Abstract
Subsurface flow garden constructed wetland (SFGCW) is a type of constructed wetland with garden characteristics. This study explores the efficiency of SFGCW in the removal of ammonia nitrogen (NH4+-N) and total nitrogen (TN) from domestic sewage and examines the structure of the rhizosphere microbial community and prevalence of nitrifying and denitrifying bacteria. An L4(23) orthogonal experiment was conducted using different factors such as substrates, plants, and hydraulic retention times (HRT). The results of range and variance analyses revealed that HRT and substrate considerably influenced nitrogen removal by SFGCWs, with plant factors playing a notable role. The use of fluidized bed slag as the substrate enhanced nitrogen removal, particularly when HRT was set at 3 or 6 days. At the phylum level, Proteobacteria predominated the rhizosphere microbial abundance, comprising 42.58%–55.38% of the microbial population, followed by Chloroflexi (7.19%–17.16%). It exhibited higher counts in winter than in autumn. Anaerolineaceae, which belongs to Chloroflexi, was predominant in each wetland group. Seasonal variations significantly impacted the abundance of ammonia-oxidizing and nitrite-oxidizing bacteria among nitrifying bacteria in the rhizosphere microbial community, with higher levels observed in autumn than in winter, and played a crucial role in NH4+-N transformation. Additionally, a correlation was observed between the nitrogen removal effectiveness and abundance of rhizosphere microbial nitrifying bacteria, providing technical insights for further optimization of the structure and operational parameters of SFGCW.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A constructed wetland is a type of sewage biological treatment technology. Microorganisms in wetland are primarily attached to the substrate and surface of plant roots. The presence of plants creates favorable conditions for microbial growth in the rhizosphere (Choi et al., 2021). This interaction between substrate and plant renders the wetland system crucial for nutrient cycling and the removal of nitrogen, phosphorus, and heavy metals (Zeng et al., 2014). Nitrogen removal efficiency of constructed wetlands has been a focus of research. The mechanisms of nitrogen removal in wetlands include volatilization, ammonification, nitrification, denitrification, plant uptake, and substrate adsorption. Microbial nitrification and denitrification cooperatively eliminate nitrogen from wastewater (Al-Saedi et al., 2018; Inamori et al., 2007). Specifically, under aerobic conditions, ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) convert ammonia nitrogen (NH4+-N) to nitrite nitrogen (NO2--N) and nitrate nitrogen (NO3--N) , while denitrifying bacteria subsequently reduce nitrate to nitrogen (N2) under hypoxic conditions. The function and structure of microbial communities are influenced by plants, especially in the rhizosphere (Herrmann et al., 2008).
Previous studies demonstrated that plants absorb nitrogen from sewage through their roots, converting it into plant nitrogen for purification purposes (Wei et al., 2019). Abbasi et al. (2019) highlighted a significant correlation among the nitrogen removal, plant roots, and biomass. The unique biological–physical–chemical environment of the plant rhizosphere influences the microbial community, resulting in a very specific rhizosphere microbial community (Hein et al., 2008). (Xu et al., 2022) proposed that plant roots enhance microbial nitrification and denitrification , while rhizosphere microorganisms facilitate nitrogen removal via these processes (Lu et al., 2018). Zhai et al. (2013) showed that organic carbon exuded from plant roots substantially contribute to the carbon source required for microbial denitrification in wetlands. Furthermore, Guo et al. (2023) demonstrated that the enhancement in the plant photosynthetic capacity would significantly affect the metabolic activities of microorganisms, and total nitrogen (TN) removal rate was 63.32% in the appropriate photoperiod (15h). The dominant microbial communities in constructed wetland ecosystems comprise microbes such as Proteobacteria, Chloroflexi, Bacteroidetes, and Firmicutes, which typically prevail in wetland sediments. However, factors such as water pollution levels, plant species, and wetland types can induce variations in their abundance and diversity (Ansola et al., 2014; Cao et al., 2017; Micallef et al., 2009). Each phylum comprises various nitrifying and denitrifying bacteria including Nitrosomonas and Nitrosococcus (nitrifying bacteria) and Thauera and Denitratisoma (denitrifying bacteria), which are all related to the Proteobacteria phylum (Gu et al., 2023).
The distribution of nitrifying and denitrifying bacteria in constructed wetlands is influenced by various wetland configurations, particularly wetland plants (Chi et al., 2021; Zhang et al., 2022). Deeper wetland areas, often characterized by anoxic/anaerobic environments, provide favorable conditions for denitrifying bacteria, thereby facilitating denitrification (Pelissari et al., 2017). Salvato et al. (2012) demonstrated that Pseudomonas, a genus of denitrifying bacteria, showed a higher abundance on the root surface of Phragmites australis and Phalaris arundinacea L., and their roots secreted more dissolved organic carbon, resulting in the denitrification rates of 38% and 34%, respectively. Fu et al. (2016) employed quantitative PCR to study the abundance of functional genes related to nitrogen removal and concluded that both AOB and denitrifying bacteria are predominant in the nitrogen removal process. Therefore, the structure of the constructed wetlands presents distinct advantages for nitrogen removal.
