Abstract
In the present study, the performance of surface flow constructed wetlands (SFCWs) added with different dosage of biochar (group A 0%, group B 10%, group C 20%; v/v) was investigated, to evaluate the effect of biochar on nitrogen removal of a constructed wetland. No significant difference was observed in NH4+-N removal among three groups even during different seasons. Labile organic carbon released from biochar distinctly enhanced denitrification process, which improved NO3−-N removal efficiency by 4.58% in group B and 10.33% in group C. More importantly, compared with group A, biochar addition increased plant N removal by 82.24% and 192.11% in groups B and C, respectively. This result indicated that biochar could increase the accumulation of plant net biomass. In addition, TN removal of group A was much lower at low temperature (4.9 °C). However, no obvious influence of temperature on TN removal was observed in groups B and C with biochar addition. Microbial community analysis showed that, compared with that in group A, the total relative abundance of the main denitrification bacteria (Proteobacteria, Firmicutes, and Bacteroidetes) increased by 0.81% in group B and 13.63% in group C. These results provide a reasonable strategy for improving the performance of SFCWs under cold climate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater reclamation from sewage treatment plants (STPs) has gained considerable attention over the past 30 years in China (Lyu et al. 2016). However, discharge of sewage effluent containing a high level of nutrients such as nitrogen (N) has been a key restraint for the development of wastewater reclamation (Boonchai and Seo 2015). In China, the grade III national surface water standards (GB3838-2002) for total nitrogen (TN) was standardized to 1 mg L−1. Therefore, it is urgent to achieve satisfactory N removal performance from sewage before being discharged into aquatic ecosystems.
Constructed wetlands (CWs) were acknowledged as a promising ecological technology to treat wastewater and widely applied in aquatic ecosystems due to their low cost, effective self-adaption, and eco-friendliness (De Rozari et al. 2016). N removal in CWs is a complex process that mainly includes substrate adsorption, plant absorption, and microbial immobilization. However, the treatment performance of CWs was subject to changes in seasonal temperature variation (Rai et al. 2015). Previous studies have shown that N removal efficiency obviously declined in cold winter compared with that in warm seasons (Wang et al. 2012; Wang and Li 2015). This was mainly because most of the aquatic plants easily went into dormancy and decayed in winter, such as Phragmites australis, Typha orientalis, lotus, Acorus calamus, Canna generalis, and Juncus effusus (Fan et al. 2016). Moreover, microbial abundance and activities could also be inhibited when at low temperature (Wu et al. 2015). Thus, research on overwintering plant selection and sustainable nitrogen removal under seasonal temperature variation are crucial for the enhancement of treatment performance of CWs. Oenanthe javanica (O. javanica) is a typical macrophyte capable of rooting well and tolerating cold temperature in winter (Zhou et al. 2010). It can be applied to CWs for treating wastewater. Furthermore, little attention has been paid to determine the relationship between plants and substrate, and the influence of substrate on total biomass change and N accumulation in O. javanica is still unclear.
Biochar is a carbon-rich material pyrolyzed from biological residues under anoxic or anaerobic conditions (Manyà et al. 2012). Lately, it is of rising interest in biochar as a multifunctional adsorbent for wastewater treatment (Mohan et al. 2014). Biochar is also distinguished for its application of soil amendment. It can not only promote plants growth and reduce nutrient leaching and N2O emission, but also impact soil microbial community and stimulate N transformation (Xu et al. 2014; Lehmann and Joseph 2015). However, few studies have been conducted to apply biochar as CWs substrate, and most of the studies only focused on improvement of nitrogen and phosphorus removal efficiencies (Gupta et al. 2015; De Rozari et al. 2016). Furthermore, no efforts have been made to estimate the influence of biochar on plants growth and N transformation under seasonal variation in CWs. Therefore, it is essential to develop biochar-added CWs concentrated on wetland plant growth characteristics and N removal processes under seasonal variation. On the one hand, the application of biochar produced from wetland plant residuals can significantly increase N retention in plants as nutrients. On the other hand, the characteristics of high porosity and large surface area of biochar can increase microbial attachment sites and then stimulate N transformation. The hypothesis of this study is that CWs combined with biochar addition could enhance plants growth and N transformation and then further enhance N removal under seasonal temperature variation.
