Abstract
To improve the nitrogen and phosphorus removal efficiency of surface flow constructed wetlands (SFCWs), biochar was added to an SFCW matrix. The effects of adding different amounts of biochar on water purification, the growth of Vallisneria natans (V. natans), and microbial mechanisms were explored through SFCW simulation experiments. The results showed that through the joint action of biochar and V. natans, the concentrations of total nitrogen, total phosphorus, and ammonia nitrogen in the effluent significantly decreased. The total biomass, relative growth rate, and chlorophyll content of V. natans were significantly reduced by adding biochar (≥20%, v/v), as the root activity and the root to leaf biomass ratio slightly increased at first and then decreased. The carbon and nitrogen contents of V. natans slightly increased with the addition of biochar (≥10%, v/v), but the phosphorus content slightly decreased. Moreover, the nitrogen content of the matrices decreased significantly over time (P<0.05), and the phosphorus content in the matrix showed an increasing trend in the same period. In addition, the microbial 16S rDNA sequencing results indicated that the diversity and abundance of the microbial community in the matrix of the biochar-added SFCW tended to decrease. Nevertheless, the abundance of functional bacteria related to nitrogen and phosphorus removal (i.e., Pseudomonas and Dechloromonas) slightly increased, which would benefit denitrification and dephosphorization in the SFCW. Hence, the addition of biochar to the SFCW matrix facilitated the improvement of effluent water quality, while excessive biochar addition (≥10%, v/v) restrained the growth of V. natans but did not cause death. This conclusion provides valid data support regarding the ability of biochar-added SFCW to purify lightly contaminated water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid urbanization and economic development have led to the continuous deterioration of urban rivers in developing countries (Ge et al. 2015; Wei et al. 2020; Xu et al. 2019b). Constructed wetlands (CWs) are engineered systems designed to utilize natural processes for wastewater treatment and have been in use since the 1960s. They have been widely used around the world for several decades due to their low external energy requirements, simple operation and maintenance, high pollutant removal efficiency, and effective recycling of water resources for domestic, industrial, and agricultural wastewater (Stefanakis 2020; Fu et al. 2019). Notably, constructing CWs inside effluent-fed urban rivers, which can enhance wastewater natural treatment efficiency, is a viable option for improving the rate of water recycling and minimizing the additional areal requirements of CWs (Vymazal 2011; Kadlec and Wallace 2008). Based on their hydrological characteristics and flow forms, CWs can be divided into two broad categories: surface flow constructed wetlands (SFCWs) and subsurface flow constructed wetlands (SSFCWs) (Abedi and Mojiri 2019; Zheng et al. 2016). Compared with SSFCWs (Nuamah et al. 2020; Liu et al. 2019), SFCWs are simpler and easier to construct and operate but lack processing efficiency (Guo et al. 2017; Ding et al. 2018). Therefore, the approach of improving water purification by SFCWs should be explored.
Submerged plants, which are not only the main producers in aquatic ecosystems but also the basis for the structural complexity, spatial heterogeneity, and functional stability of aquatic ecosystems, can be regarded as an effective means for maintaining the stability of the clear-water state in CWs (Gao et al. 2017; Nixon 2009). Through a series of biotic interactions and physicochemical reactions with water and the CW matrix, submerged plants remove nutrients (especially nitrogen and phosphorus) to improve the effluent water quality (Ciurli et al. 2009; Dai et al. 2014). Biochar, a carbon-rich multifunctional material that has high physical stability, high porosity, a large surface area, and a high cation exchange capacity (Beesley et al. 2011; Mohan et al. 2014), has been widely applied for pollutant removal, soil quality improvement, and carbon sequestration (Dai et al. 2017a; Dai et al. 2017b; Zhang et al. 2018). For instance, biochar can affect water quality directly via the strong adsorption/release of compounds (Li et al. 2020; Laird et al. 2010) and indirectly by modifying the oxygen conditions, soil enzymes, and microbial activities (Song et al. 2020; Tian et al. 2016). Consequently, biochar incorporation into the substrate of CWs can serve as an efficient way to improve the reduction of ammonia nitrogen, total nitrogen, chemical oxygen demand (COD), and organic compounds in wastewater (Wang et al. 2020; Feng et al. 2020; Nguyen et al. 2020). However, there are still few studies on the application of biochar to aquatic plants in CWs. Therefore, it is of great significance to explore the effects of adding biochar on the purification of lightly contaminated water and the effects of submerged plants in CW.
Carbon, nitrogen, and phosphorus, the most important and essential elements for plants (Elser et al. 2007; Vitousek and Howarth 1991), have strongly connected biochemical processes. Moreover, as a key component in CWs, microbes play a core role in purifying wastewater and thus affect the growth of plants (Fu et al. 2019; Song et al. 2019). Likewise, plants can affect the activity and composition of microbial communities by altering the physical environment during root growth, modifying nutrient availability through nutrient uptake, and affecting substrate availability through root exudation (Jiang and Guo 2019; Sun et al. 2012). In addition, there may be some functional bacteria in the matrix used for potential bioremediation. Therefore, it is necessary to explore how biochar could influence microbial communities in the CW matrix, in order to analyse its influences on submerged plants whose growth process and nutrient cycles interact with microbial communities in the matrix.
In this study, a SFCW supplemented with biochar was set up to evaluate its long-term performance in treating lightly contaminated water. An artificial substrate constructed through the addition of different amounts of biochar was set up to investigate the treatment effect of the SFCW on lightly contaminated water and the growth response of Vallisneria natans (V. natans), a typically submerged plant, to the substrate conditions as well as to explore the prospect of applying biochar in CWs to treat lightly contaminated water.