Subsurface flow garden constructed wetland (SFGCW) is a specific type of constructed wetland designed for sewage resource conversion, employing garden trees/shrubs and wetland substrates (Wen et al., 2015). Willow is a woody plant that is favored in wastewater treatment because of its rapid biomass growth rate, nutrient and trace element absorption and accumulation capabilities, and high transpiration rate (Frédette et al., 2019; Listosz et al., 2018). Consequently, willow is selected as the wetland plant for the construction of SFGCW. Research has indicated that greater biomass of wetland plants, such as in reed wetland sewage treatment systems, enhance the nitrogen removal capacity in sewage purification (Wang et al., 2021). Considering the association between plant nitrogen and phosphorus accumulation and biomass, this study aimed to examine the nitrogen removal efficacy of willow-based constructed wetlands. Additionally, this study sought to investigate the rhizosphere microbial community structure of wetland plants and its impact on nitrogen removal, thereby providing a theoretical foundation for the development of SFGCWs with enhanced nitrogen removal capabilities.
2 Materials and Methods
2.1 Experimental Facility
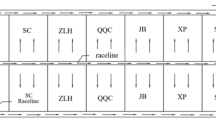
Figure 1 illustrates the experimental device used for designing the SFGCW. It comprised a plexiglass pool, measuring 1.8 m × 0.4 m × 0.7 m and remained operational for 3 years (Wen et al., 2015). Substrate layer has a depth of 50 cm, and on the substrate layer separated by permeable nonwoven covering 5 cm sand layer. Seedlings were purchased from the local market and planted at a density of 2 saplings/pool. As the structure of wetland primarily comprised willow trees and a matrix, Salix babylonica L. and S. matsudana Koidz. were selected as willow trees and fluidized bed slag (bottom slag of the circulating fluidized bed waste incinerator, 0.2-8mm, BET surface area of 8.16m2/g) and gravel (0.2-8mm, BET surface area of 3.30m2/g) were used as substrates. The designed hydraulic retention time (HRT) was set at 3 and 6 days. A peristaltic pump was used for water intake and the level gauge was set for automatic drainage, enabling the maintenance of continuous inflow and outflow throughout the device operation.
2.2 Experimental Design
The experimental design used L4(23) orthogonal table (Table 1) to make four groups with different combination structure: CW1, CW2, CW3, and CW4. The experimental input was sourced from campus domestic sewage produced in the dormitory area, and it was diluted. After dilution, the TN and NH4+-N concentrations were 45–115 mg/L and 35–55 mg/L, respectively. The detailed influent concentrations of TN and NH4+-N for each month are shown in Fig. 3. Water quality analysis was conducted in accordance with relevant standards, among which NH4+-N levels were determined using Nessler’s reagent spectrophotometry and TN was quantified using the alkaline potassium persulfate digestion UV spectrophotometric method. Water samples from the inflow and outflow of all four wetland groups were collected, with each index of the samples replicated thrice. In addition, the ambient temperature, water temperature and dissolved oxygen (DO) values were determined, and the results are shown in Fig. 2. The wetland had a seasonal duration from April to December, with an average annual ambient temperature of 23.8°C. Furthermore, an average temperature difference of 2.8°C exists between the ambient temperature and water temperature, with the water temperature generally being lower than the ambient temperature in summer and autumn, but higher in winter and spring. Rhizosphere substrate samples (from sections where the thickness of the filter material layer was <35 cm) of four groups of wetlands were collected in September and December (marked as Q and D, respectively). Following collection, high-throughput sequencing was performed on all eight sample groups.
2.3 Microbial Sampling and Analysis of Rhizosphere
The OMEGA soil DNA kit (D5625) was used to extract DNA from rhizosphere microorganisms, and the integrity and concentration of the DNA was assessed using agarose gel electrophoresis. PCR was employed to amplify the V3–V4 region of bacterial DNA, using forward primer 5'-ACTCCTACGGGAGGCAGCAG-3' and reverse primer 5'-GGACTACHVGGGTWTCTAAT-3'. Subsequently, the PCR products were evaluated through agarose electrophoresis, and DNA was extracted using the agarose gel extraction kit (cat: SK8131). The extracted DNA was precisely quantified using the Qubit2.0 DNA assay kit, and all samples were mixed in a 1:1 ratio. Thereafter, the samples were rigorously shaken, and the amplified products were analyzed through high-throughput sequencing on the Illumina Miseq sequencing platform by Majorbio Biotech Co., Ltd (Shanghai, China).
Based on the high-throughput sequencing results, complete cluster analysis was used to cluster similar samples, and sequences exhibiting 97% similarity were clustered into operational taxonomic units (OTUs). Microbial diversity was assessed by estimating Alpha diversity, and the measurement indexes were Shannon, ACE, and Chao1.
2.4 Data Analysis
The removal rates of NH4+-N and TN were determined using the chronological average method. The impact of different factors on NH4+-N and TN removal rates in SFGCWs was investigated using extreme difference and variance analysis, with equations (1) serving as the computational formula for the range of factors.
In equation (1), yjk denotes the experimental result corresponding to the j-th factor k level, \({\overline{y} }_{jk}\) (%) represents the chronological average value, and Rj (range value) reflects the variation in the horizontal influence of the experimental indexes of factor j.
In this experiment, Microsoft Excel and Origin 2021 software were employed for all data analysis and chart illustration, and SPSS 22.0 was used for statistical analysis of data.