In the present study, three groups of surface flow constructed wetlands (SFCWs) added with biochar and common sand were set up to evaluate the long-term treatment performance of STPs effluent. The main objectives of this study were as follows: (1) to compare N removal performance of three groups under seasonal temperature variation; (2) to investigate the effects of various amounts of biochar addition on plant growth characteristics and the corresponding N removal performance in SFCWs; and (3) to study the retention, transformation, and export of N in SFCWs by presence of biochar.
Materials and methods
Design and setup of batch-operated SFCWs
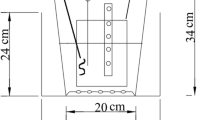
Three groups (each group has two parallels) were set up and performed under the transparent shelter in the central campus of Shandong University in Jinan, China (36° 40′ 53″ N, 117° 03′ 58″ E), where there has obvious seasonal change. Group A was packed with the sand substrate as control. Group B was packed with 10% biochar and 90% sand (v/v) substrate. Group C was packed with 20% biochar and 80% sand (v/v) substrate. Each group was 50 cm in height and 25 cm in diameter, and the substrate depth was 25 cm. Bed frame structure of substrate was divided into two layers. The main substrate layer was 20 cm filled with sand and biochar. The supporting layer was 5 cm filled with washed gravel. The schematic diagram of the experimental setup is shown in Fig. 1.
In the present study, O. javanica with mainly similar biomass was planted in the three groups, at a density of 12 rhizomes per reactor. Plants were transplanted into the SFCWs. Each group was kept flooded for about 2 months until the plants were well established. Biochar was prepared from the decayed wetland plant, Arundo donax, collected from Nansi Lake wetland, Shandong province, which was pyrolyzed under an anaerobic condition at 300 °C (Zheng et al. 2013). The reasons for selecting A. donax biochar were (1) environmental and renewable raw material; (2) high yield as well as developed aerenchyma; and (3) release of dissolved organic matter (DOM), which can provide essential carbon sources (Bruun et al. 2012).
Experiment procedure
The experimental CW microcosms were operated for 8 months at a temperature ranging from − 2 to 27 °C. The experiment involved the following two phases: phase I (with average temperature 4.9 °C) from 28 October 2016 to 11 March 2017 and phase II (with average temperature 19.4 °C) from 12 March 2017 to 15 May 2017. In order to minimize variability in the experiment, STP effluent (class IA) was simulated with synthetic wastewater. The detailed influent compositions are shown in Table 1. Due to poor treatment performance of CWs in the cold season (Spieles et al. 1999), the hydraulic retention time (HRT) of each group was 7 days in phase I and 3 days in phase II based on previous studies (Huang et al. 2000), corresponding to a hydraulic loading of 0.13 m3 m−2 batch−1.
Sampling and analysis
Water quality monitoring
In each batch, water samples were collected from the influent tank and the effluent of each group at 8:00–9:00. Chemical oxygen demand (COD), NH4+-N, NO3−-N, and NO2−-N were analyzed in the present study according to the methods described in APHA-AWWA-WPCF (2001). Dissolved oxygen (DO) and temperature were measured by a DO Meter (HQ30d, Hach, USA). The pH value was determined using a pH meter (SG2, METTLER TOLEDO, Switzerland).
Plant monitoring and analyses
Plant height was monitored during the whole experimental stage. Relative growth rate (RGR) per plant per day was calculated with Eq. (1):
where W1 and W2 are the initial dry biomass at t1 (beginning of the experiment) and harvested dry biomass at t2 (end of the experiment), respectively (Liu et al. 2012).
At the end of the experiment, plant aboveground and underground samples were separately harvested to estimate N retention in plants. The plant samples were washed with tap water to remove dust and then were dried in an oven at 65 °C for 72 h to obtain biomass dry weight. After grinding, all dried samples were sieved to pass through a 100-mesh sieve for performing the element content by the elemental analyzer (Vario EL cube, Germany).
The total N uptake (g m−2) was estimated with Eq. (2):
where N1 and N2 are the initial N content and ultimate N content in plant, respectively, and S (m2) is the surface area of the SFCW.
The percentage of plant removal efficiency was calculated with Eq. (3):
where U is total N uptake, and Inload and Efload are the N loads in influent and effluent.
