Abstract
The identification of fecal contamination in coastal marine ecosystems is one of the main requirements for evaluation of potential risks to human health. The objective of this study was to investigate the occurrence and distribution of fecal indicators and pathogenic bacteria in seawaters and mussels collected monthly during a period of 1 year from four different sites in Northeastern Algeria (sites S1 to S4), through biochemical and molecular analyses. Our research is the first to use molecular analysis to unambiguously identify the potentially pathogenic bacteria present in Algerian Perna perna mussels. The obtained results revealed that the levels of fecal indicator bacteria (FIB) from both P. perna and seawater samples largely exceeded the permissible limits at S2 and S3. This is mainly related to their location close to industrial and coastal activity zones, which contain a mixture of urban, agricultural, and industrial pollutants. Besides, P. perna collected from all sites were severalfold more contaminated by FIB than seawater samples, primarily during the warm season of the study period. Biochemical and molecular analyses showed that isolated bacteria from both seawater and mussels were mainly potentially pathogenic species such as E. coli, Salmonella spp., Staphylococcus spp., Klebsiella spp., Pseudomonas spp., and Proteus spp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For many decades, the coastal marine ecosystems have been continuously threatened by several anthropogenic activities such as improper sewage disposal, urban runoff, and massive discharges of agricultural and industrial effluents (Ghozzi et al. 2017; Damak et al. 2020). Coastal waters are often the receiving environment for all kinds of wastewater discharges containing many microorganisms that are harmful to human health, especially in bathing beaches and shellfish production areas (Perkins et al. 2014). Thus, the impact on health is more than worrying, placing microbiological pollution as a major public health problem.
Due to their sessile lifestyle, resistance to environmental stressors, and efficient water clearance ability, bivalves, especially mussels, have been widely used as bioindicators of coastal pollution (Belabed et al. 2013; Jia et al. 2018; Ozkan et al. 2017). These invertebrates have the potential to accumulate large quantities of microorganisms from their surrounding waters, including opportunistic bacteria (Aeromonas spp., Vibrio spp., Pseudomonas spp.), protozoan parasites (Cryptosporidium, Giardia), and viruses (adenoviruses, hepatoviruses), as well as pathogenic bacteria (E. coli, Salmonella) (Ghozzi et al. 2017). They may therefore jeopardize human health, especially when they are consumed as seafood (Stabili et al. 2005; Zannella et al. 2017; Vincy et al. 2017).
Numerous studies have reported that many serious illnesses such as acute gastroenteritis and hepatitis E virus infections are related to the presence of pathogenic microorganisms in bivalves, especially when they are eaten raw or undercooked (Le Guyader et al. 2006; O’Hara et al. 2018; Kobayashi et al. 2019; Fouillet et al. 2020). Hence, there is an urgent need for an overall assessment to predict the presence of these infectious agents related to waterborne outbreaks, and to prevent the impacts of fecal contamination on human and environmental health.
The brown mussel Perna perna (Linnaeus, 1758) is a bivalve mollusc belonging to the Mytilidae family. It is widely distributed in tropical and subtropical regions of the Mediterranean Sea, as well as in the Atlantic and Indian oceans (dos Santos et al. 2018; Neves et al. 2019). Because of its importance as a valuable source for human nutrition, P. perna is considered as one of the key aquaculture species worldwide, and the production in Brazil alone is about 18,000 tons (dos Santos et al. 2018; FAO 2019; Krampah et al. 2020). This wide geographical distribution also makes P. perna appropriate for determination of pollution levels, especially in regions that are not economically and technically prepared to monitor aquatic contamination through more sophisticated analysis (Sokolowski et al. 2004; Francioni et al. 2004).
In Algeria, P. perna mussels are harvested directly from the rocky beaches of the coastal regions where no legislation for consumption exists. This species is widely used as a bioindicator of coastal pollution to identify and classify the most suitable sites for mussel aquaculture (Belabed et al. 2013; Boudjema et al. 2014; Kadri et al. 2017; Kerdoussi et al. 2017; Abderrahmani et al. 2020). Like all North African countries, mussel aquaculture along the Algerian coasts faces important constraints such as the underdeveloped markets, low availability of good sites, lack of qualified personnel, and financial. However, universities in the Algerian cities of Oran, Mostaganem, Algiers, and Annaba are making significant efforts to develop this sector (Kara et al. 2018).
The Gulf of Annaba is one of the most valuable coastal regions of Northern Algeria, because of its great touristic and economic importance (Ouali et al. 2018). However, it is highly vulnerable to several types of pollutants, primarily related to the intensive agricultural and industrial discharges and the presence of domestic wastes, especially on the outskirts of the city where there is a high population (Soltani et al. 2012; Amri et al. 2017; Ouali et al. 2018). Other natural environmental contaminants such as the leaching of soils, animal excreta and river discharges, as well as the problems of climate change and global warming, are also likely responsible for fecal contamination in the Gulf of Annaba. According to Barreras Jr et al. 2019, rising sea surface temperatures is a consequence of the expected climate change, and this will extend the time period during which fecal bacteria can survive, which again will lead to increased bacterial load.
During recreational activities, water contaminated with fecal pollutants can pose a significant risk to human health, as many enteric pathogens are often associated with fecal matter (Oliveira et al. 2016). In case of direct or indirect contact with water, users may be exposed to a variety of waterborne diseases of ears, eyes, and skin, as well as gastrointestinal and upper respiratory illness (Maipa et al. 2001; Chávez-Díaz et al. 2020)
Despite this increasing pollution pressure, few studies have been carried out on fecal contamination and its impact on human health in the Gulf of Annaba (Kadri et al. 2015, 2017). Therefore, this study aimed to evaluate the occurrence and the distribution of fecal indicators and pathogenic bacteria in seawater and the mussel Perna perna samples by implementing a spatial–temporal sampling strategy, and to assess the impact of physicochemical variables on the abundance of fecal indicator bacteria (FIB). It should also be emphasized that our research is the first to use molecular analysis to identify with certainty the pathogenic bacteria present in Perna perna mussels collected in the Gulf of Annaba.