3 Results and Discussion
3.1 Seasonal Characteristics and Difference Analysis of Nitrogen Removal
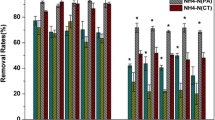
During the experiment, the removal rates for NH4+-N and TN in the four groups of SFGCWs demonstrated fluctuations in response to changes in influent concentration, as depicted in Fig. 3. Each group exhibited distinct seasonal characteristics in terms of NH4+-N and TN removal effectiveness. From October to December, the average removal rates of both NH4+-N and TN exhibited decline.
The maximum NH4+-N removal rates of the CW1, CW2, CW3, and CW4 groups were 70.34%, 68.01%, 59.94% and 63.37%, respectively, which occurred from July to August, i.e., during summer and autumn. During winter (November and December), the NH4+-N removal rate of the four groups of wetlands significantly decreased, which was the lowest among the four seasons. The highest TN removal rate in CW1, CW2, and CW4 was observed in September (66.63%), April (66.11%), and July (71.51%), respectively, whereas the highest TN removal rate of CW3 was 54.55% in December. In spring (April–May), summer (June–August), autumn (September–October), and winter (November–December), the removal effects of NH4+-N and TN in the four groups of wetlands (except the TN removal rate of CW3) in winter were unsatisfactory, and their values ranged from 37.42%–43.10% and 36.76%–51.24%, respectively.
Table 2 presents the results of the range and variance analyses regarding the NH4+-N and TN removal effects for the four SFGCW groups. The analysis revealed that the type of substrate significantly impacted the removal of NH4+-N, whereas the influence of plant species was not significant. The order of significance for NH4+-N removal effectiveness was substrate, HRT, and plant. The optimal SFGCW configuration for NH4+-N removal was based on the fluidized bed slag as the substrate, Salix babylonica L. as the plant, and an HRT of 6 days. The influence of SFGCW on TN removal differed from that on NH4+-N, with both HRT and substrate having significant impacts. The order of influence was HRT, substrate, and plant. The most effective SFGCW setup for TN removal involved fluidized bed slag as the substrate, Salix matsudana Koidz. as the plant, and an HRT of 3 days.
As observed in Fig. 3a, the NH4+-N concentration in the effluent of CW1 and CW2 was notably lower than that of CW3 and CW4 from April to October, indicating that the NH4+-N removal efficiency in SFGCWs having fluidized bed slag as a substrate surpassed those using limestone. These findings suggest that the NH4+-N removal efficacy in SFGCWs is primarily influenced by the type of substrate, which is consistent with the results of extreme difference and variance analysis. However, no significant difference in NH4+-N removal rates was observed among all groups.
Figure 3b displays the TN removal rates for the four SFGCW groups. Unlike NH4+-N, CW1 exhibited a relatively stable removal rate, whereas CW2, CW3, and CW4 demonstrated significant variability, with variations in CW4 being particularly pronounced. Among the four groups, CW4 had the lowest NH4+-N removal rate of 47.34%. Nevertheless, the TN removal rate in CW4 was 56.21%, followed by that of CW1 (58.62%). One-way analysis of variance test conducted on the NH4+-N and TN removal rates of the four SFGCW groups from April to October revealed no significant difference in NH4+-N removal rates among the groups (p = 0.21 > 0.05). The differences in TN removal rates were highly significant (p = 0.0024 < 0.01). The differential analysis results between each pair of CW1, CW2, CW3, and CW4 groups are presented in Table 3. When compared with the TN removal efficacy of CW3, CW1 and CW4 exhibited highly significant differences, followed by CW2 (p = 0.034 < 0.05). These results indicate that enhancing the TN removal efficiency in SFGCWs requires not only the selection of a suitable substrate but also an optimal setting of HRT.
3.2 Rhizosphere Microbial Community Structure Response
3.2.1 α-diversity Analysis
Based on high-throughput sequencing data, the rhizosphere microbial abundance and diversity in eight groups of SFGCW samples were analyzed, with the results including Shannon, ACE, and Chao1 indices. These results are presented in Table 4. The species richness and diversity indices for CW1, CW2, CW3, and CW4 indicated no significant seasonal variation between winter and autumn. The α-diversity analysis results based on ACE and Chao1 indices revealed the highest species richness in the microbial community of CW1D, whereas CW1Q exhibited the lowest species richness. The Shannon index showed that the microbial community α diversity was notably higher in SFGCWs using limestone as a substrate (CW3, CW4), and significantly different from HRT and plant species in fluidized bed slag as substrate for SFGCWs (CW1, CW2).
3.2.2 Species Composition Analysis
The rhizosphere microbial communities of the four SFGCW groups in autumn and winter underwent principal component analysis at both phylum and genus levels. As depicted in Fig. 4, CW1D and CW2D exhibited similar microbial community structures, similar to CW3D and CW4D. CW2Q, CW3Q, and CW4Q were closely related, whereas CW1Q was considerably different from the other three groups. These findings indicate that season and substrate types are key factors influencing the bacterial community structure of SFGCWs. OTU cluster analysis revealed that rhizosphere microorganisms in SFGCW encompassed 30 phyla (Fig. 5a). The predominant microorganism across all eight sample groups was Proteobacteria, with an average abundance of 55.38% and 50.12% in autumn and winter, respectively.