Substrate sampling and analyses
Substrate samples of each group were collected for element content and microbial analysis. After collection, the substrate samples were dried at − 60 °C using a freeze dryer (Unicryo MC 2-L freeze dryer, Germany) for 36 h and then sieved (0.154 mm) and stored at − 20 °C for further analysis.
N2O sampling and analyses
Closed chamber method was used to take gas samples. Gas samples were collected at 0, 8, 24, 32, 48, and 56 h in each typical cycle. The thermometers and gas fans were installed into the transparent shelter collection boxes (100 cm in height and 28 cm in diameter). The air temperature outside and in the box was recorded simultaneously. All collected samples were measured by gas chromatography (GC, Agilent Technologies 7890A, USA) to detect N2O concentration (Xia et al. 2013).
Microbial community composition
Quantitative polymerase chain reaction
PowerSoil™ DNA Isolation Kit (MOBIO) was used to extract the total genomic DNA from the collected substrate. Quantitative analysis was conducted on bacterial 16S rRNA and nitrification functional genes (amoA, NSR) as well as denitrification functional genes (nirS, nirK, narG, nosZ) (Cheng et al. 2016).
High-throughput sequencing
To determine the microbial community of three groups, high-throughput sequencing was conducted at the Yuanxu Biotechnology Company (Shanghai, China). The sequences length shorter than 250 bp and quality score less than 30 were eliminated from the pyrosequencing-derived data sets.
Statistical analysis
All measurements were performed via the statistical program SPSS 11.0 (SPSS, Chicago, USA). The variance (ANOVA) was used to test the significance of the statistic. The results were considered to be statistically significant when p < 0.05 and no significant when p > 0.05.
Results and discussion
Water quality and N removal performance of SFCWs
Water quality
Table 1 shows the influent and effluent pH value and DO concentration throughout the experimental period. Similar effluent pH values were observed in three groups. It kept relatively stable between the two phases in each group, indicating that there was no obvious impact of biochar on pH values. The effluent DO concentrations of groups B and C were much higher than that of group A, indicating biochar addition facilitated oxygen diffusion. In phase II, the effluent DO concentrations of three groups were slightly increased compared with phase I. The possible reason may be that radial oxygen loss of wetland plants was slightly improved of the three groups as plants grow.
Nitrogen removal
The influent and effluent concentrations of various N form are shown in Table 1. The effluent concentrations of NH4+-N were all below 0.5 mg L−1 in three groups, which satisfied the standards of grade-III (NH4+-N 1 mg L−1) national surface water. From Table 1, NH4+-N removal efficiencies of the three groups were all above 92%. There was no significant difference (p > 0.05) of NH4+-N removal efficiency of each group between the two phases, illustrating that SFCWs in the present study still keep satisfying NH4+-N removal performance when the season changed. In addition, NH4+-N removal efficiency (92.85–95.33%) in the present study was superior to other studies reported at similar operation condition (Jing et al. 2001; Gao et al. 2014).
In the present study, much higher NO3−-N removal efficiency was observed in groups B and C. The NO3−-N removal efficiencies of three groups were 62.18 ± 15.31%, 81.56 ± 9.76%, and 91.27 ± 6.30% in phase I and 84.04 ± 2.24%, 87.89 ± 3.78%, and 92.72 ± 3.04% in phase II (p < 0.01, Table 1). The present results demonstrated that biochar addition could significantly improve NO3−-N treatment performance. As a result of promotion in nitrification and denitrification, the highest TN removal efficiency was obtained in group C (91.66%, 93.26%) at phases I and II, followed by group B (85.72%, 90.23%) and group A (73.58%, 88.01%). TN removal efficiencies of groups B and C were no obvious change between phases I and II. In phase I, however, TN removal declined by 16.40% in group A compared with phase II. These results demonstrated that biochar could be a promising choice for sustainable operation under seasonal temperature variation. TN removal efficiency in the present study was greater than other conventional and enhanced CWs (Vymazal et al. 2007; Zhi et al. 2014; Fan et al. 2016).
Plant growth
The individual plant height and the air temperature change with time are shown in Fig. 2. The plants in the SFCWs grew normally and without obvious symptom of toxicity in wastewater after domestication time has passed. It was observed that the growth of O. javanica was rather slow when the temperature dropped, but still showed good growth tendency, indicating O. javanica has the capacity to deal with seasonal temperature variation. Fast growth was also obtained in three groups as temperature increased. No significant difference was observed in the individual height (p > 0.05) of three groups. The net growth sizes were 79.70 cm, 88.05 cm, and 92.32 cm for plant height in three groups, suggesting that biochar was beneficial to the improvement of plants height.