Materials and methods
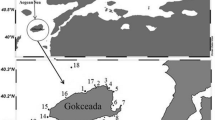
The Gulf of Annaba is located in the northeast of Algeria. It stretches over 40 km from Cap de Garde (36°96′N, 7°79′E) in the west to Cap Rosa (36°68′N, 8°25′E) in the east. It is a heavily polluted ecosystem, due to a variety of agricultural, industrial, and urban discharges, in addition to massive domestic wastes from a large part of the city of Annaba (Abdennour et al. 2000). Four sampling sites were strategically selected for the present study, based on different potential pollution sources in these areas: S1 ‘Cap de Garde’; S2 ‘Rezgui Rachid’; S3 ‘Sidi Salem’; and S4 ‘Lahnaya’ (Fig. 1). The characteristics of the selected sites are shown in Table 1.
Map showing the location of the Gulf of Annaba and sampling sites. The small map shows the overall location of Annaba with respect to Algeria and the Mediterranean Sea. The large map shows the exact locations of the four sampling sites (S1 to S4), which are indicated with black circles. S1, Cap de Garde; S2, Rezgui Rachid; S3, Sidi Salem; S4: Lahnaya
Sampling protocol
Samples of seawater and Perna perna mussels were monthly and simultaneously collected at low tide at each site, in the period from January to December 2018. Low tides are generally associated with higher concentrations of fecal indicator bacteria in coastal areas. This increase of FIB levels is the result of the mobilization of sediment-associated indicator bacteria as tidal waters recede (USEPA 2010).
Water samples were obtained at a depth of 30-50 cm below the surface of the water to avoid sunlight exposure using 250-ml sterile glass bottles. P. perna mussels (43–110mm in length, 20–35mm in width, and 10–30mm in height) were harvested by hand near the water collecting points at a rate of 10–20 individuals (depending on size). All samples were immediately placed in a clean cooler containing ice cubes (4°C) and transported to the laboratory within the following 2–4h. At each site and each month, seawater environmental variables including temperature (T), pH, salinity (Sal), and dissolved oxygen (DO) were measured in situ using a multiparameter probe (Multi 340i/SET-82362, WTW®, Germany). The determination of seawater suspended solids (SS) was performed as described by Aminot and Chaussepied (1983).
Bacteriological analysis
For seawater samples, a volume of 100 ml was directly analyzed without any prior treatment (Rodier et al. 2009). Immediately upon return to the laboratory, the mussels were cleaned and cleared of encrusting organisms, after which they were opened with a sterile scalpel. The tissue and intravalvular liquid (25 g) were mixed and homogenized with 225-ml sterile physiological water in a sterile laboratory blender (standard NF EN ISO 6887). The levels of FIB such as total coliforms (TC) and Escherichia coli (EC), as well as fecal streptococci (FS), were estimated by three-tube decimal dilution using the most probable number (MPN) method (standard NF V 08-021 (1993)/ISO 7402 and NF V 08-020 (1994)/ISO 7251). All results were statistically expressed as MPN per 100 ml of the sample according to Mac Grady’s tables (Rodier et al. 2009). For the isolation of potentially pathogenic bacteria, standard microbial methods were carried out (Rodier et al. 2009). Bacterial isolates were biochemically identified at the species level through Analytical Profile Index (API 20E, API20NE, API Staph) and further confirmed by 16S rRNA gene sequencing, multilocus sequence typing (MLST), and phylogenetic analysis.
DNA extraction and 16S rRNA gene amplification
Twenty-five bacterial isolated were selected, based on their potential to be human pathogens. Bacterial colonies were picked from overnight LB (Lysogeny Broth) agar plates and transferred into 1.5-ml Eppendorf tubes containing 50 μl of 1xTE buffer (10 mM Tris-HCl pH 8, 1 mM EDTA) supplemented with approximately 100 mg of 0.1 mm Zirconia beads. The tubes were incubated at 37°C for 15 min and then strongly vortexed for 3 min to disrupt the cells. The resulting bacterial lysate served as a template for the 16S rRNA gene amplification. The 25 μl PCR mixture contained 0.5 μl DNA template, 2.5 μl Dream Taq buffer (10x), 1.5 μl dNTPs (2.5 mM each), 0.5μl Dream Taq DNA polymerase (Thermo ScientificTM) and 1.5 μl 10 μM of each universal primers 27F and 1492R (Table 2). PCR cycling was carried out as described by da Silva et al. (2013). Amplification products were visualized by electrophoresis on 1% agarose gel in 1x TBE buffer after staining with SYBR Safe (Invitrogen) and subsequently purified with Gene Jet Gel Extraction Kit (Thermo Scientific TM).
16S rRNA sequence analysis
The PCR-amplified regions of the 16S rRNA genes were Sanger-sequenced using primer 27F (Table 2). The obtained partial sequences of the 16S rRNA gene were compared with the GenBank NCBI database through the BLAST software, to confirm the species of the isolates. After that, a multiple sequence alignment was carried out using the Clustal X software integrated into the MEGA 7 program (Kumar et al. 2016). Finally, the phylogenetic tree was constructed by the neighbor-joining method with 1000 bootstrap replications.
Multilocus sequence typing analysis (MLST analysis)
To investigate the source of the detected E. coli strains, DNA from isolates EM3, EM18, EM97, EM102, and MM6 were subjected to PCR amplification targeting seven specific genes (trpA, trpB, dinB, polB, putP, pabB, and icdA) using suitable primers (Table 2), and following the same procedures used for 16S rRNA genes. The amplification program was carried out as follows: initial denaturation of 4 min at 94°C, followed by 30 cycles of 30s at 94°C, 30s at 52°C, and 2 min at 72°C, and a final extension at 72°C for 4 min. The phylogenetic tree is based on 2758 bp concatenated partial sequences of the seven genes from EM3, EM18, EM97, EM102, and MM6, as well as the equivalent loci in closely related strain. The sequences were aligned with Clustal Omega with default settings on the EBI server, and the guide-tree was visualized using iTOL (Letunic and Bork 2019; Madeira et al. 2019).
Statistical analysis
Statistical analysis was carried out with the R software version 3.1.2. Normality distribution and homogeneity of data variances were first tested by the Shapiro–Wilk test (Shapiro and Francia 1972). Since data were not normally distributed, we had to choose nonparametric tests for data analysis. First, the Spearman correlation coefficient was evaluated to investigate possible relationships between our data sets. Then, the Kruskal–Wallis test was applied to assess the intersite and intermonth comparisons. Comparisons between the groups with significant differences were identified by the Wilcoxon tests. In all tests, the significance level was set to p value < 0.05.
Finally, principal component analysis (PCA) was used as a descriptive method to characterize the four sampling sites in the study area and to assess the contribution percentage of measured environmental variables on the abundance of the fecal indicators employing the FactoMineR package. In PCA analysis, the square cosine (cos2) indicates the importance of the contribution of a component to the distance squared from the initial observation. The components with a high cos2 value contribute significantly to the total distance, and these components are therefore the important contributors (Abdi and Williams 2010).