Phyla with relative abundances exceeding 1% in the SFGCWs include Chloroflexi (9.065%, 17.04%), Bacteroidetes (9.15%, 5.55%), Planctomycetes (5.14%, 5.55%), Actinobacteria (5.59%, 3.63%), Firmicutes (2.98%, 3.74%), Acidobacteria, Cyanobacteria, Chlamydiae, Nitrospirae, Verrucomicrobia, and Gemmatimonadetes. Numerous studies have established Proteobacteria’s significant role in TN removal, with most denitrifying bacteria categorized as Proteobacteria (Miao et al., 2015; Werner et al., 1994). Across the four SFGCW groups, Proteobacteria maintained a consistent abundance within the rhizosphere microorganisms; however, a marginal decrease was observed in CW1 and CW2 during winter.
The abundance of Chloroflexi was higher in winter than in autumn, which is potentially linked to the dissolved oxygen levels in wetlands. Certain research indicates that Chloroflexi are characterized by anoxic photosynthesis and unique carbon fixation metabolism (Shih et al., 2017). Conversely, the abundance of Bacteroidetes was higher in autumn than in winter. Despite Bacteroidetes being typical denitrifying bacteria, the autumnal trend of TN removal rate did not correlate with their higher abundance in CW3 and CW4. Therefore, the TN removal efficacy in SFGCWs was influenced by HRT and substrate and significantly associated with the microbial community structure.
Planctomycetes exhibited the highest abundance in autumn and winter in CW2. These microorganisms are prevalent in anaerobic environments and primarily contribute to anaerobic ammonia oxidation (Zhu et al., 2021). In CW1Q, Actinobacteria exhibited the highest abundance, followed by CW3Q, indicating that the rhizosphere area of Salix babylonica L. might be more conducive to their enrichment.
In the SFGCW rhizosphere, the dominant bacterial genus (Fig. 5b) was Anaerolineaceae, with an average abundance of 5.86% and 11.83% in autumn and winter, respectively. At the genus level, significant differences were observed in the average abundance of microorganisms between autumn and winter. Few genera with an average rhizosphere microorganism abundance exceeding 2% in autumn such as Pseudomonas (5.10%), Thauera (2.99%), Blastocatella (2.38%), Chitinophagaceae (2.25%), and Comamonadaceae (2.02%). While in winter, this included Denitratisoma (3.87%), Thauera (3.62%), Thiobacillus (3.1%), Acidovorax (2.88%), Anaerolinea (2.02%), and Planctomycetaceae (2.01%). Anaerolineaceae is a genus comprising anaerobic microorganisms that belong to the Chloroflexi family (Liang et al., 2015). It induces the effect of fermentation and provides available carbon sources for denitrifying bacteria to enhance the denitrification process (Jia et al., 2023; Meng et al., 2019). Pseudomonas offers adequate heterotrophic nitrification and aerobic denitrification performance, which is crucial for the removal of NH4+-N and TN (Pang et al., 2022). The abundance of Pseudomonas in the four groups of SFGCWs was >1% in autumn, and the abundance in CW1 (14.78%) was considerably higher than that in other groups, but it decreased rapidly in winter. The results indicated that the appropriate HRT in autumn was conducive to the enrichment of nitrogen-removing microorganisms. Additionally, the abundance of Blastocatella, Chitinophagaceae, and Comamonadaceae, which tend to be in aerobic environment, decreased to less than 1% in winters. The abundance of Thiobacillus, Acidovorax, Anaerolinea, and Planctomycetaceae exceeded 2%, indicating great changes in the oxygen environment of the four groups of SFGCWs.
3.3 Relationship Between Nitrifying and Denitrifying Bacteria and Nitrogen Removal
Some studies reported that nitrifying and denitrifying bacteria are key contributors to the nitrogen cycle within constructed wetlands (He et al., 2018). Correlation analysis between the relative abundance of these bacteria in SFGCWs and nitrogen removal efficacy was conducted (Fig. 6a). A significant positive correlation was observed between the relative abundance of nitrifying bacteria and the NH4+-N removal rate (p < 0.05). However, no significant correlation was observed between denitrifying bacteria and the removal rates of NH4+-N and TN (p > 0.01). This result suggests that NH4+-N removal in SFGCWs is predominantly driven by the nitrification process of nitrifying bacteria. The functional microflora associated with rhizosphere microbial nitrification include AOB and NOB. It was found that there were two AOB lineages of Nitrosomonas and Nitrosococcus. The relative abundance of Nitrosomonas in each wetland ranged from 0.19% to 1.78%, and AOB showed significant enrichment in each wetland. Additionally, Nitrospira (0.97%–2.15%) was the sole NOB detected in each wetland, aligning with the findings of (Wang et al., 2016) in subsurface flow constructed wetlands. The correlation analysis between the detected nitrifying bacteria (Nitrosomonas, Nitrosococcus, and Nitrospira) and the denitrification efficacy is presented in Fig. 6b. A significant positive correlation was found between Nitrospira and NH4+-N removal (p < 0.05), demonstrating a higher competitiveness than the other nitrifying bacteria in NH4+-N removal.