Materials and methods
SFCW system design
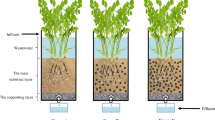
A customized rectangular frame was made of polyvinyl chloride plexiglass, with a length, width, and height of 30, 15, and 30 cm, respectively. As shown in Fig. 1, five plant-culture bottles (a diameter of 7.5 cm and a height of 8 cm) filled with a matrix mixed with different proportions of biochar (Suichang County, Zhejiang Province) were placed at the bottom of the structure. The main component of the matrix was a sandy soil substrate taken from the lightly contaminated river on the Xianlin Campus of Nanjing University (Nanjing City, Jiangsu Province, China, 118° 56′ E, 32° 07′ N). The matrix was collected from 0 to 20 cm depth and air-dried. The biochar had a diameter ranging from 1 to 3 mm and was made from bamboo poles (Phyllostachys heterocycla (Carr.) Mitford cv. Pubescens) that were heated under controlled oxygen conditions for 3 days and then heated at 800 °C for 48 h (Gao et al. 2018). Correspondingly, five biochar proportions, ranging from 0 to 20% (v/v) with a 5% interval, were established in the bottles; the experimental setup was designed by referring to the literature (Li et al. 2018; Rozari et al. 2016; Rozari et al. 2018; Liang et al. 2020). Then, the bottles were divided into five groups and labelled B0 (control), B1 (5%), B2 (10%), B3 (15%), and B4 (20%), with three duplicates in each group.
The seeds of V. natans used in the experiment were provided by Nanjing Gucheng Lake Farm. They were cultivated in the laboratory for approximately 2 months, and then V. natans plants of approximately 15 cm with 3 to 4 leaves were selected for the experiment. They were planted at a density of 6 rhizomes per bottle. The lightly contaminated water added to the test device was collected from the same river used to obtain the matrix material. The influent concentration is shown in Table 1. Then the equipment was placed in a glass greenhouse with a daily temperature of 16 to 23 °C under natural light. In addition, the positions of the different groups in the structure were rotated frequently.
Measurements of water chemicals and plant growth parameters
The concentrations of total nitrogen, total phosphorus, ammonia nitrogen, and COD were measured every 3 days, and all the tests were performed according to the criteria of the national standard method formulated by China’s State Environmental Protection Administration (SEPA 2002). In addition, the water in each device was set to a 54-day experimental period with a hydraulic retention time of 3 days.
Simultaneously, the total biomass, leaf biomass, root biomass, root to leaf biomass ratio, relative growth rate (RGR), and root activity were selected as the growth indicators of V. natans, while the total chlorophyll was measured as a subsidiary physiological index. The biomass of V. natans was measured as follows: the plants were divided into leaves and roots and oven-dried at 60 °C to constant weight, and then the dried weight of each part was measured to record the biomass. The root to leaf biomass ratio and RGR were calculated according to the following formulas:
where W1 is the root dry weight (g), W2 is the leaf dry weight (g), W3 is the final dry weight (g), W4 is the initial dry weight (g), and dt is the growth time (d).
Root activity was determined by the triphenyl tetrazolium chloride (TTC) method (Liu et al. 2014), and calculated according to the following formula:
where C is the reduction of tetrazolium in the root of V. natans (g), W5 is the root weight (g), h is the chromogenic time (h), m is the dilution ratio of the extraction solution, and triphenylformazan (TTF) is a red insoluble compound obtained by reduction of TTC.
Furthermore, the ethanol extraction method (Peng et al. 2013) was adopted to measure the chlorophyll content in the leaves of V. natans. In short, 0.5 g fresh leaves were immersed in an incubator at 37 °C for 24 h with 95% ethanol and shaken several times during the soaking process. Then, when the leaves were completely whitened, the supernatants were measured on a spectrophotometer at 665 nm and 649 nm. Finally, the chlorophyll content in leaves was expressed in mg pigment/g fresh weight.
Carbon, nitrogen, and phosphorus in the matrix and V. natans
The concentrations of carbon, nitrogen, and phosphorus in the matrix were measured every 14 days. According to the national standard method formulated by the Ministry of Environmental Protection, nitrogen, phosphorus, and carbon were measured with the Kjeldahl method (Bremner 2009), molybdenum antimony method (Dick and Tabatabai 1977), and potassium dichromate oxidation spectrophotometry (Schumacher 2002), respectively. The carbon and nitrogen contents of V. natans were determined through an elemental analyser (CHN-O-Rapid, Heraeus Inc., Germany), and the phosphorus content was H2SO4-H2O2 digested first and then analysed by ammonium molybdate ascorbic acid methods (Chen et al. 2007).
DNA extraction and high-throughput sequencing of bacteria in the matrix
Matrix samples were collected at the end of the experiment and stored at −80 °C for microbial community analysis. High-throughput sequencing of microbial 16S rDNA in the matrix was performed by Sangon Biotech, Inc. (Shanghai, China). Genomic DNA was extracted by using an E.Z.N.A. Soil DNA Kit (Omega, USA) following the manufacturer’s instructions. PCR was started immediately after the DNA extraction. The 16S rRNA V3-V4 amplicons were amplified using KAPA HiFi Hot Start Ready Mix (2×) (TaKaRa Bio Inc., Japan). Then, high-throughput sequencing was performed using the Illumina MiSeq system (Illumina MiSeq, USA) according to the manufacturer’s instructions.
Subsequently, the data were collected as follows: the MiSeq sequences included the barcode sequence, as well as the primer and linker sequences added during sequencing. First, the primer linker sequence was removed, and then the pairs of reads were merged into a sequence according to the overlapping relationship between PE reads. Next, the samples were identified and differentiated according to the barcode label sequence. Finally, quality control filtration was performed on each sample to obtain valid data for the sample.
Statistical analysis
All data were presented as the mean ± SE. Data analysis was performed using GraphPad Prism 7 software (GraphPad Software, Inc., La Jolla, CA, USA) by Student’s paired t-test (Zhu et al. 2015) and one-way analysis of variance (ANOVA) (Gao et al. 2017). The significance level for the statistical analysis was α=0.05.