Results
Physicochemical analysis of sampled water
The monthly variation in seawater environmental variables obtained throughout the sampling period is presented in Fig. 2. As expected, the annual temperature and salinity cycles showed similar seasonal fluctuations across the four study sites. Seawater temperature ranged from 10.3°C at S4 in February to 28.6°C at S2 in August, while salinity varied from 34.9 g/L at S3 in March to 41.6 g/L at S2 in August. The variations of these two parameters are primarily influenced by the climatic conditions of the area. The high values of temperature and salinity recorded at S2 (temperature: 28.6 °C; salinity: 41.61 g/L), and S3 (temperature: 28°C; salinity: 41.6 g/L) would be due to the fact that these sites are located well within the Gulf and are protected from currents. Relatively high evaporation, especially in summer, also contributed to the increase in salinity. The pH remained relatively constant and alkaline during the sampled months, with a slight increase in spring. An inverse relationship between dissolved oxygen and temperature vs. salinity was observed. The highest value (12.6 mg/L) of dissolved oxygen was recorded during winter at S4, while the lowest one (5 mg/L) was detected during summer at S3 and S2. Indeed, the application of Spearman’s correlation test revealed a strong negative and significant correlation between dissolved oxygen and the temperature (r = −0.84, p < 0.0001) and between the same variable and the salinity (r = −0.65, p < 0.0001) (Table 3). Levels of suspended solids were lower at S1 and S4 as compared with the other two sites. The highest value (0.42mg/L) was recorded two times in February at S3 and in December at S2.
Bacteriological analysis of isolated bacteria
As shown in Fig. 3, the results of the bacteriological analysis revealed that the fecal contamination varied over time and among the four sampling sites in the Gulf of Annaba (p < 0.05).
Spatial and temporal variations of fecal indicator bacteria in seawater and mussels. a and b Total coliforms per 100 ml. c and d Escherichia coli per 100 ml. e and f Fecal streptococci per 100 ml. Note that the scales are different on each diagram. S1, Cap de Garde; S2, Rezgui Rachid; S3, Sidi Salem; S4, Lahnaya
The levels of all FIB (TC, EC, and FS) in seawater samples were alarmingly high at S2 and S3 (4.6×103 MPN/100 ml), and largely exceeded the limits defined by the Algerian law as 500 TC/100 ml, 100 EC/100 ml, and 100 FS/100 ml (JORA 1993, 2006). The minimum levels of FIB were recorded at S1 and S4 (0 MPN/100ml) (Fig. 3). The Wilcoxon test was used to examine potential differences in detected TC, EC, and FS between the four study sites. The most significant differences between the four study sites were between S3 and S4 (p=0.0004), and between S2 and S4 (p=0.0011). No statistically significant differences were found between S2 and S3 (p > 0.05) (Table S1).
As expected, P. perna mussels from all sites were severalfold more contaminated by FIB than the seawater samples. TC concentrations ranged from 9×103 MPN/100g at S4 to 3×105 MPN/100g at S3. For E. coli, 100% of the P. perna samples at S3 showed loads of more than 4.6×104 MPN/100g. FS was present throughout the entire study period, and the highest concentration (2.5×104 MPN/100g) was detected in the mussels of S2 (Fig. 3).
Depending on the seasons of sampling (winter = December, January, and February; spring = March, April, and May; summer = June, July, and August; autumn = September, October, and November), the levels of FIB in both compartments were generally higher during the warmer months of the year. The maximum levels of TC in seawater (4.6×104 MPN/100ml) and P. perna mussels (3×105 MPN/g) were both recorded in summer. The highest concentrations of E. coli in seawater (1.2 × 104 MPN/100ml) and P. perna (2.5×104 MPN/100g) were detected in autumn, respectively. Finally, fecal contamination by FS was most pronounced in autumn in both compartments: 2.1×104 MPN/100ml in seawater, and 2.5×105 MPN/g in P. perna mussels (Fig. 3).
The Wilcoxon test was applied to test the significance of these differences. For TC, the most significant differences between the sampling seasons were between the autumn and winter (p=0.028), and between the autumn and spring (p=0.041). For E. coli, a significant difference was found between the autumn and winter (p=0.029). Finally, for FS, the most significant differences were found between the autumn and winter (p=0.023), and between the summer and winter (p=0.028) (Table S2).
Based on Spearman's correlation results, FIB-levels were highly correlated with each other (p < 0.0001) (Table 3). TC were found to be positively and significantly correlated with EC (r= 0.77, p<0.0001) and FS (r=0.56, p<0.0001). In addition, EC also showed positive and significant correlation with FS (r=0.45, p=0.0012)
The results of Spearman’s correlation analysis between FIB and physicochemical variables are given in Table 3. According to the correlation coefficients, FS appeared to be the most correlated indicator with all environmental variables except pH and SS: DO (r=−0.72, p<0.0001); temperature (r=0.64, p<0.0001) and salinity (r=0.46, p<0.01). In contrast, EC and TC were found to be positively and significantly correlated with SS (r=0.51, p<0.001; 0.57, p<0.001; respectively) and negatively correlated with DO (r=−0.46, p<0.01; r=−0.37, p<0.01; respectively).
Spatial–temporal abundance of potentially pathogenic bacteria in the Gulf of Annaba
During the entire study period, a total of 208 bacterial isolates (142 from mussels and 66 from seawater) belonging to 22 genera and 46 species were identified using biochemical tests. The most ubiquitous and abundant microorganism among all the environmental samples was E. coli (41.4%), followed by Aeromonas hydrophila (5.8%), Klebsiella pneumoniae (3.9%), Pseudomonas aeruginosa (3.4%), Enterobacter cloacae (2.9%), Vibrio parahaemolyticus (2.9%), Burkholderia cepacia (2.4%), Morganella morganii (2.4%), Micrococcus spp (1.9%) Pseudomonas luteola (1.9%), Staphylococcus sciuri (1.9%), Staphylococcus xylosus (1.9%), Providencia rettgeri (1.4%), Salmonella spp (1.4%), and Yersinia enterolittica (1.4%). Figure 4 shows the abundance of potentially pathogenic bacteria in both compartments (seawater and Perna perna samples), and in each sampling site throughout the entire study period. It only indicates bacteria found with more than two isolates per sample (see Table 1). In the seawater samples of S1 and especially of S4, the number of different potentially pathogenic strains did not exceed 14 whereas in S2 and S3, their number was 16 and 27, respectively. In P. perna samples, the number of these infectious agents was 16 in S4, 24 in S1, and 43 and 59 in S2 and S3, respectively (Table S3). The members of the family Enterobacteriaceae were dominant and presented the highest occurrence of all potentially pathogenic microorganisms (65.4%). This large group of bacteria was extensively found in all samples of P. perna mussels and its presence was most pronounced in S2 and S3 (Fig. 4; Table S3).