Kraiem et al. (2019) demonstrated that Nitrospira critically influences the nitrification process of vertical flow constructed wetlands. In autumn, the relative abundance of nitrifying bacteria in CW1, CW2, CW3, and CW4 was 2.26%, 2.71%, 2.9%, and 2.05%, respectively, which decreased by 1.17%, 1.56%, 2.08%, and 1.21% in winter, respectively (Fig. 7). Higher temperatures in autumn are more conducive to the growth of nitrifying bacteria. (Li et al., 2018) investigated seasonal effects on nitrification and denitrification microbial activity in surface flow constructed wetlands demonstrated that nitrification activity is dependent on seasonal temperature variations. Second, the decrease in DO during winter contributes to the reduced relative abundance of nitrifying bacteria. Li et al. (2023) compared nitrogen removal effects under different oxygen supply modes and confirmed that regions more conducive to DO transfer and reoxygenation exhibited higher relative abundances of AOB and NOB.
The relative abundances of Nitrosomonas, Nitrosococcus, and Nitrospira in each wetland system during autumn and winter are presented in Fig. 7. In autumn, the relative abundance of nitrifying bacteria in CW3 was 0.78%–1.25% higher than that in other wetlands. This observation corresponds with the significantly different TN removal rate of CW3 compared with that of CW1, CW2, and CW4, as outlined in Table 3. The predominant nitrifying bacteria in CW3 varied seasonally, with Nitrosomonas being more abundant in autumn and Nitrospira in winter. Notably, CW3 exhibited the highest TN removal rate in winter, which was different from the other three wetlands with unsatisfactory TN removal in winter. Nitrosomonas was significantly enriched in autumn and did not show superior nitrogen removal effect. Su et al. (2018) also observed in their comprehensive study on constructed wetlands that enhancing AOB activity could increase NH4+-N removal rates. However, a high relative abundance of AOB does not necessarily improve AOB activity, thus achieving the purpose of increasing NH4+-N removal rate, especially when Nitrosomonas predominated AOB. In autumn, wetlands with different substrates comprised varying dominant nitrifying bacteria. Nitrospira was the dominant nitrobacterium in CW1 and CW2, whereas Nitrosomonas was the dominant nitrobacterium in CW3 and CW4. In winter, there was no difference in the dominant nitrifying bacteria in each wetland, Nitrospira being the dominant bacteria in all wetlands. Fluidized bed slag-CWs exhibited a high NH4+-N removal rate (58.05%), which may be attributed to the influence of Nitrospira as a nitrite oxidizer that facilitates the complete nitrification of NH4+-N.
Investigations revealed the presence of heterotrophic denitrifying bacteria such as Thauera and Denitratisoma, along with autotrophic denitrifying bacteria like Thiobacillus and Rhizobium, in the wetland. Additionally, various common denitrifying bacteria were identified, such as Azospira, Comamonadaceae, and Acidovorax. The relative abundance differences of each bacterial genus are depicted in the heatmap in Fig. 8a. Thauera, known for short-range denitrification, exhibited significant enrichment in fluidized bed slag-based CWs, i.e., CW1 and CW2, with the highest relative abundances of 5.42% and 6.58%, respectively. The gravel-CWs (CW3, CW4) exhibited high relative abundances of Thiobacillus, Denitratisoma, and Acidovorax, peaking at 4.28%, 3.06%, and 3.56%, respectively (Fig. 8a). These findings suggest that the type of substrate was also a significant factor affecting the enrichment of denitrifying bacteria. (Liu et al., 2020) observed microbial communities enriched in different substrates in tidal flow constructed wetlands and revealed that Dechloromonas (3.25%) was significantly enriched in shale ceramsite substrate and Acidovorax (14.53%) was significantly enriched on the active alumina substrate.
The relative abundance changes of denitrifying bacteria such as Azospira, Thauera, Thiobacillus, Acidovorax, and Denitratisoma were compared across wetland groups in autumn and winter, revealing that lower winter temperatures did not inhibit their growth. This suggests the presence of a weak correlation between the relative abundance of denitrifying bacteria and seasonal temperature changes, which is consistent with the finding that temperature has no significant effect on denitrifying bacteria communities in the environmental factors (Wang et al., 2022). The relative abundances of Rhizobium, Azospira, and Comamonadaceae were similar across all groups. Notably, Denitratisoma and Acidovorax exhibited significantly higher relative abundances in CW4 than in the other three groups, ranging from 2.69% to 6.18% and from 3.07% to 4.21%, respectively.
Figure 8b indicates that Denitratisoma and Acidovorax were the key denitrifying bacteria in CW4, distinctly differentiating its bacterial structure from the other groups. This finding indicates that both the choice of substrate and the setting of HRT critically impacted the abundance and population structure of denitrifying bacteria. Therefore, variations in substrate types and HRT may alter the DO environment, thereby influencing the nitrogen removal efficiency of wetlands (Fu et al., 2020; Wang et al., 2020).