Results and discussion
Pollutant removal in SFCW with biochar and V. natans
SFCWs are engineered wastewater treatment systems that have been designed and constructed to utilize natural processes including biological, chemical, and physical processes, within a controlled environment. The results showed that SFCW amended with biochar and V. natans had a positive impact on the water purification effect with time. High removal efficiencies were observed for total nitrogen and ammonia nitrogen (Fig. 2a, c). Especially, after 24 days, the total nitrogen removal rate was in the range of 20.0–33.8%, which is generally consistent with the results of earlier studies (Wei et al. 2020; Gunes et al. 2012). In addition, the removal rate of ammonia nitrogen was as high as 92.8± 4.2%. The ammonia nitrogen purification capacity attained in this study was higher than the results in Rozari et al.’s (2018) report, which demonstrated a reduction of 65–79%. The plants used in that study were paperbark trees (Melaleuca quinquenervia) and lemongrass (Cymbopogon citratus) in wetlands. These results suggest that V. natans played a positive role in the nitrogen removal process of the SFCW. Nitrogen in SFCWs is mainly removed through plant uptake, nitrification-denitrification activities, sediment storage, and volatilization (Sawaittayothin and Polprasert 2007). Fig. 2 b shows that the removal efficiency of total phosphorus was in the range of 56.5–83.3%. This could be explained by the potential of biochar to absorb and deposit phosphorous, while plants and microorganisms can also promote phosphorus removal in CWs (Huett et al. 2005; Du et al. 2017). The effluent water quality was obviously better than that of the influent, which corroborates multiple studies that have reported that adding biochar is extremely effective for total nitrogen and total phosphorus removal (Li et al. 2020; Dai et al. 2014; Jia et al. 2020). This is due to the strong adsorption affinity of biochar for soluble nutrients such as ammonium, nitrate, phosphate, and other solutes (Yuan et al. 2019). However, the removal efficiency for COD was low or even negative during the entire test period (Fig. 2d). This is because the low concentration of COD, ranging from 8 to 11 mg/L, the low concentration of influent organic matter, and the insufficient bioavailable organic carbon were not conducive to the removal of organic pollutants (Huang et al. 2012). The results of this study were consistent with those of previous studies. For example, Li et al. (2008) and Wang et al. (2016) also found that the organic matter removal performance was undesirable when the influent COD concentrations were 7.63 mg/L and 5.99 mg/L, respectively. In addition, the organic root exudates from V. natans increase the effluent COD.
Growth of V. natans in SFCW with biochar
Nutrient acquisition by roots and leaves are two dominant pathways for the growth of submerged macrophytes (Rattray et al. 1991; Xu et al. 2019a), and lower nitrogen and phosphorus contents in water may lead to slower growth of V. natans. To explore the optimal amount of biochar to add during the joint remediation process, the effects of the biochar content of the matrix on the growth of V. natans were further discussed. Here, total biomass, RGR, root activity, and the biomass allocation ratio of leaves and roots (leaf mass ratio, root mass ratio, and the ratio of roots to leaves) were used as indicators to evaluate the growth status of V. natans. According to the measurement results for the total biomass in the different experimental groups (Fig. 3a), the total biomass of V. natans in the B2 (10%), B3 (15%), and B4 (20%) groups was significantly lower than that in the B0 (control) and B1 (5%) groups (P<0.05). At the same time, the RGR of V. natans had a decreasing trend with increasing biochar content; in particular, the RGR of the B4 (20%) group was significantly lower than that of the control group (Fig. 3b, P<0.05). These results were accord with those of a study on the growth of the seedlings, shoots, and roots of Robinia pseudoacacia L. by Bu et al. (2020). However, Li et al. (2018) found that the development of the macrophyte Arundo donax was significantly promoted in SFCWs with 20% (v/v) biochar addition. Since the roots of emergent plants are submerged, they can absorb nutrients from water directly and without interacting with the biochar and microorganisms in the matrix.
Effects of sediment with different levels of biochar addition on the biomass of Vallisneria natans (V. natans). a Total biomass. b Relative growth rate. Five biochar proportions, from 0 to 20% (v/v) with 5% intervals, are labelled B0, B1, B2, B3, and B4. Bars represent the mean ± SE (n=3). Different letters indicate statistically significant differences (P<0.05).
To determine why biochar inhibited the growth of V. natans leaves, the root activity, chlorophyll content, leaf mass ratio, root mass ratio, and the root to leaf biomass ratio were further studied. The root activity and the ratio of roots to leaves slightly increased and then decreased as the biochar content increased, although the difference was not significant (Fig. 4a, Table 2). An increase in the proportion of roots to the total mass can increase the exposed absorptive surface area in nutrient-insufficient sediment, which is advantageous for nutrient acquisition in adverse environments (Chen et al. 2017). The results of this study revealed that root activity was stressed by biochar, which led to a higher root to leaf biomass ratio and a lower RGR. Additionally, chlorophyll content is an important parameter for evaluating the photosynthetic activity of plants, and these measured values can be used as indicators of pollutant-induced plant stress (Zeng et al. 2019). Therefore, chlorophyll was used as an auxiliary physiological index to evaluate the effect of biochar on the growth of V. natans. As shown in Fig. 4b, with the increase in biochar content in the matrix, the chlorophyll content in the leaves of V. natans tended to decrease. In particular, the chlorophyll content in group B4 (20%) was significantly lower than that in group B0 (P<0.05), with a difference of 0.20 mg/g.
Effects of matrices with different biochar addition levels on the root activity (a) and chlorophyll content (b) of V. natans. Five biochar proportions, from 0 to 20% (v/v) with 5% intervals, are labelled B0, B1, B2, B3, and B4. Bars represent the mean ± SE (n=6). Different letters indicate statistically significant differences (P<0.05).