The results of this study also revealed a seasonal pattern in the diversity of potentially pathogenic bacteria. For both P. perna and seawater samples, the diversity increased during the warm-water months (between June and October), with the highest diversity of potentially pathogenic bacteria recorded in summer (June, July, August), closely followed by autumn (September, October, November) (Fig. 5).
Seasonal differences in the diversity of potentially pathogenic bacteria in both P. perna and seawater samples. The vertical axis represents the number of different potentially pathogenic strains identified from both P. perna and seawater samples collected from the four sampling sites combined. Summer: June, July, and August. Autumn: September, October, and November. Winter: December, January, and February. Spring: March, April, and May
Molecular identification of selected isolates
In addition to biochemical identification, 25 isolates of either Staphylococci or γ-proteobacteria were chosen for further identification via their 16S rRNA genes. Universal primers 27F and 1492R were used to PCR-amplify the 16S rRNA genes, and the products of approximately 1500 bp (Fig. 6) were Sanger sequenced. The 16S rRNA sequences were compared to the NCBI database, using BLAST. All sequences had between 97 and 100% identity to known bacterial species, permitting the identification of the analyzed strains (Table 4). A phylogenetic tree was generated to visualize the evolutionary placement of our environmental bacteria with respect to their closest studied relatives (Fig. 7). A main clade, with high bootstrap value (100% bootstrap), grouped 17 isolates of seven genera within the family of Enterobacteriaceae, namely, Escherichia/Shigella spp., Klebsiella spp., Enterobacter spp., Citrobacter spp., Proteus spp., and Morganella spp. The Staphylococci were only represented by two isolates (BM2 and BS4) which were close to type strain S. epidermidis ATCC 10145T (100% bootstrap). Overall, most (18/25) of the 16S rRNA gene sequence identification results matched with the genus identification using API tests (Table 4).
Phylogeny of the 25 isolates with molecular identification. 16S rRNA sequences from the 25 selected isolates, together with the best hit from the GenBank database for each of the sequences, were compared in a Clustal X multiple sequence alignment (Kumar et al. 2016). Accession numbers of the reference sequences are in parentheses, and Halobacterium sp. A1T was used to root the tree
Multilocus sequence typing analysis (MLST)
E. coli comprised more than 40% of the isolated strains, and several individual E. coli isolates came from the very same environmental context (i.e., same sampling-site, sample-date, and environmental compartment). We therefore wondered whether these E. coli isolates were due to multiple separate contamination events or were caused by a single highly abundant E. coli strain which was able to thrive and outcompete other bacteria in the given condition. To test whether the five isolates (EM3, EM18, EM97, EM102, and MM6) obtained from P. perna mussels at S3 on the same sampling date (January 15, 2018) belonged to the same strain of E. coli, we PCR-amplified and Sanger-sequenced sections of seven conserved genes (trpA, trpB, dinB, polB, putP, pabB and icdA) (Fig. 8). A tree based on a multiple alignment of the concatenated sequences from our five strains (and the equivalent gene-sections from other E. coli strains) revealed that our isolates were not identical, and thus were probably not from a single contamination event. However, the isolates were closely related to each other, and slightly more distantly to E. coli strains K12 and SCU-103 (Fig. 9).
Principal component analysis (PCA)
Principal component analysis (PCA) revealed that the three first main components together explain 92.4% of the total information (Fig. 10a and b). The first principal component (PC), which represents 56.3% of the variance, was the most significant component of the latter. It was mainly loaded by the Temperature (r = 0.95), Salinity (r = 0.89), Dissolved Oxygen (r = −0.77), SS (r = −0.66) and FS (r = 0.55). The second PC, representing 22.4% of the variation, was found to be positively correlated by pH (r = 0.86). The third PC, representing 13.7%, was positively correlated with TC and EC (r = 0.61 and r =0.52, respectively). According to the PCA plot a clear opposition was observed between the 12 months of sampling and the distribution of the four sites on the first two axes. S2 and S3 were strongly correlated with each other and showed maximum variations of fecal contamination during the warm months of the year, whereas S1 and S4 demonstrated lower fecal contamination variations during the cold months (Fig. S1).
Principal component analysis performed on data from seawater samples. a Correlation of environmental variables with the two first axes of the standard PCA. b Correlation of environmental variables with the first and third axes of the standard PCA. The main variables are indicated with solid arrows, and the supplemental variables are indicated with dotted arrows. DO, dissolved oxygen; Sal, salinity; T, water temperature; SS, suspended solids; EC, Escherichia coli; TC, total coliforms; and FS, fecal streptococci
Discussion
Bacteriological analyses of FIB in the Gulf of Annaba
Although our previous study detected a recent increase in bacterial contamination of the Gulf of Annaba (Hidouci et al. 2014; Kadri et al. 2015, 2017), the results of the present study revealed a highly alarming further increase of fecal contamination in the area, to a level that is higher than other coastal regions of the Mediterranean Sea (Bouhayene and Djebar 2014; Boutaib et al. 2015; Dallarés et al. 2018; Rincé et al. 2018). Much of this difference is probably due to the continuous pollution pressure in the Gulf, mainly related to anthropogenic activities, as well as rapid urbanization over the last few years. According to Inal et al. (2018), more than 40% of the Algerian population (more 19 million people) is living along the Gulfs near the largest agglomerations, of which Annaba is the third most populated, with almost 400 thousand inhabitants within the city itself (https://en.populationdata.net/countries/algeria/).
In the current study, these impacts were differently manifested depending on the local sources of pollution at each site.
The strong presence of FIB at S3 could be explained by significant urban, industrial and agricultural discharges that Wadi Seybouse (Fig. 1) drains from its catchment basin of about 6470 km2 (ABH-CSM 1999-2000; Mebarki 2000). Further contribution to bacterial contamination at S3 presumably comes from untreated wastewater effluents from a large part of Annaba city and its outskirts, including a nearby slaughterhouse, which are discharged directly into the sea via Wadi Bedjima (Fig. 1). These contamination sources should be added to the natural contamination stemming from a large colony of seabirds and animals (Telailia 2014).