4 Conclusions
The nitrogen removal efficiency of SFGCW is a fundamental property. The results from the orthogonal experiment indicated that the factors influencing NH4+-N removal, in order of significance, were substrate, HRT, and plant (optimal combination: fluidized bed slag + Salix babylonica L.+ 6 days). The sequence of factors affecting TN removal was HRT, substrate, plant (optimal combination: fluidized bed slag + Salix matsudana Koidz. + 3 days). There was a significant correlation between plants and TN removal rate; however, no correlation of plants with NH4+-N removal was observed. The overall microbial diversity in limestone wetlands surpassed that in fluidized bed slag wetlands. Proteobacteria and Chloroflexi emerged as the dominant rhizosphere microorganisms across all four groups of wetlands. Although the abundance of denitrifying bacteria exhibited only a slight connection with seasonal variations, the abundance of nitrifying bacteria was strongly correlated with seasons. There was a significant correlation between the NH4+-N removal rate and type of nitrifying bacteria.
The influence of various factors on the plant rhizosphere microbial community structure was thoroughly analyzed, and the process of plant rhizosphere microbial community formation in SFGCW was revealed, providing theoretical and technical support for the further optimization of sustainable nitrogen removal in these systems.
Data Availability
No data was used for the research described in the article.
Abbreviations
- AOB:
-
Ammonia-oxidizing bacteria
- CW:
-
Constructed wetland
- HRT:
-
Hydraulic retention time
- NOB:
-
Nitrite-oxidizing bacteria
- OTU:
-
Operational taxonomic units
- SFGCW:
-
Subsurface flow garden constructed wetland
- NH4+-N:
-
ammonia nitrogen
- TN:
-
total nitrogen
- NO2--N:
-
nitrite nitrogen
- NO3--N:
-
nitrate nitrogen
References
Abbasi, H. N., Xie, J., Hussain, S. I., Lu, X. (2019) Nutrient removal in hybrid constructed wetlands spatial-seasonal variation and the effect of vegetation. Water Science & Technology. https://doi.org/10.2166/wst.2019.196
Al-Saedi, R., Smettem, K., & Siddique, K. H. M. (2018). Nitrogen removal efficiencies and pathways from unsaturated and saturated zones in a laboratory-scale vertical flow constructed wetland. Journal of Environmental Management, 228, 466–474. https://doi.org/10.1016/j.jenvman.2018.09.048
Ansola, G., Arroyo, P., & Saenz de Miera, L. E. (2014). Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Science of the Total Environment, 473–474, 63–71. https://doi.org/10.1016/j.scitotenv.2013.11.125
Cao, Q., Wang, H., Chen, X., Wang, R., & Liu, J. (2017). Composition and distribution of microbial communities in natural river wetlands and corresponding constructed wetlands. Ecological Engineering., 98, 40–48. https://doi.org/10.1016/j.ecoleng.2016.10.063
Chi, Z., Hou, L., & Li, H. (2021). Effects of pollution load and salinity shock on nitrogen removal and bacterial community in two-stage vertical flow constructed wetlands. Bioresource Technology, 342, 126031. https://doi.org/10.1016/j.biortech.2021.126031
Choi, K., Khan, R., & Lee, S. W. (2021). Dissection of plant microbiota and plant-microbiome interactions. Journal of Microbiology, 59(3), 281–291. https://doi.org/10.1007/s12275-021-0619-5
Frédette, C., Grebenshchykova, Z., Comeau, Y., & Brisson, J. (2019). Evapotranspiration of a willow cultivar (Salix miyabeana SX67) grown in a full-scale treatment wetland. Ecological Engineering, 127, 254–262. https://doi.org/10.1016/j.ecoleng.2018.11.027
Fu, G., Yu, T., Ning, K., Guo, Z., & Wong, M.-H. (2016). Effects of nitrogen removal microbes and partial nitrification-denitrification in the integrated vertical-flow constructed wetland. Ecological Engineering, 95, 83–89. https://doi.org/10.1016/j.ecoleng.2016.06.054
Fu, G., Wu, J., Han, J., Zhao, L., Chan, G., & Leong, K. (2020). Effects of substrate type on denitrification efficiency and microbial community structure in constructed wetlands. Bioresource Technology, 307, 123222. https://doi.org/10.1016/j.biortech.2020.123222
Gu, X., Peng, Y., Yan, P., Fan, Y., Zhang, M., Sun, S., He, S. (2023). Microbial response to nitrogen removal driven by combined iron and biomass in subsurface flow constructed wetlands with plants of different ages. Science of The Total Environment, 875. https://doi.org/10.1016/j.scitotenv.2023.162692
Guo, M., Yang, G., Meng, X., Zhang, T., Li, C., Bai, S., Zhao, X.,2023. Illuminating plant–microbe interaction: How photoperiod affects rhizosphere and pollutant removal in constructed wetland? Environment International, 179. https://doi.org/10.1016/j.envint.2023.108144
He, S., Wang, Y., Li, C., Li, Y., & Zhou, J. (2018). The nitrogen removal performance and microbial communities in a two-stage deep sequencing constructed wetland for advanced treatment of secondary effluent. Bioresource Technology, 248, 82–88. https://doi.org/10.1016/j.biortech.2017.06.150