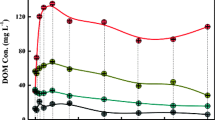
RGR and plant net growth values were employed to investigate the influence of biochar on plants growth situation (Table 2). The RGR values of plant aboveground and underground portion during the entire experimental period were 0.011 and 0.008 day−1 in group A, 0.013 and 0.009 day−1 in group B, and 0.014 and 0.010 day−1 in group C. The RGR was influenced by the plant portion as well as biochar addition, with the RGR order as follows: aboveground portion > underground portion; group A < group B < group C. These results reflected that biochar could accelerate plants growth, especially for an aboveground portion. RGR values in the present study were significantly superior to other studies obtained in winter (Gao et al. 2014) and even higher than other studies investigated under warm climate (Liu et al. 2012). Changes in plant biomass occurred with net growth, which was calculated by subtracting the initial measured value from the value measured at the end of the experiment. There was a significant difference (p < 0.05) of the plant net biomass accumulation among the three groups. Compared with group A, biochar addition increased plant biomass by 61.72% on average in group B and 104.43% in group C. This observable increase may be a function of the release of carbon and nitrogen elements from biochar to provide nutrients (Fig. 3). Furthermore, the net growth of aboveground biomass was larger than underground biomass in all groups, illustrating that nutrients mainly stored in aboveground portion via advanced root and mature plant, which was consistent with the results of RGR.
Microbial analysis
The quantity of nitrogen-related genes
The absolute abundance of bacterial 16S rRNA, amoA, NSR, narG, nirK, nirS, and nosZ were quantified at the end of the experiment to determine the influence of biochar on microbial population and nitrogen transformation process in all groups (Fig. S5). The quantitative polymerase chain reaction (qPCR) data showed that biochar could slightly increase the absolute abundance of bacterial 16S rRNA (p > 0.05), demonstrating its stimulation of microbes. The amoA, NSR, narG, nirK, nirS, and nosZ genes were related to NH4+-N, NO2−-N, and NO3−-N transformation in the processes of nitrification and denitrification. The amounts of nitrification functional genes showed no significant difference among the three groups (p > 0.05), suggesting that biochar addition has no obvious impact on nitrification. The amounts of denitrification functional genes of group C were slightly higher than that of group B, and obviously higher than that of group A. The results indicated that biochar addition strengthened denitrification process. The performance was improved as the amounts of biochar increased in the present study. These results were consistent with the results of various forms of N removal. Therefore, biochar could meaningfully increase the absolute abundance of microbes and functional genes quantities and then enhance N removal. Furthermore, the microbial community structure also played a very important role in N transformation of CWs (Truu et al. 2009).
Bacterial community structure
Illumina high-throughput sequencing of the 16S rRNA gene of the three groups was adopted to further analyze the microbial mechanism. Each sample contained 52,173–83,753 reads with a read length of 409–410 bp. Based on the Good coverage index of 99.1 to 99.8%, the sequence number was capable to describe the bacterial community, with OTUs ranging from 1105 to 1216 (Table 3). According to Chao and ACE index, the addition of biochar in groups B and C could obviously increase the community richness. On the basis of community diversity estimators of the Shannon and Simpson index, the diversity followed the order of group B > group A > group C. These results indicated that the addition of biochar could have a positive influence on the bacterial community, which was consistent with N removal (Tables 1 and 2). Notably, the Shannon and Simpson index dropped slightly while the N removal performed well and the quantities of functional genes were larger than the other two groups in group C. The decrease demonstrated that larger amount of biochar addition promoted the development of the functional microbial community in SFCWs. Bacterial community composition was further investigated to explain this phenomenon.