The carbon, nitrogen, and phosphorus contents of V. natans and the matrix
The carbon, nitrogen, and phosphorus contents of V. natans were used to determine the limiting nutrients in the soil. Here, changes in the carbon, nitrogen, and phosphorus contents of V. natans and the matrix are further discussed. Fig. 5 a–c show the effects of different amounts of biochar on the contents of carbon, nitrogen, and phosphorus in V. natans. When compared with the carbon and nitrogen contents of V. natans in the B0 (control) group, those in the three experimental groups (with 10%, 15%, and 20% biochar) all increased (carbon increased 29.17–34.80 mg/g and nitrogen increased 0.27–3.27 mg/g). Many studies have shown that adding low concentrations of biochar is beneficial to plant growth (Liang et al. 2019; Li et al. 2021) because biochar can not only absorb nutrients and provide nutrients for plants but can also reduce the mechanical resistance of plant root growth and directly or indirectly promote the growth of plant roots (Backer et al. 2017). This study also found that the addition of biochar somewhat increased the nitrogen content in V. natans (Fig. 5b), which demonstrated that biochar can promote plant nitrogen absorption; this result is consistent with previous studies (Liang et al. 2019; Li et al. 2021). However, compared with the phosphorus content in the B0 (control) group, those in the three experimental groups (B2–B4) decreased by 0.25–1.26 mg/g (Fig. 5c). At the same time, Wu et al. (2021) found that magnetic biochar reduced plant biomass and photosynthetic capacity and inhibited the absorption of phosphorus, which is similar to our findings (Fig. 3; Fig. 4b; Fig 5c).
Effects of matrices with different biochar addition levels on the carbon (a), nitrogen (b), and phosphorus (c) contents of V. natans. Five biochar proportions, from 0 to 20% (v/v) with 5% intervals, are labelled B0, B1, B2, B3, and B4. Bars represent the mean ± SE (n=3). Different letters indicate statistically significant differences (P<0.05).
The carbon, nitrogen, and phosphorus contents in the matrix of the SFCW were further discussed. In this study, matrix samples were collected every 14 days, and the contents of total organic carbon, total nitrogen, and total phosphorus were measured. As shown in Fig. 6a, the carbon content apparently increased in the matrix of the SFCW, but it did not change much over time. According to the measurement results for nitrogen in the different groups, there was no obvious change in the nitrogen content of the matrices in the different groups in the same period; however, over time, the nitrogen content of the matrices decreased significantly (Fig. 6b, P<0.05). In addition, the phosphorus content in the matrix showed an increasing trend in the same period (Fig. 6c) because biochar can absorb phosphorus from water (Qiu et al. 2019). Which means, with more biochar added in the matrix, phosphorus concentration, especially available phosphorus for V. natans utilization, would decrease and thus caused V. natans to grow slowly.
Carbon (a), nitrogen (b), and phosphorus (c) contents in matrices with different amounts of biochar. Five biochar proportions, from 0 to 20% (v/v) with 5% intervals, are labelled B0, B1, B2, B3, and B4. Bars represent the mean ± SE (n=3). Different letters indicate statistically significant differences: the uppercase letters show the comparison of the same dose at different times, and the lowercase letters show the comparison of different doses at the same time (P<0.05).
Microbial communities in the matrices of SFCW
PCR cloning of the 16S rRNA gene was applied to detect the microbial community structures in the different biochar groups in order to understand the microbial community composition and its potential effects on the SFCW. The statistical results for the diversity indexes of the microbial community structure in the matrix are presented in Table 3. The Shannon index of the B0–B3 group decreased as the amount of added biochar increased. The Simpson index showed the opposite trend. These results indicate that the more biochar was added, the less diverse the microbial communities were, and the fewer species there were in the matrix, even though the differences were not significant (P<0.05). Similarly, the community richness indexes (ACE and Chao) (Table 3) indicated the same phenomenon. Microbial communities are important for the health and function of ecosystems (Liang et al. 2018), and the results demonstrate that the addition of biochar can partially reduce the microbial biomass and reshape the microbial community structure in the substrate (Table 3; Fig. 7).
Distribution of the microbial community in the matrix at the genus level (a) and distribution of dominant bacteria related to nitrogen transformation (b) and phosphorus removal (c) in the matrix at the genus level. Five biochar proportions, from 0 to 20% (v/v) with 5% intervals, are labelled B0, B1, B2, B3, and B4.
The microbial community structure was further analysed, and it was found that Acidobacteria Gp6 was the most abundant genus in every group, followed by Sphingomonas, Gemmatimonas, Thiobacillus, and Dechloromonas (Fig. 7a). These five genera accounted for 16.03 to 18.33% of the total microbial communities. Gp6 is an acidophilic bacterium that is widely used in the field of sewage treatment because of its prolific enzymatic system and strong metabolic capacity (Boopathy et al. 2015). Moreover, the abundances of the dominant microbial taxa of denitrifying bacteria (i.e., Pseudomonas, Nitrosospira, Fig. 7b) and polyphosphate-accumulating bacteria (i.e., Dechloromonas, Pseudomonas, Fig. 7c) increased in the matrices with higher biochar content. As the abundance of the dominant denitrification and dephosphorization bacteria increased, the microbial community diversity decreased. Among them, Dechloromonas is a facultative anaerobic bacterium capable of accumulating polyphosphate within cells that plays an important role in the degradation of organic compounds and nitrogen and phosphorus removal (Terashima et al. 2016). Pseudomonas is not only beneficial for nitrogen removal but also good for phosphorus removal (Tan et al. 2021). These results confirmed that the increase in functional bacteria was conducive to the treatment of lightly contaminated water and was in line with previous results showing that the combined effect of biochar and V. natans contributed to the purification of water quality.