Hein, J. W., Wolfe, G. V., & Blee, K. A. (2008). Comparison of rhizosphere bacterial communities in mutants for systemic acquired resistance. Microbial ecology, 55(2), 333–343. https://doi.org/10.1007/s00248-007-9279-1
Herrmann, M., Saunders, A.M., Schramm, A. (2008). Archaea Dominate the Ammonia-Oxidizing Community in the Rhizosphere of the Freshwater Macrophyte
Inamori, R., Gui, P., Dass, P., Matsumura, M., Xu, K. Q., Kondo, T., Ebie, Y., & Inamori, Y. (2007). Investigating CH4 and N2O emissions from eco-engineering wastewater treatment processes using constructed wetland microcosms. Process Biochemistry, 42(3), 363–373. https://doi.org/10.1016/j.procbio.2006.09.007
Jia, L., Zhou, Q., Li, Y., & Wu, W. (2023). Integrated treatment of suburb diffuse pollution using large-scale multistage constructed wetlands based on novel solid carbon: Nutrients removal and microbial interactions. The Journal of Environmental Management, 326(Pt B), 116709. https://doi.org/10.1016/j.jenvman.2022.116709
Kraiem, K., Kallali, H., Wahab, M. A., Fra-vazquez, A., Mosquera-Corral, A., & Jedidi, N. (2019). Comparative study on pilots between ANAMMOX favored conditions in a partially saturated vertical flow constructed wetland and a hybrid system for rural wastewater treatment. Science of The Total Environment, 670, 644–653. https://doi.org/10.1016/j.scitotenv.2019.03.220
Li, X., Zhang, M., Liu, F., Chen, L., Li, Y., Li, Y., Xiao, R., & Wu, J. (2018). Seasonality distribution of the abundance and activity of nitrification and denitrification microorganisms in sediments of surface flow constructed wetlands planted with Myriophyllum elatinoides during swine wastewater treatment. Bioresource Technology, 248, 89–97. https://doi.org/10.1016/j.biortech.2017.06.102
Li, L., Zhang, J., Shi, Q., & Lu, S. (2023). Comparison of nitrogen removal performance and mechanism from low-polluted wastewater by constructed wetlands with two oxygen supply strategies: Tidal flow and intermittent aeration. Chemosphere, 313, 137364. https://doi.org/10.1016/j.chemosphere.2022.137364
Liang, B., Wang, L. Y., Mbadinga, S. M., Liu, J. F., Yang, S. Z., Gu, J. D., & Mu, B. Z. (2015). Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation. AMB Express, 5(1), 117. https://doi.org/10.1186/s13568-015-0117-4
Listosz, A., Kowalczyk-Juśko, A., Jóźwiakowski, K., Marzec, M., Urban, D., Tokarz, E., & Ligęza, S. (2018). Productivity and chemical properties of Salix viminalis in a horizontal subsurface flow constructed wetland during long-term operation. Ecological Engineering, 122, 76–83. https://doi.org/10.1016/j.ecoleng.2018.07.024
Liu, C., Li, X., Yang, Y., Fan, X., Tan, X., Yin, W., Liu, Y., Zhou, Z. (2020). Double-layer substrate of shale ceramsite and active alumina tidal flow constructed wetland enhanced nitrogen removal from decentralized domestic sewage. Science of The Total Environment, 703. https://doi.org/10.1016/j.scitotenv.2019.135629
Lu, B., Xu, Z., Li, J., & Chai, X. (2018). Removal of water nutrients by different aquatic plant species: An alternative way to remediate polluted rural rivers. Ecological Engineering, 110, 18–26. https://doi.org/10.1016/j.ecoleng.2017.09.016
Meng, D., Li, J., Liu, T., Liu, Y., Yan, M., Hu, J., Li, X., Liu, X., Liang, Y., Liu, H., & Yin, H. (2019). Effects of redox potential on soil cadmium solubility: Insight into microbial community. Journal of Environmental Science China, 75, 224–232. https://doi.org/10.1016/j.jes.2018.03.032
Miao, Y., Liao, R., Zhang, X. X., Wang, Y., Wang, Z., Shi, P., Liu, B., & Li, A. (2015). Metagenomic insights into Cr(VI) effect on microbial communities and functional genes of an expanded granular sludge bed reactor treating high-nitrate wastewater. Water Research, 76, 43–52. https://doi.org/10.1016/j.watres.2015.02.042
Micallef, S. A., Shiaris, M. P., & Colón-Carmona, A. (2009). Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. Journal of Experimental Botany, 60(6), 1729–1742. https://doi.org/10.1093/jxb/erp053
Pang, Q., Xu, W., He, F., Peng, F., Zhu, X., Xu, B., Yu, J., Jiang, Z., & Wang, L. (2022). Functional genera for efficient nitrogen removal under low C/N ratio influent at low temperatures in a two-stage tidal flow constructed wetland. Science of The Total Environment, 804, 150142. https://doi.org/10.1016/j.scitotenv.2021.150142
Pelissari, C., Avila, C., Trein, C. M., Garcia, J., de Armas, R. D., Sezerino, P. H. (2017). Nitrogen transforming bacteria within a full-scale partially saturated vertical subsurface flow constructed wetland treating urban wastewater. Science of the Total Environment, 574, 390–399. https://doi.org/10.1016/j.scitotenv.2016.08.207