Bacterial community composition is known as an important factor to evaluate N removal mechanism in SFCWs (Truu et al. 2009; Xu et al. 2018). Ten primary phyla were assigned to analyze bacterial community composition, which is shown in Fig. 5. Proteobacteria was the dominant phylum in three groups (with the relative abundance of 36.51%, 52.16%, and 30.20% for group A, group B, and group C, respectively), followed by Firmicutes (34.91%, 19.93%, and 50.41%, respectively), Actinobacteria (12.05%, 10.50%, and 5.33%, respectively), and Bacteroidetes (5.42%, 5.37%, and 6.70%, respectively). Proteobacteria, the major phylum in the three groups, was further analyzed under class level through sequencing. In the present study, the branches of alpha-, beta-, delta-, and gamma-Proteobacteria were particularly evaluated (Table 4). The percentage of alpha-Proteobacteria in group C increased to 19.68% compared to that in group A, suggesting that alpha-Proteobacteria was adapted well under biochar-added environment. The gamma- and beta-Proteobacteria were related to the nutrient biodegradation (Heylen et al. 2006; Manz et al. 1994). The proportions of gamma- and beta-Proteobacteria in group C increased by 76.22% and 19.07% compared to group A, respectively. The result implied that the changes in this relative abundance were likely responsible for the enhanced denitrification in SFCWs of group C. These results are consistent with the qPCR analysis.
N retention, transformation, and export
The present study focused on N retention, transformation, and export under seasonal temperature variation to clearly identify effects of biochar amounts on N processing in SFCWs. According to the “Nitrogen removal” section about N removal (Table 1), TN removal efficiencies varied among groups and seasons ranging from 73.58 ± 9.64% (group A, phase I) to 93.26 ± 2.09% (group C, phase II). This result could be attributed to the two factors.
On the one hand, plants uptake occupied the largest proportion of TN removal in three groups (more than 50%, Table 2). That is to say, most of N from influent and substrate was assimilated and stored in plants as nutrients (Table 2, Fig. 3). Table 2 illustrates the N content and N uptake of the plant after the whole experimental period in three groups. The initial N content of plant was 1.15%. At the end of the experiment, it increased by 11.84% in group B and 48.03% in group C compared with group A. A significant increase was benefited from biochar addition (Prendergast-Miller et al. 2014). Furthermore, plant N uptake was mainly determined by biomass and N content. On the basis of the highest biomass accumulation and N content, the plant N uptake in group C was 88.8 g m−2, which was higher than that in group B (55.4 g m−2), followed by that in group A (30.4 g m−2). Higher plant N uptake usually led to more efficient N removal efficiency in SFCWs. The plant N uptake accounted for 56.47%, 60.25%, and 78.78% of mass N removal in three groups, respectively. Compared with non-biochar-added SFCWs, biochar increased N removal by 82.24% and 192.11% in groups B and C. This significant improvement highlights the direct effect of biochar on N retention in plants.
On the other hand, N transformation and export was influenced by nitrification-denitrification processes. Supporting results was obtained in Table 1. The export of NH4+-N was no significant difference among the three groups, even between two phases (p > 0.05). The results indicated that biochar did not show apparent adsorption for NH4+-N. Previous studies reported that nitrification was inhibited when the temperature was below 10 °C (Werker et al. 2002; Kuschk et al. 2003). While O. javanica in three groups retained favorable absorption capability for NH4+-N as well as enhanced oxygenation via the rhizosphere (Zhou et al. 2010), resulting in stable NH4+-N removal under seasonal temperature variation. The export of NO3−-N varied according to biochar addition (higher in group A, lower in group C), which demonstrated that the NO3−-N removal performance was positive correlated with the amount of biochar addition in the present study. This phenomenon could be supported by the results of COD effluent concentration, carbon content change of biochar, and N2O emission in three groups (Table 1, Figs. 3 and 4). Firstly, there was no significant difference of COD effluent concentration among the three groups, while carbon element content of biochar reduced by 11.31% and 12.19% in group B and group C after the experiment. These results indicated the liable organic carbon release from biochar was consumed for denitrification (Saeed et al. 2012). Secondly, Fig. 4 shows the average N2O fluxes and the percentage of N2O-N emission in TN removal. The N2O emission fluxes in group A was 1.6 and 2.3 times higher than that in groups B and C, indicating that denitrification was completely accomplished due to adequate carbon source released from biochar in groups B and C. Moreover, the characteristics of high porosity and large surface area of biochar could also create more microbial attachment sites for denitrification bacteria (Kizito et al. 2017). The previous study reported that the three typical phyla of Proteobacteria, Firmicutes, and Bacteroidetes strains were important for the denitrification process (Miao et al. 2015). As shown in Fig. 5, compared with group A, the total relative abundance values of the three phyla increase by 0.81% in group B and 13.63% in group C, respectively. The result suggested that biochar could increase many types of denitrification bacteria and then heightened denitrification process in SFCWs. Apart from enhanced denitrification process, NO3−-N export was also influenced by plant presence. Nitrate is also one of the most essential N forms for plant assimilation (Kadlec and Knight 1996), so NO3−-N produced from nitrification and in influent was converted into organic compounds serving as nutrients for plant growth.