As shown in Fig. 8, V. natans plants acquire nutrients such as nitrogen and phosphorus through their leaves and roots. Biochar promoted the adsorption of nitrogen and phosphorus in water by the matrix, which caused the nitrogen and phosphorus contents in the matrix to increase with the increase in the amount of biochar. Dissolved organic matter released from biochar has great potential to provide essential carbon sources for denitrification (Li et al. 2018). Furthermore, biochar was beneficial to the growth of the nitrogen and phosphorus removal microbial community, and there was a cooperative and competitive relationship between microorganisms and V. natans in the substrate. Plants can not only assimilate nitrogen and phosphorus to promote their growth but can also offer attachment sites for microorganisms and affect microbial nitrification and denitrification by releasing oxygen from roots (Huang et al. 2013). Plants and microbial interactions play a major role in CWs (Li et al. 2019). Nitrifying bacteria convert ammonia ions into nitrite ions and nitrate ions through nitrification. Denitrifying bacteria convert nitrate ions into nitrite ions, nitrous oxide, and nitrogen oxide (Kuypers et al. 2018), which results in a decrease in the available nitrogen content in the matrix and leads to a decrease in nitrogen uptake by the root system, further causing the nitrogen content in V. natans to decrease with the increase in biochar. Additionally, major phosphorus removal processes include sorption, precipitation, plant uptake, and microorganismal absorption and utilization (Vymazal 2007; Xu et al. 2020). The absorption of phosphorus by phosphorus-accumulating bacteria reduced the amount of phosphorus in the matrix that could be used by V. natans. This caused the phosphorus content of V. natans to decrease with the increase in biochar content, which damaged the photosynthetic system of V. natans; eventually, the biomass of V. natans decreased significantly, i.e., its growth was inhibited. In a word, from the perspective of nutrients, due to the effective removal of nitrogen and phosphorus from water by biochar, the absorption of nitrogen and phosphorus from water by V. natans may be reduced, and the growth of V. natans is inhibited to a certain extent. However, there are still other factors which can influence the growth of V. natans, such as allelochemical, inserted algae, and hazardous substance. Further research will be determined.
Potential mechanism of the effects of surface flow constructed wetlands with biochar on V. natans and lightly contaminated water. Biochar promoted the removal of nitrogen from the matrixes of the surface flow constructed wetland by microbes, reduced the content of available nutrients, and restricted the growth of V. natans.
Conclusion
By simulating an SFCW, the authors investigated the effects of adding biochar on water purification and V. natans growth. The growth, physiological and biochemical indexes of V. natans, water quality parameters, and microbial community structure in the matrix were measured. The results showed that adding different amounts of biochar could positively affect the growth of nitrogen- and phosphorus-removing bacteria. The addition of biochar and V. natans to the SFCW provided well effect of water purification over time. Although adding biochar slightly restrained the growth of V. natans, the tested biochar concentrations did not cause plant death. In summary, the combination of biochar with V. natans has strong application potential for improving the purification capacity of SFCWs.
References
Abedi T, Mojiri A (2019) Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment. Environ Technol Innov 16:100472. https://doi.org/10.1016/j.eti.2019.100472
Backer RGM, Saeed W, Seguin P, Smith DL (2017) Root traits and nitrogen fertilizer recovery efficiency of corn grown in biochar-amended soil under greenhouse conditions. Plant Soil 415:465–477. https://doi.org/10.1007/s11104-017-3180-6
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282. https://doi.org/10.1016/j.envpol.2011.07.023
Boopathy R, Kern C, Corbin A (2015) Use of Bacillus consortium in waste digestion and pathogen control in shrimp aquaculture. Int Biodeter Biodegr 102:159–164. https://doi.org/10.1016/j.ibiod.2015.02.001
Bremner JM (2009) Determination of nitrogen in soil by the Kjeldahl method. J Agr Sci 55:11–33. https://doi.org/10.1017/S0021859600021572
Bu X, Xue J, Wu Y, Ma W (2020) Effect of biochar on seed germination and seedling growth of Robinia pseudoacacia L. in karst calcareous soils. Commun Soil Sci Plant 51:352–363. https://doi.org/10.1080/00103624.2019.1709484
Chen A, Hu J, Sun S, Xu G (2007) Conservation and divergence of both phosphate- and mycorrhiza-regulated physiological responses and expression patterns of phosphate transporters in solanaceous species. New Phytol 173:817–831. https://doi.org/10.1111/j.1469-8137.2006.01962.x
Chen J, Hu X, Cao T, Zhang X, Xi Y, Wen X, Su H, de Silva W, Zhu T, Ni L, Xie P (2017) Root-foraging behavior ensures the integrated growth of Vallisneria natans in heterogeneous sediments. Environ Sci Pollut Res 24:8108–8119. https://doi.org/10.1007/s11356-017-8473-z
Ciurli A, Zuccarini P, Alpi A (2009) Growth and nutrient absorption of two submerged aquatic macrophytes in mesocosms, for reinsertion in a eutrophicated shallow lake. Wetl Ecol Manag 17:107–115. https://doi.org/10.1007/s11273-008-9091-9