Our present results further demonstrated that the overall contamination was exceptionally high at S2, revealing that the waters of this site should be considered as unsafe for bathing in accordance with the Algerian Bathing Water executive decrees (JORA 1993, 2006). Similar to S3, these high levels of FIB were primarily due to the domestic wastewaters from nearby homes, which are discharged directly into the sea without prior treatment (Kadri et al. 2017).
The lower concentrations observed in S1 and especially S4 are presumably due to their remoteness from the city area and their strong water flux, which may contribute to the dispersion of fecal pollutants in the water column (Kadri et al. 2015). The coastal current of the Gulf of Annaba is associated with important North African upwelling processes (Arnone et al. 1990). This current permanently enters the Gulf of Annaba near Cap de Garde and flows to the east in the direction of the northeast part of the bay (Ouali et al. 2018). Thus, S1 and S4 are under the influence of dominant north-westerly winds that induce strong hydrodynamics and promote mixing of the coastal waters of the Gulf with less contaminated seawater. In contrast, S2 and S3 are characterized by relatively low hydrodynamic conditions and slow vortices (Hafsaoui et al. 2016; Ouali et al. 2018).
It is important to note that S1 and S4 are often visited by bathers in summer (just like S2), which is a possible explanation for the increasing levels of FIB during this period of the year (Kadri et al. 2017). This is due to the direct or indirect discharge of waste from toilets and tourist resorts located along the coast, the absence of water treatment plant, as well the lack of awareness among tourists regarding solid waste and wastewater collection and disposal (Torres-Bejarano et al. 2018).
According to several studies, fecal contamination at bathing beaches can be hazardous to humans because many pathogenic bacteria could be ingested during recreational water activities leading to various waterborne diseases (Marion et al. 2010; Santhiya et al. 2011; Arnold et al. 2016). The governments should therefore develop solutions to minimize the risks associated with the use of contaminated recreational water such as, the installation of wastewater treatment plants, prohibiting direct industrial and agricultural discharges, improving public awareness, and developing control measures by means of quality criteria (Kacar and Omuzbuken 2017).
Fecal contamination in Perna perna mussels
Data obtained showed that the levels of FIB were even higher in P. perna than in the surrounding seawaters during the entire study period. This is consistent with reports from other coastal regions worldwide, suggesting that this strong accumulation capacity is mainly related to the filter-feeding behavior of these sentinel organisms, which make them one of the best bioindicators of fecal pollution in coastal waters, even when pollutants are at low concentrations (Stabili et al. 2005; Martinez and Oliveira 2010; Jayme et al. 2016; Bozcal and Dagdeviren 2020). It has furthermore been demonstrated that the mussels were contaminated with E. coli for a long period of time after a brief exposure to fecally contaminated water (Ho et al. 2000).
Similar to the study conducted by Stabili et al. (2005) and Cavallo et al. (2008) in the Northern Ionian Sea of Italy, the bacterial community of P. perna mussels from all sites in our study was very similar to that of surrounding waters but with higher abundance (Fig. 4). The levels of intestinal indicators in all sampling sites were well above the permissible limits recommended according to Regulation (854/2004/EC) of 29 April 2014 for human consumption, which recommends less than 230 E. coli/100g. Thus, the mussels inhabiting the Gulf of Annaba would be unfit for direct consumption.
FS were present in mussel and seawater samples throughout the entire sampling period with concentrations higher than E. coli in almost all samples, and this is also in agreement with previous reports (Tiefenthaler et al. 2009; Zegmout et al. 2011; Kadri et al. 2017; Islam et al. 2017). These FIB are known to have a better survival period than thermotolerant coliforms in surface waters, as well as in the digestive tract of bivalves (Geldreich and Litsky 1976, Noble et al. 2004).
Influence of environmental variables on fecal indicators in the Gulf of Annaba
In addition to anthropogenic activities, the survival was also influenced by a multitude of natural variables, among them; the seasonal variations of temperature were very likely responsible for the observed differences in bacterial concentrations in our samples. Indeed, our results demonstrated that elevated bacterial loads during the warmer months of the year (between June and October) were associated with maximum FIB rates in both compartments. Another probable reason for the high levels of FIB in P. perna in this period is probably the physiology of this sentinel species. Burge et al. (2016) have indicated that elevated temperatures promote an increase in the filtration rates in mussels and, therefore, they can retain more microorganisms from the surrounding waters. According to the Intergovernmental Panel on Climate Change (IPCC) Fifth Assessment Report (2013), Algeria will experience an increase in temperatures between 1°C and 3.7°C over the next few years. Consequently, this may extend the time period during which FIB can persist in the water (Barreras Jr et al. 2019). This hypothesis was supported by the significant and positive correlations between E. coli and FS, and the temperature revealed by Spearman’s correlation test (p < 0.05) and PCA analysis in the current study, all of which is consistent with other studies (Koirala et al. 2008; Gutiérrez-Cacciabue et al. 2014; Abia et al. 2015; Islam et al. 2017).
In addition to elevated temperatures, summer also brings a large number of bathers to the beaches, which increase the influx of bacterial contaminants. On top of that, the presence of FIBs is affected by the local hydrodynamic patterns, which are calmer during summer, thus reducing the dispersion of fecal bacteria in the water column (Lacaze 1996).
In our study, only FS showed a significant positive correlation (p < 0.001) with salinity (Table 3). These FIB are known for their high resistance to harsh environmental stressors and tolerance to high concentrations to salt, making them powerful indicators of fecal contamination in seawater (Byappanahalli et al. 2012). Conversely, DO show the strongest correlations with all groups of FIB, mainly due to the bacterial degradation of detritus which consumes a lot of oxygen. This biodegradation was more important with the increase in temperature in summer (5 mg/L) (Fig. 2), especially in highly contaminated sites, which receive massive quantities of domestic discharges and industrial effluents. These findings are in agreement with those of a recent study by Chávez-Díaz et al. (2020), which found negative correlations between DO and FIB. The latter were found to be positively correlated with SS, which, according to the literature, play a protective role for intestinal bacteria against solar radiation and predators (Walters et al. 2014; Kadri et al. 2017). This appears to be the case for the SS-rich waters of S2 and S3.
Numerous studies have indicated that FIB can be used as surrogates to estimate the possible presence of pathogenic microorganisms, especially when they are found at high levels (Wilkes et al. 2011; Shoults and Ashbolt 2018).