Salvato, M., Borin, M., Doni, S., Macci, C., Ceccanti, B., Marinari, S., & Masciandaro, G. (2012). Wetland plants, micro-organisms and enzymatic activities interrelations in treating N polluted water. Ecological Engineering, 47, 36–43. https://doi.org/10.1016/j.ecoleng.2012.06.033
Shih, P. M., Ward, L. M., & Fischer, W. W. (2017). Evolution of the 3-hydroxypropionate bicycle and recent transfer of anoxygenic photosynthesis into the Chloroflexi. Proceedings of the National Academy of Sciences, 114(40), 10749–10754. https://doi.org/10.1073/pnas.1710798114
Su, Y., Wang, W., Wu, D., Huang, W., Wang, M., & Zhu, G. (2018). Stimulating ammonia oxidizing bacteria (AOB) activity drives the ammonium oxidation rate in a constructed wetland (CW). Science of The Total Environment, 624, 87–95. https://doi.org/10.1016/j.scitotenv.2017.12.084
Wang, P., Zhang, H., Zuo, J., Zhao, D., Zou, X., Zhu, Z., Jeelani, N., Leng, X., An, S. (2016). A Hardy Plant Facilitates Nitrogen Removal via Microbial Communities in Subsurface Flow Constructed Wetlands in Winter. Scientific Reports, 6(1). https://doi.org/10.1038/srep33600
Wang, J., Hou, J., Xia, L., Jia, Z., He, X., Li, D., Zhou, Y. (2020). The combined effect of dissolved oxygen and COD/N on nitrogen removal and the corresponding mechanisms in intermittent aeration constructed wetlands. Biochemical Engineering Journal, 153. https://doi.org/10.1016/j.bej.2019.107400
Wang, J., Chen, G., Fu, Z., Qiao, H., Liu, F. (2021). Assessing wetland nitrogen removal and reed (Phragmites australis) nutrient responses for the selection of optimal harvest time. Journal of Environmental Management, 280. https://doi.org/10.1016/j.jenvman.2020.111783
Wang, R., Cui, L., Li, J., Li, W., Zhu, Y., Hao, T., Liu, Z., Lei, Y., Zhai, X., Zhao, X. (2022). Response of nir-type rhizosphere denitrifier communities to cold stress in constructed wetlands with different water levels. Journal of Cleaner Production, 362. https://doi.org/10.1016/j.jclepro.2022.132377
Wei, W., Tong, J., Hu, B.X. (2019). Study on ecological dynamic model for phytoremediation of farmland drainage water. Journal of Hydrology, 578. https://doi.org/10.1016/j.jhydrol.2019.124026
Wen, K., Zhang, Y., Wu, L., Li, Q., & Yan, Q. (2015). Efficiency of nitrogen and phosphorus removal in subsurface flow garden constructed wetland based on orthogonal design. Environmental Science & Technology, 38(09), 113–118. +126.
Werner, M., Michael, W., Rudolf, A., Karl-Heinz, S. (1994) In situ characterization of the microbial consortia active in two wastewater treatment plants. Water Research, 28(8). https://doi.org/10.1016/0043-1354(94)90243-7
Xu, J., Huang, X., Luo, P., Zhang, M., Li, H., Gong, D., Liu, F., Xiao, R., & Wu, J. (2022). Root exudates release from Myriophyllum aquaticum and effects on nitrogen removal by constructed wetlands. Journal of Cleaner Production, 375, 134095. https://doi.org/10.1016/j.jclepro.2022.134095
Zeng, Y., Yu, Z., & Huang, Y. (2014). Combination of culture-dependent and -independent methods reveals diverse acyl homoserine lactone-producers from rhizosphere of wetland plants. Current Microbiology, 68(5), 587–593. https://doi.org/10.1007/s00284-013-0513-4
Zhai, X., Piwpuan, N., Arias, C. A., Headley, T., & Brix, H. (2013). Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecological Engineering, 61, 555–563. https://doi.org/10.1016/j.ecoleng.2013.02.014
Zhang, Q., Huang, J., Dzakpasu, M., Gao, Z., Zhou, W., Zhu, R., & Xiong, J. (2022). Assessment of plants radial oxygen loss for nutrients and organic matter removal in full-scale constructed wetlands treating municipal effluents. Bioresource Technology, 360, 127545. https://doi.org/10.1016/j.biortech.2022.127545
Zhu, T., Gao, J., Huang, Z., Shang, N., Gao, J., Zhang, J., & Cai, M. (2021). Comparison of performance of two large-scale vertical-flow constructed wetlands treating wastewater treatment plant tail-water: Contaminants removal and associated microbial community. Journal of Environmental Management, 278(Pt 1), 111564. https://doi.org/10.1016/j.jenvman.2020.111564
Acknowledgments
This research was supported by the Natural Science Foundation of Tianjin, China (No. 15JCZDJC40100) and Tianjin Municipal Science and Technology Program (No. 20YDTPJC01100).
Author information
Authors and Affiliations
Contributions
Writing – original draft, Baishi Wang; Conceptualization, Liping Wu; Resources, Ruoqiao Wang; Supervision, Jiangbo Huo; Visualization, Zhou Yi, Zexin Wang and Hongzhou Zhang; Writing – review & editing, Liping Wu and Jiangbo Huo.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, B., Wu, L., Wang, R. et al. Rhizosphere Microorganisms in Subsurface Flow Garden Constructed Wetland and their Influence on Nitrogen Removal Efficiency. Water Air Soil Pollut 235, 428 (2024). https://doi.org/10.1007/s11270-024-07243-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07243-w