Dai L, Fan L, Liu Y, Ruan R, Wang Y, Zhou Y, Zhao Y, Yu Z (2017a) Production of bio-oil and biochar from soapstock via microwave-assisted co-catalytic fast pyrolysis. Bioresour Technol 225:1–8. https://doi.org/10.1016/j.biortech.2016.11.017
Dai L, Tan F, Li H, Zhu N, He M, Zhu Q, Hu G, Wang L, Zhao J (2017b) Calcium-rich biochar from the pyrolysis of crab shell for phosphorus removal. J Environ Manage 198:70–74. https://doi.org/10.1016/j.jenvman.2017.04.057
Dai Y, Tang H, Chang J, Wu Z, Liang W (2014) What’s better, Ceratophyllum demersum L. or Myriophyllum verticillatum L., individual or combined? Ecol Eng 70:397–401. https://doi.org/10.1016/j.ecoleng.2014.06.009
Dick WA, Tabatabai MA (1977) An alkaline oxidation method for determination of total phosphorus in soils. Soil Sci Soc of Am J 41:511–514. https://doi.org/10.2136/sssaj1977.03615995004100030015x
Ding X, Xue Y, Zhao Y, Xiao W, Liu Y, Liu J (2018) Effects of different covering systems and carbon nitrogen ratios on nitrogen removal in surface flow constructed wetlands. J Clean Prod 172:541–551. https://doi.org/10.1016/j.jclepro.2017.10.170
Du L, Chen Q, Liu P, Zhang X, Wang H, Zhou Q, Xu D, Wu Z (2017) Phosphorus removal performance and biological dephosphorization process in treating reclaimed water by integrated vertical-flow constructed wetlands (IVCWs). Bioresour Technol 243:204–211. https://doi.org/10.1016/j.biortech.2017.06.092
Elser JJ, Bracken ME, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x
Feng L, Liu Y, Zhang J, Li C, Wu H (2020) Dynamic variation in nitrogen removal of constructed wetlands modified by biochar for treating secondary livestock effluent under varying oxygen supplying conditions. J Environ Manage 260:110152. https://doi.org/10.1016/j.jenvman.2020.110152
Fu W, Wang Y, Wei W, Li P (2019) Species diversity and functional prediction of soil bacterial communities in constructed wetlands with different plant conditions. Curr Microbiol 76:338–345. https://doi.org/10.1007/s00284-019-01634-7
Gao H, Qian X, Wu H, Li H, Pan H, Han C (2017) Combined effects of submerged macrophytes and aquatic animals on the restoration of a eutrophic water body-a case study of Gonghu Bay, Lake Taihu. Ecol Eng 102:15–23. https://doi.org/10.1016/j.ecoleng.2017.01.013
Gao Y, Zhang W, Gao B, Jia W, Miao A, Xiao L, Yang L (2018) Highly efficient removal of nitrogen and phosphorus in an electrolysis integrated horizontal subsurface-flow constructed wetland amended with biochar. Water Res 139:301–310. https://doi.org/10.1016/j.watres.2018.04.007
Ge Y, Wang X, Zheng Y, Dzakpasu M, Zhao Y, Xiong J (2015) Functions of slags and gravels as substrates in large-scale demonstration constructed wetland systems for polluted river water treatment. Environ Sci Pollut Res 22:12982–12991. https://doi.org/10.1007/s11356-015-4573-9
Gunes K, Tuncsiper B, Ayaz S, Drizo A (2012) The ability of free water surface constructed wetland system to treat high strength domestic wastewater: a case study for the Mediterranean. Ecol Eng 44:278–284. https://doi.org/10.1016/j.ecoleng.2012.04.008
Guo C, Cui Y, Dong B, Luo Y, Liu F, Zhao S, Wu H (2017) Test study of the optimal design for hydraulic performance and treatment performance of free water surface flow constructed wetland. Bioresour Technol 238:461–471. https://doi.org/10.1016/j.biortech.2017.03.163
Huang L, Gao X, Guo J, Ma X, Liu M (2013) A review on the mechanism and affecting factors of nitrous oxide emission in constructed wetlands. Environ Earth Sci 68:2171–2180. https://doi.org/10.1007/s12665-012-1900-z
Huang L, Gao X, Liu M (2012) Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Ecol Eng 46:98–106. https://doi.org/10.1016/j.ecoleng.2012.06.004
Huett DO, Morris SG, Smith G, Hunt N (2005) Nitrogen and phosphorus removal from plant nursery runoff in vegetated and unvegetated subsurface flow wetlands. Water Res 39(14):3259–3272. https://doi.org/10.1016/j.watres.2005.05.038
Jia W, Sun X, Gao Y, Yang Y, Yang L (2020) Fe-modified biochar enhances microbial nitrogen removal capability of constructed wetland. Sci Total Environ 740:139534. https://doi.org/10.1016/j.scitotenv.2020.139534
Jiang Y, Guo X (2019) Stoichiometric patterns of soil carbon, nitrogen, and phosphorus in farmland of the Poyang Lake region in Southern China. J Soil Sediment 19:3476–3488. https://doi.org/10.1007/s11368-019-02317-3
Kadlec RH, Wallace SD (2008) Treatment wetlands. CRC Press, Boca Raton
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276. https://doi.org/10.1038/nrmicro.2018.9
Laird D, Fleming P, Wang B, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158:436–442. https://doi.org/10.1016/j.geoderma.2010.05.012
Li D, Chu Z, Huang M, Zheng B (2019) Multiphasic assessment of effects of design configuration on nutrient removal in storing multiple-pond constructed wetlands. Bioresour Technol 290:121748. https://doi.org/10.1016/j.biortech.2019.121748
Li J, Fan J, Zhang J, Hu Z, Liang S (2018) Preparation and evaluation of wetland plant-based biochar for nitrogen removal enhancement in surface flow constructed wetlands. Environ Sci Pollut Res 25:13929–13937. https://doi.org/10.1007/s11356-018-1597-y
Li L, Li Y, Biswas DK (2008) Potential of constructed wetlands in treating the eutrophic water: Evidence from Taihu Lake of China. Bioresour Technol 99(6):1656–1663. https://doi.org/10.1016/j.biortech.2007.04.001
