth. Ind Crop Prod 44:283–289. https://doi.org/10.1016/j.indcrop.2012.11.026
Singh J, Suhag M, Dhaka A (2015) Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: a review. Carbohydr Polym 117:624–631. https://doi.org/10.1016/j.carbpol.2014.10.012
Sukumaran RK, Singhania RR, Mathew G, Pandey A (2009) Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew Energy 34(2):421–424. https://doi.org/10.1016/j.renene.2008.05.008
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58. https://doi.org/10.1016/j.biortech.2015.08.061
Taherzadeh MJ, Gustafsson L, Niklasson C, Lidén G (1999) Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J Biosci Bioeng 87(2):169–174. https://doi.org/10.1016/S1389-1723(99)89007-0
Tu Y, Wang L, Xia T, Sun D, Zhou S, Wang Y, Peng L (2017) Mild chemical pretreatments are sufficient for complete saccharification of steam-exploded residues and high ethanol production in desirable wheat accessions. Biores Technol 243:319–326. https://doi.org/10.1016/j.biortech.2017.06.111
Xia A, Cheng J, Song W, Yu C, Zhou J, Cen K (2013) Enhancing enzymatic saccharification of water hyacinth through microwave heating with dilute acid pretreatment for biomass energy utilization. Energy 61:158–166. https://doi.org/10.1016/j.energy.2013.09.019
Yan J, Wei Z, Wang Q, He M, Li S, Irbis C (2015) Bioethanol production from sodium hydroxide/hydrogen peroxide-pretreated water hyacinth via simultaneous saccharification and fermentation with a newly isolated thermotolerant Kluyveromyces marxianu strain. Bioresour Technol 193:103–109. https://doi.org/10.1016/j.biortech.2015.06.069
Zhang Q, Weng C, Huang H, Achal V, Wang D (2016) Optimization of bioethanol production using whole plant of water hyacinth as substrate in simultaneous saccharification and fermentation process. Front Microbiol 6:1411. https://doi.org/10.3389/fmicb.2015.01411
Zhao S, Liang X, Hua D, Ma TS, Zhang H (2011) High-yield cellulase production in solid-state fermentation by Trichoderma reesei SEMCC-3.217 using water hyacinth (Eichhornia crassipes). Afr J Biotechnol 10(50):10178–10187. https://doi.org/10.5897/AJB10.748
Acknowledgements
Figueroa-Torres L. A. thanks Consejo Nacional de Ciencia y Tecnología, México for the scholarship No. 446317.
Author information
Authors and Affiliations
Contributions
RH conceptualization. LAF and NL and ECV did the analytical work and methodology. RH, LAF and EF Data analysis. MAL validation. RH and MAL helped in preparing and editing the MS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Figueroa-Torres, L.A., Lizardi-Jiménez, M.A., López-Ramírez, N. et al. Saccharification of water hyacinth biomass by a combination of steam explosion with enzymatic technologies for bioethanol production. 3 Biotech 10, 432 (2020). https://doi.org/10.1007/s13205-020-02426-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02426-8