Abundance of potentially pathogenic bacteria in the Gulf of Annaba
For all samples in the study, it was consistently Proteobacteria which was the most dominant phylum (88/208, 46%). Within this phylum the most frequently isolated family was Enterobacteriaceae, which indicated that domestic wastes, especially from the most polluted sites are most likely the primary source of pollution in the Gulf of Annaba since enteric bacteria are mainly derived from the excrement of warm-blooded animals, including humans (Poharkar et al. 2017). The enteric bacteria, Salmonella spp., Shigella spp. and, E. coli are major causes of human gastrointestinal tract infections, with 1.7 billion cases of human diarrhea recorded each year, primarily caused by pathogenic strains of E. coli (Yang et al. 2017), and 450 per 100,000 children in India and Pakistan contracting enteric fever caused by Salmonella typhi (Sánchez-Vargas et al. 2011; Neogi et al. 2014).
Environmental bacteria such as Pseudomonas spp., Aeromonas spp., and Shewanella spp. were also identified in both environmental compartments. Species of the genus Aeromonas are widely isolated from aquatic environments and frequently reported to cause waterborne and seafood infections (gastroenteritis and septicemia) (Chopra and Houston 1999; Joseph et al. 2013; Hamid et al. 2016). Pseudomonas spp. are ubiquitous microorganisms found in marine shellfish and recreational waters (Maravić et al. 2018; Goh et al. 2019). These opportunistic pathogens are frequently multidrug-resistant and associated with diarrhea, intraabdominal and nosocomial infections, particularly in immune-compromised patients (Morrissey et al. 2013; Streeter and Katouli 2016). The results of biochemical identification also revealed the detection of different species of the genus Vibrio in the four sampling sites, of which V. paraheamolyticus was the most isolated microorganism. Vibrio. spp are waterborne bacteria naturally found in estuarine and coastal environments. Yet, certain species can be pathogenic to humans and marine organisms such as bivalves (Eggermont et al. 2017; Rincé et al. 2018; Bozcal and Dagdeviren 2020). In August 2018, the Algerian Ministry of Health reported a cholera outbreak in Blida and five other regions (Algiers, Tipaza, Bouira, Médéa, and Ain Defla) in the north of the country. This devastating and strictly human epidemic caused mainly by V. cholerae O1 or O139 can cause severe dehydrating diarrhea and even death (Feldhusen 2000). In the Gulf of Annaba, V. cholerae was found in S3 mussels in the same period as the outbreak, classifying this site as the area of highest risk.
In addition to Proteobacteria, 24 isolates of the genus Staphyloccocus were also detected during the study period. These potentially pathogenic bacteria, including S. aureus, are well-known causative agents of several human diseases, such as skin rashes, pneumonia, ear and eye infections, endocarditis, and meningitis (Schets et al. 2020; Yaghoubzadeh et al. 2020). According to Pomykała et al. (2012), some coagulase-positive staphylococci are common seafood pathogens and may pose a significant risk to human health through improper consumption of bivalve mollusks. Furthermore, methicillin-resistant S. aureus (MRSA), which is one of the most harmful pathogens to human health, has also been frequently detected on several recreational beaches in the United States (Abdelzaher et al. 2010; Levin-Edens et al. 2012; Plano et al. 2013; Thapaliya et al. 2017).
Biochemical and molecular identification of bacteria
Strains of Enterobacteriaceae are known to be difficult to distinguish by conventional methods (Nhung et al. 2007; Hamdi et al. 2017). Besides, the use of biochemical identification alone can be problematic as some new taxa may not be included in available databases (Janda and Abbott 2002). For this reason, additional molecular identification targeting the 16S rRNA gene was performed on 25 strains, including two Gram-positive bacteria isolated from P. perna mussels of S3. In general, the biochemical identification at the genus level was confirmed in 72% of the cases by the 16S rRNA gene sequencing (Table 4). All strains exhibited more than 97% sequence similarities with their matching sequences retrieved from the GenBank database.
The ribosomal 16S rRNA gene has highly conserved regions in all bacterial cells, interspersed with nine hyper-variable stretches of sequences (named V1–V9), and is a molecular fingerprint for bacterial identification and taxonomic classification (Benga et al. 2014; Jo et al. 2016; Monticelli et al. 2019). Sequencing of the 16S ribosomal RNA (rRNA) gene to study bacterial taxonomy and phylogeny has been the most widely used technique for several reasons: the detection and identification of fastidious, noncultured, and slow-growing bacterial pathogens, as well as novel isolates, its presence in all bacteria, and also its large size (approximately 1500 bp) which contains statistically valid and relevant sequence information (Patel 2001; Clarridge 2004; Janda and Abbott 2007). Despite its accuracy, sequence analysis of the 16S rRNA gene has disadvantages when comparing closely related species, where the16S rRNA genes might not be sufficiently different to distinguish the two species. It is therefore often desirable to complement the 16S rRNA analysis with multilocus sequence typing (MLST). This technique examines multiple protein coding genes, which are more susceptible to genomic drift than the 16S rRNA gene. For some species, there are even standardized MLST approaches, which can be compared directly to public MLST databases (Sabat et al. 2013). However, the main disadvantage of this technique is its relatively high coast, since it requires more PCR and Sanger DNA sequencing reactions per analyzed strain (Boers et al. 2012; Sabat et al. 2013).
For the strains (EM3, EM18, EM97, EM102, and MM6) identified as E. coli using API tests and 16S rRNA gene sequencing, MLST was performed to further understand their phylogenetic relationships. Interestingly, the results indicate that our isolates were very similar to each other but nevertheless distinct, and therefore did not belong to the same strain of E. coli (Fig. 8). This suggests that they came from a variety of separate human and animal sources of fecal contamination, since E. coli is mainly found in the fecal wastes of warm-blooded mammals (Poharkar et al. 2017). Therefore, the use of Microbial Source Tracking techniques to identify both human and animal specific markers in future studies will be an important tool for understanding the origin of fecal pollution in the Gulf of Annaba, and for assessing the associated health risks related to the presence of pathogenic microorganisms.
To summarize, this study indicated that identification systems based on molecular techniques provide the means for accurate identification of potentially pathogenic bacteria in coastal waters, and are rapid when the required tools are available and the techniques have been established in the analyzing laboratory. In contrast, biochemical analyses are simpler to use, have an acceptable level of accuracy, and are less expensive compared with molecular techniques, but these techniques are time consuming, and their results are not always easy to interpret (Gracias and McKillip 2004; Russell et al. 1997). It is therefore prudent to use molecular identification when the results from biochemical identification are ambiguous.