Li QW, Liang JF, Zhang XY, Feng JG, Song MH, Gao JQ (2021) Biochar addition affects root morphology and nitrogen uptake capacity in common reed (Phragmites australis). Sci Total Environ 766:144381. https://doi.org/10.1016/j.scitotenv.2020.144381
Li W, Zhou J, Ding H, Fu H, Liu J, Chen Y, Dai T, Lou Q, Zhong X, Fan H, Zhong J (2020) Low-dose biochar added to sediment improves water quality and promotes the growth of submerged macrophytes. Sci Total Environ 742:140602. https://doi.org/10.1016/j.scitotenv.2020.140602
Liang JF, An J, Gao JQ, Zhang XY, Song MH, Yu FH (2019) Interactive effects of biocharand AMF on plant growth and greenhouse gas emissions from wetland microcosms. Geoderma 346:11–17. https://doi.org/10.1016/j.geoderma.2019.03.033
Liang X, Chen L, Liu Z, Jin Y, He M, Zhao Z, Liu C, Niyungeko C, Arai Y (2018) Composition of microbial community in swine-manure biochar amended soils and linkage to heavy metal accumulation in rice at harvest. Land Degrad Dev 29:2189–2198. https://doi.org/10.1002/ldr.2851
Liang Y, Wang Q, Huang L, Liu M, Wang N, Chen Y (2020) Insight into the mechanisms of biochar addition on pollutant removal enhancement and nitrous oxide emission reduction in subsurface flow constructed wetlands: microbial community structure, functional genes and enzyme activity. Bioresour Technol 307:123249. https://doi.org/10.1016/j.biortech.2020.123249
Liu G, He T, Liu Y, Chen Z, Li L, Huang Q, Xie Z, Xie Y, Wu L, Liu J (2019) Study on the purification effect of aeration-enhanced horizontal subsurface-flow constructed wetland on polluted urban river water. Environ Sci Pollut Res 26:12867–12880. https://doi.org/10.1007/s11356-019-04832-9
Liu JJ, Wei Z, Li JH (2014) Effects of copper on leaf membrane structure and root activity of maize seedling. Bot Stud 55:47. https://doi.org/10.1186/s40529-014-0047-5
Mohan D, Sarswat A, Ok YS, Pittman CU Jr (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent-a critical review. Bioresour Technol 160:191–202. https://doi.org/10.1016/j.biortech.2014.01.120
Nguyen XC, Tran TCP, Hoang VH, Nguyen TP, Bach QV (2020) Combined biochar vertical flow and free-water surface constructed wetland system for dormitory sewage treatment and reuse. Sci Total Environ 713:136404. https://doi.org/10.1016/j.scitotenv.2019.136404
Nixon SW (2009) Eutrophication and the macroscope. Hydrobiologia 629:5–19. https://doi.org/10.1007/s10750-009-9759-z
Nuamah LA, Li Y, Pu Y, Nwankwegu AS, Haikuo Z, Norgbey E, Banahene P, Bofah-Buoh R (2020) Constructed wetlands, status, progress, and challenges. The need for critical operational reassessment for a cleaner productive ecosystem. J Clean Prod 269:122340. https://doi.org/10.1016/j.jclepro.2020.122340
Peng G, Xie XL, Jiang Q, Song S, Xu CJ (2013) Chlorophyll a/b binding protein plays a key role in natural and ethylene-induced degreening of Ponkan (Citrus reticulata Blanco). Sci Hortic-Amsterdam 160:37–43. https://doi.org/10.1016/j.scienta.2013.05.022
Qiu GQ, Zhao YL, Wang H, Tan XF, Chen FX, Hu XJ (2019) Biochar synthesized via pyrolysis of Broussonetia papyrifera leaves: mechanisms and potential applications for phosphate removal. Environ Sci Pollut Res 26:6565–6575. https://doi.org/10.1007/s11356-018-04095-w
Rattray MR, Howard-Williams C, Brown JMA (1991) Sediment and water as sources of nitrogen and phosphorus for submerged rooted aquatic macrophytes. Aquat Bot 40:225–237. https://doi.org/10.1016/0304-3770(91)90060-I
Rozari PD, Greenway M, Hanandeh AE (2016) Phosphorus removal from secondary sewage and septage using sand media amended with biochar in constructed wetland mesocosms. Sci Total Environ 569-570:123–133. https://doi.org/10.1016/j.scitotenv.2016.06.096
Rozari PD, Greenway M, Hanandeh AE (2018) Nitrogen removal from sewage and septage in constructed wetland mesocosms using sand media amended with biochar. Ecol Eng 111(1-10):1–10. https://doi.org/10.1016/j.ecoleng.2017.11.002
Sawaittayothin V, Polprasert C (2007) Nitrogen mass balance and microbial analysis of constructed wetlands treating municipal landfill leachate. Bioresour Technol 98:565–570. https://doi.org/10.1016/j.biortech.2006.02.002
Schumacher BA (2002) Methods for the determination of total organic carbon (TOC) in soils and sediments. Ecological Risk Assessment Support Center, US
SEPA (2002) Water and wastewater monitoring and analysis methods. China Environmental Science Press
Song M, Peng W, Du H, Xu Q (2019) Responses of soil and microbial C:N:P stoichiometry to vegetation succession in a karst region of Southwest China. Forests 10:755. https://doi.org/10.3390/f10090755
Song X, Razavi BS, Ludwig B, Zamanian K, Gunina A (2020) Combined biochar and nitrogen application stimulates enzyme activity and root plasticity. Sci Total Environ 735:139393. https://doi.org/10.1016/j.scitotenv.2020.139393
Stefanakis AI (2020) Constructed wetlands for sustainable wastewater treatment in hot and arid climates: opportunities, challenges and case studies in the Middle East. Water 12:1665. https://doi.org/10.3390/w12061665
Sun X, Kang H, Du H, Hu H, Zhou J, Hou J, Zhou X, Liu C (2012) Stoichiometric traits of oriental oak (Quercus variabilis) acorns and their variations in relation to environmental variables across temperate to subtropical China. Ecol Res 27:765–773. https://doi.org/10.1007/s11284-012-0953-5
Tan X, Yang YL, Liu YW, Yin WC, Fan XY (2021) The synergy of porous substrates and functional genera for efficient nutrients removal at low temperature in a pilot-scale two-stage tidal flow constructed wetland. Bioresour Technol 319:124135. https://doi.org/10.1016/j.biortech.2020.124135