Conclusion
Biochar played an important role in N processing (retention, transformation, and export) of constructed wetlands. It not only could increase the accumulation of plant net biomass, but also released the labile organic carbon source for denitrification bacteria to enhance TN removal. Moreover, the characteristics of high porosity and large surface area of biochar created more microbial attachment sites and correspondingly improved microbial abundance, which resulted in satisfactory N removal performance. During both summer and winter seasons, with the average temperature at 4.9 °C and 19.4 °C, respectively, TN removal efficiencies followed the order of group C > group B > group A. Group B and group C showed a stable treatment performance under seasonal temperature variation. Overall, for N removal from STP effluent, a combined biochar substrate-Oenanthe javanica microbe strategy was suitable for application in SFCWs over winter.
References
APHA-AWWA-WPCF (2001) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Boonchai R, Seo G (2015) Microalgae membrane photobioreactor for further removal of nitrogen and phosphorus from secondary sewage effluent. Korean J Chem Eng 32(10):2047–2052
Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H (2012) Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol Biochem 46:73–79
Cheng C, Xie H, Yang E, Shen X, Dai P, Zhang J (2016) Nutrient removal and microbial mechanisms in constructed wetland microcosms treating high nitrate/nitrite polluted river water. RSC Adv 6(75):70848–70854
De Rozari P, Greenway M, El Hanandeh A (2016) Phosphorus removal from secondary sewage and septage using sand media amended with biochar in constructed wetland mesocosms. Sci Total Environ 569:123–133
Fan J, Zhang J, Ngo HH, Guo W, Yin X (2016) Improving low-temperature performance of surface flow constructed wetlands using Potamogeton crispus L. plant. Bioresour Technol 218:1257–1260
Gao J, Wang W, Guo X, Zhu S, Chen S, Zhang R (2014) Nutrient removal capability and growth characteristics of Iris sibirica in subsurface vertical flow constructed wetlands in winter. Ecol Eng 70:351–361
Gupta P, Ann TW, Lee SM (2015) Use of biochar to enhance constructed wetland performance in wastewater reclamation. Environ Eng Res 21(1):36–44
Heylen K, Vanparys B, Wittebolle L, Verstraete W, Boon N, De Vos P (2006) Cultivation of denitrifying bacteria: optimization of isolation conditions and diversity study. Appl Environ Microbiol 72(4):2637–2643
Huang J, Reneau RB, Hagedorn C (2000) Nitrogen removal in constructed wetlands employed to treat domestic wastewater. Water Res 34(9):2582–2588
Jing SR, Lin YF, Lee DY, Wang TW (2001) Nutrient removal from polluted river water by using constructed wetlands. Bioresour Technol 76(2):131–135
Kadlec RH, Knight RL (1996) Treatment wetlands. CRC. Baca Raton, FL
Kizito S, Lv T, Wu S, Ajmal Z, Luo H, Dong R (2017) Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: role of media and tidal operation. Sci Total Environ 592:197–205
Kuschk P, Wiessner A, Kappelmeyer U, Weissbrodt E, Kästner M, Stottmeister U (2003) Annual cycle of nitrogen removal by a pilot-scale subsurface horizontal flow in a constructed wetland under moderate climate. Water Res 37(17):4236–4242
Lehmann J, Joseph S (eds) (2015) Biochar for environmental management: science, technology and implementation. Routledge
Liu X, Huang S, Tang T, Liu X, Scholz M (2012) Growth characteristics and nutrient removal capability of plants in subsurface vertical flow constructed wetlands. Ecol Eng 44:189–198
Lyu S, Chen W, Zhang W, Fan Y, Jiao W (2016) Wastewater reclamation and reuse in China: opportunities and challenges. J Environ Sci 39:86–96
Manyà JJ (2012) Pyrolysis for biochar purposes: a review to establish current knowledge gaps and research needs. Environ Sci Technol 46(15):7939–7954