Conclusion
The results obtained in this study allowed assessing the presence and distribution of fecal indicators and potentially pathogenic bacteria in Perna perna and seawater samples from the Gulf of Annaba. The bacteriological concentrations in P. perna were significantly higher than in seawater samples, and were well above the permitted limits for human consumption and recreational water in Sidi Salem (S3) and Rezgui Rachid (S2). Discharges from Wadi Seybouse and Wadi Bedjima, as well as anthropogenic activities, are likely to be important factors in the fecal contamination in the Gulf of Annaba. Additionally, the bacteriological quality of the water was influenced by multiple physico-chemical variables such as temperature, salinity, and dissolved oxygen, combined with the local hydrodynamic conditions. The survival and the presence of infectious agents in P. perna are a matter of great concern with regard to epidemic diseases that they may occur when mussels are consumed by humans. Therefore, the implementation of necessary measures should be carried out, especially in highly polluted sites in order to protect environmental resources and human health.
Data availability
The datasets used and analyzed during the current study are available, upon reasonable request, from Mouna Boufafa (email: mouna_boufafa@yahoo.fr), the corresponding author.
References
Abdelzaher AM, Wright ME, Ortega C, Solo-Gabriele HM, Miller G, Elmir S, Newman X, Shih P, Bonilla JA, Bonilla TD, Palmer CJ, Scott T, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano C, Gidley M, Plano LRW, Zhu X, Wang JD, Fleming LE (2010) Presence of pathogens and indicator microbes at a non-point source subtropical recreational marine beach. Appl Environ Microbiol 76:724–732. https://doi.org/10.1128/AEM.02127-09
Abdennour C, Smith B, Boulakoud M, Samraoui B, Rainbow PS (2000) Trace metals in marine, brackish and freshwater prawns (Crustacea, Decapoda) from northeast Algeria. Hydrobiologia 432:217–227. https://doi.org/10.1023/A:1004027204088
Abderrahmani K, Boulahdid M, Bendou N, Aissani A (2020) Seasonal distribution of cadmium, lead, nickel, and magnesium in several tissues of mussels from the Algerian coasts. Environ Sci Pollut Res 27:22547–22567. https://doi.org/10.1007/s11356-020-08682-8
Abdi H, Williams LJ (2010) Principal component analysis. Wiley Interdiscip Rev Comput Stat 2:433–459. https://doi.org/10.1002/wics.101
ABH-CSM (1999-2000) The agency's notebooks. In: River Basin Agency Constantinois Seybouse-Mellegue, Constantine, the Seybouse basin 1: 35
Abia ALK, Ubomba-Jaswa E, Momba MNB (2015) Impact of seasonal variation on Escherichia coli concentrations in the riverbed sediments in the Apies River, South Africa. Sci Total Environ 537:462–469. https://doi.org/10.1016/j.scitotenv.2015.07.132
Aminot A, Chaussepied M (1983) Manual of marine chemical analysis. CNEXO, Brest (in French)
Amri S, Samar MF, Sellem F, Ouali K (2017) Seasonal antioxidant responses in the sea urchin Paracentrotus lividus (Lamarck 1816) used as a bioindicator of the environmental contamination in the South-East Mediterranean. Mar Pollut Bull 122:392–402. https://doi.org/10.1016/j.marpolbul.2017.06.079
Arnold BF, Wade TJ, Benjamin-Chung J, Schiff KC, Griffith JF, Dufour AP, Weisberg SB, Colford JM Jr (2016) Acute gastroenteritis and recreational water: highest burden among young US children. Am J Public Health 106:1690–1697. https://doi.org/10.2105/AJPH.2016.303279
Arnone RA, Wiesenburg DA, Saunders KD (1990) The origin and characteristics of the Algerian current. J Geophys Res Oceans 95:1587–1598. https://doi.org/10.1029/JC095iC02p01587
Barreras H Jr, Kelly EA, Kumar N, Solo-Gabriele HM (2019) Assessment of local and regional strategies to control bacteria levels at beaches with consideration of impacts from climate change. Mar Pollut Bull 138:249–259. https://doi.org/10.1016/j.marpolbul.2018.10.046
Belabed BE, Laffray X, Dhib A, Fertouna-Belakhal M, Turki S, Aleya L (2013) Factors contributing to heavy metal accumulation in sediments and in the intertidal mussel Perna perna in the Gulf of Annaba (Algeria). Mar Pollut Bull 74:477–489. https://doi.org/10.1016/j.marpolbul.2013.06.004
Benga L, Benten WPM, Engelhardt E, Köhrer K, Gougoula C, Sager M (2014) 16S ribosomal DNA sequence-based identification of bacteria in laboratory rodents: a practical approach in laboratory animal bacteriology diagnostics. Lab Anim 48:305–312. https://doi.org/10.1177/0023677214538240
Bjedov I, Lecointre G, Tenaillon O et al (2003) Polymorphism of gene encoding SOS polymerases in natural populations of Escherichia coli. DNA Repair 2:417–426. https://doi.org/10.1016/S1568-7864(02)00241-0
Boers SA, Van der Reijden WA, Jansen R (2012) High-throughput multilocus sequence typing: bringing molecular typing to the next level. PLoS One 7:e39630
Boudjema K, Kordali S, Bounakous N, Meknachi A, Badis A (2014) Tilising caging techniques to investigate catalase response, accumulated fecal bacteria and heavy metals in perna perna (linnaeus, 1758) for active biomonitoring coastal waters: a study applied in the bay of Bou-Ismail (Algeria). Adv Environ Biol 8:215–224
Bouhayene S, Djebar AB (2014) Evaluation of the microbiological quality of the seawater of the main beaches of Skikda (East-Algerian). Ann Biol Res 5:40–45
Boutaib R, Azhari H, Abid M, Marhraoui M (2015) Comparison of Escherichia coli levels in shellfish from Mediterranean coast, Morocco. Glob Adv Res J Microbiol 4:10
Bozcal E, Dagdeviren M (2020) Bacterial metagenome analysis of Mytilus galloprovincialis collected from Istanbul and Izmir coastal stations of Turkey. Environ Monit Assess 192:1–18. https://doi.org/10.1007/s10661-020-8129-1