Terashima M, Yama A, Sato M, Yumoto I, Kamagata Y, Kato S (2016) Culture-dependent and-independent identification of polyphosphate-accumulating Dechloromonas spp. predominating in a full-scale oxidation ditch wastewater treatment plant. Microbes environ 31(4):449–455. https://doi.org/10.1264/jsme2.ME16097
Tian J, Wang J, Dippold M, Gao Y, Blagodatskaya E, Kuzyakov Y (2016) Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci Total Environ 556:89–97. https://doi.org/10.1016/j.scitotenv.2016.03.010
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115. https://doi.org/10.1007/BF00002772
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380:48–65. https://doi.org/10.1016/j.scitotenv.2006.09.014
Vymazal J (2011) Constructed wetlands for wastewater treatment: five decades of experience. Environ Sci Technol 45:61–69. https://doi.org/10.1021/es101403q
Wang H, Xu J, Sheng L (2020) Preparation of straw biochar and application of constructed wetland in China: a review. J Clean Prod 273:123131. https://doi.org/10.1016/j.jclepro.2020.123131
Wang W, Zheng J, Wang Z (2016) Performance of pond-wetland complexes as a preliminary processor of drinking water sources. J Environ Sci 39:119–133. https://doi.org/10.1016/j.jes.2015.11.006
Wei D, Singh RP, Li Y, Fu D (2020) Nitrogen removal efficiency of surface flow constructed wetland for treating slightly polluted river water. Environ Sci Pollut Res 27:24902–24913. https://doi.org/10.1007/s11356-020-08393-0
Wu J, Huang R, Zhou Q, Lu H, Feng L, Wu K, Li Z (2021) Magnetic biochar reduces phosphorus uptake by Phragmites australis during heavy metal remediation. Sci Total Environ 758:143643. https://doi.org/10.1016/j.scitotenv.2020.143643
Xu X, Zhou Y, Han R, Song K, Zhou X, Wang G, Wang Q (2019a) Eutrophication triggers the shift of nutrient absorption pathway of submerged macrophytes: implications for the phytoremediation of eutrophic waters. J Environ Manage 239:376–384. https://doi.org/10.1016/j.jenvman.2019.03.069
Xu Y, Wu Y, Esquivel-Elizondo S, Dolfing J, Rittmann BE (2020) Using microbial aggregates to entrap aqueous phosphorus. Trends Biotechnol 38:1292–1303. https://doi.org/10.1016/j.tibtech.2020.03.012
Xu Z, Xu J, Yin H, Jin W, Li H, He Z (2019b) Urban river pollution control in developing countries. Nat Sustain 2:158–160. https://doi.org/10.1038/s41893-019-0249-7
Yuan P, Wang J, Pan Y, Shen B, Wu C (2019) Review of biochar for the management of contaminated soil: preparation, application and prospect. Sci Total Environ 659:473–490. https://doi.org/10.1016/j.scitotenv.2018.12.400
Zeng P, Guo Z, Xiao X, Peng C, Feng W, Xin L, Xu Z (2019) Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci Total Environ 650:594–603. https://doi.org/10.1016/j.scitotenv.2018.09.055
Zhang C, Liu L, Zhao M, Rong H, Xu Y (2018) The environmental characteristics and applications of biochar. Environ Sci Pollut R 25:21525–21534. https://doi.org/10.1007/s11356-018-2521-1
Zheng Y, Wang X, Dzakpasu M, Zhao Y, Ngo HH, Guo W, Ge Y, Xiong J (2016) Effects of interspecific competition on the growth of macrophytes and nutrient removal in constructed wetlands: a comparative assessment of free water surface and horizontal subsurface flow systems. Bioresour Technol 207:134–141. https://doi.org/10.1016/j.biortech.2016.02.008
Zhu ZJ, Yuan HZ, Wei Y, Li PS, Zhang PH, Xie D (2015) Effects of ammonia nitrogen and sediment nutrient on growth of the submerged plant Vallisneria natans. Clean-Soil Air Water 43:1653–1659. https://doi.org/10.1002/clen.201300878
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Funding
This work was supported by grants from the National Special Program of Water Environment (2017ZX07204002), National Natural Science Foundation of China (51908277), and Jiangsu Provincial Natural Science Foundation of China (BK20190320).
Author information
Authors and Affiliations
Contributions
Chaoqun Zheng: Conceptualization, methodology, investigation, formal analysis, and writing original draft. Xuanwen Zhang: Conceptualization, methodology, and investigation. Lin Gan: Conceptualization, methodology, and formal analysis. Zhaofang He: Conceptualization and methodology. Jinling Zhu: Conceptualization and methodology. Wen Zhang: Conceptualization and methodology. Yan Gao: Funding acquisition, conceptualization, and methodology. Liuyan Yang: Funding acquisition, conceptualization, project administration, and writing-reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Alexandros Stefanakis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Too much biochar added in surface flow constructed wetland (SFCW) was not conducive to the growth of Vallisneria natans (V. natans).
• High microbial activity led to decreasing bioavailability of phosphorus when excessive biochar was added in SFCW, and it would cause slow growth of V. natans.
• The addition of biochar promoted nitrogen and phosphorus removal in SFCW, and there was no obvious effect on V. natans if the addition of biochar was less than 10% (v/v) in SFCW.
Rights and permissions
About this article
Cite this article
Zheng, ., Zhang, X., Gan, L. et al. Effects of biochar on the growth of Vallisneria natans in surface flow constructed wetland. Environ Sci Pollut Res 28, 66158–66170 (2021). https://doi.org/10.1007/s11356-021-15399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15399-9