Manz W, Wagner M, Amann R, Schleifer KH (1994) In situ characterization of the microbial consortia active in two wastewater treatment plants. Water Res 28(8):1715–1723
Miao Y, Liao R, Zhang XX, Wang Y, Wang Z, Shi P, Liu B, Li A (2015) Metagenomic insights into Cr (VI) effect on microbial communities and functional genes of an expanded granular sludge bed reactor treating high-nitrate wastewater. Water Res 76:43–52
Mohan D, Sarswat A, Ok YS, Pittman CU (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—a critical review. Bioresour Technol 160:191–202
Prendergast-Miller MT, Duvall M, Sohi SP (2014) Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur J Soil Sci 65(1):173–185
Rai UN, Upadhyay AK, Singh NK, Dwivedi S, Tripathi RD (2015) Seasonal applicability of horizontal sub-surface flow constructed wetland for trace elements and nutrient removal from urban wastes to conserve Ganga River water quality at Haridwar, India. Ecol Eng 81:115–122
Saeed T, Afrin R, Al Muyeed A, Sun G (2012) Treatment of tannery wastewater in a pilot-scale hybrid constructed wetland system in Bangladesh. Chemosphere 88(9):1065–1073
Spieles DJ, Mitsch WJ (1999) The effects of season and hydrologic and chemical loading on nitrate retention in constructed wetlands: a comparison of low-and high-nutrient riverine systems. Ecol Eng 14(1):77–91
Truu M, Juhanson J, Truu J (2009) Microbial biomass, activity and community composition in constructed wetlands. Sci Total Environ 407(13):3958–3971
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380(1):48–65
Wang L, Li T (2015) Effects of seasonal temperature variation on nitrification, anammox process, and bacteria involved in a pilot-scale constructed wetland. Environ Sci Pollut Res 22(5):3774–3783
Wang F, Liu Y, Ma Y, Wu X, Yang H (2012) Characterization of nitrification and microbial community in a shallow moss constructed wetland at cold temperatures. Ecol Eng 42:124–129
Werker AG, Dougherty JM, McHenry JL, Van Loon WA (2002) Treatment variability for wetland wastewater treatment design in cold climates. Ecol Eng 19(1):1–11
Wu H, Zhang J, Ngo HH, Guo W, Hu Z, Liang S, Liu H (2015) A review on the sustainability of constructed wetlands for wastewater treatment: design and operation. Bioresour Technol 175:594–601
Xia Y, Li Y, Li X, Guo M, She D, Yan X (2013) Diurnal pattern in nitrous oxide emissions from a sewage-enriched river. Chemosphere 92(4):421–428
Xu HJ, Wang XH, Li H, Yao HY, Su JQ, Zhu YG (2014) Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ Sci Technol 48(16):9391–9399
Xu F, Cao FQ, Kong Q, Zhou LL, Yuan Q, Zhu YJ, Wang Q (2018) Electricity production and evolution of microbial community in the constructed wetland-microbial fuel cell. Chem Eng J 339:479–486
Zheng H, Wang Z, Deng X, Xing B (2013) Impact of pyrolysis temperature on nutrient properties of biochar. In: Functions of Natural Organic Matter in Changing Environment. Springer, Dordrecht, pp 975–978
Zhi, W., & Ji, G., 2014. Quantitative response relationships between nitrogen transformation rates and nitrogen functional genes in a tidal flow constructed wetland under C/N ratio constraints. Water Res, 64, 32–41.
Zhou X, Wang G (2010) Nutrient concentration variations during Oenanthe javanica growth and decay in the ecological floating bed system. J Environ Sci 22(11):1710–1717
Funding
This work was supported by the National Natural Science Foundation of China (51878388, 51720105013, and 21507072), Natural Science Foundation of Shandong Province (ZR2018QEE006), and China Postdoctoral Science Foundation (2016M600539, 2017T100495).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(PDF 243 kb)
Rights and permissions
About this article
Cite this article
Li, J., Fan, J., Liu, D. et al. Enhanced nitrogen removal in biochar-added surface flow constructed wetlands: dealing with seasonal variation in the north China. Environ Sci Pollut Res 26, 3675–3684 (2019). https://doi.org/10.1007/s11356-018-3895-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3895-9