Burge CA, Closek CJ, Friedman CS, Groner ML, Jenkins CM, Shore-Maggio A, Welsh JE (2016) The use of filter feeders to manage disease in a changing world. Integr Comp Biol 56:573–587. https://doi.org/10.1093/icb/icw048
Byappanahalli MN, Nevers MB, Korajkic A, Staley ZR, Harwood VJ (2012) Enterococci in the environment. Microbiol Mol Biol Rev 76:685–706. https://doi.org/10.1128/MMBR.00023-12
Cavallo R, Acquaviva M, Stabili L (2008) Culturable heterotrophic bacteria in seawater and Mytilus galloprovincialis from a Mediterranean area (Northern Ionian Sea–Italy). Environ Monit Assess 149:465–475. https://doi.org/10.1007/s10661-008-0223-8
Chávez-Díaz LV, Gutiérrez-Cacciabue D, Poma HR, Rajal VB (2020) Sediments quality must be considered when evaluating freshwater aquatic environments used for recreational activities. Int J Hyg Environ Health 223:159–170. https://doi.org/10.1016/j.ijheh.2019.09.007
Chopra AK, Houston CW (1999) Enterotoxins in Aeromonas-associated gastroenteritis. Microbes Infect 1:1129–1137. https://doi.org/10.1016/S1286-4579(99)00202-6
Clarridge JE (2004) Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17:840–862. https://doi.org/10.1128/CMR.17.4.840-862.2004
da Silva MAC, Cavalett A, Spinner A, Rosa DC, Jasper RB, Quecine MC, Bonatelli ML, Pizzirani-Kleiner A, Corção G, Lima AOS (2013) Phylogenetic identification of marine bacteria isolated from deep-sea sediments of the eastern South Atlantic Ocean. Springer Plus 2:127. https://doi.org/10.1186/2193-1801-2-127
Dallarés S, Carrasco N, Álvarez-Muñoz D, Rambla-Alegre M, Solé M (2018) Multibiomarker biomonitoring approach using three bivalve species in the Ebro Delta (Catalonia, Spain). Environ Sci Pollut Res 25:36745–36758. https://doi.org/10.1007/s11356-018-3614-6
Damak M, Fourati R, Elleuch B, Kallel M (2020) Environmental quality assessment of the fish farms’ impact in the Monastir Bay (eastern of Tunisia, Central Mediterranean): a benthic foraminiferal perspective. Environ Sci Pollut Res 27:9059–9074. https://doi.org/10.1007/s11356-019-07523-7
dos Santos FS, Neves RAF, de Carvalho WF, Krepsky N, Crapez MAC (2018) Evaluation of the immune responses of the brown mussel Perna perna as indicators of fecal pollution. Fish Shellfish Immunol 80:115–123. https://doi.org/10.1016/j.fsi.2018.05.061
Eggermont M, Bossier P, Pande GSJ, Delahaut V, Rayhan AM, Gupta N, Islam SS, Yumo E, Nevejan N, Sorgeloos P, Gomez-Gil B, Defoirdt T (2017) Isolation of Vibrionaceae from wild blue mussel (Mytilus edulis) adults and their impact on blue mussel larviculture. FEMS Microbiol Ecol 93:4. https://doi.org/10.1093/femsec/fix039
Escobar-Paramo P, Giudicelli C, Parsot C, Denamur E (2003) The evolutionary history of Shigella and enteroinvasive Escherichia coli revised. J Mol Evol 57:140–148. https://doi.org/10.1007/s00239-003-2460-3
Escobar-Páramo P, Sabbagh A, Darlu P et al (2004) Decreasing the effects of horizontal gene transfer on bacterial phylogeny: the Escherichia coli case study. Mol Phylogenet Evol 30:243–250. https://doi.org/10.1016/S1055-7903(03)00181-7
FAO (2019) Fishery statistical collections. Global aquaculture production 1950–2017. FAO Fisheries and Aquaculture Department (on line), Roe. http://www.fao.org/
Feldhusen F (2000) The role of seafood in bacterial foodborne diseases. Microbes Infect 2:1651–1660
Fouillet A, Fournet N, Forgeot C, Jones G, Septfons A, Franconeri L, Ambert-Balay K, Schmidt J, Guérin P, de Valk H, Caserio-Schönemann C (2020) Large concomitant outbreaks of acute gastroenteritis emergency visits in adults and food-borne events suspected to be linked to raw shellfish, France, December 2019 to January 2020. Euro Surveil 25:2000060. https://doi.org/10.2807/1560-7917.ES.2020.25.7.2000060
Francioni E, Wagener ADLR, Calixto RDC, Bastos GC (2004) Evaluation of Perna perna (Linné, 1758) as a tool to monitoring trace metals contamination in estuarine and coastal waters of Rio de Janeiro, Brazil. J Braz Chem Soc 15:103–110
Geldreich EE, Litsky W (1976) Fecal coliform and fecal streptococcus density relationships in waste discharges and receiving waters. Crit Rev Environ Sci Technol 6:349–369. https://doi.org/10.1080/10643387609381645
Ghozzi K, Marangi M, Papini R, Lahmar I, Challouf R, Houas N, Ben Dhiab R, Normanno G, Babba H, Giangaspero A (2017) First report of Tunisian coastal water contamination by protozoan parasites using mollusk bivalves as biological indicators. Mar Pollut Bull 117:197–202. https://doi.org/10.1016/j.marpolbul.2017.01.057
Goh SG, Saeidi N, Gu X, Vergara GGR, Liang L, Fang H, Kitajima M, Kushmaro A, Gin KYH (2019) Occurrence of microbial indicators, pathogenic bacteria and viruses in tropical surface waters subject to contrasting land use. Water Res 150:200–215. https://doi.org/10.1016/j.watres.2018.11.058
Gracias KS, McKillip JL (2004) A review of conventional detection and enumeration methods for pathogenic bacteria in food. Can J Microbiol 50:883–890. https://doi.org/10.1139/W04-080
Gutiérrez-Cacciabue D, Teich I, Poma HR, Cruz MC, Balzarini M, Rajal VB (2014) Strategies to optimize monitoring schemes of recreational waters from Salta, Argentina: a multivariate approach. Environ Monit Assess 186:8359–8380. https://doi.org/10.1007/s10661-014-4010-4
Guttman DS, Dykhuizen DE (1994) Detecting selective sweeps in naturally occurring Escherichia coli. Genetics 138:993–1003
Hafsaoui I, Draredja B, Lasota R, Como S, Magni P (2016) Population dynamics and secondary production of Donax trunculus (Mollusca, Bivalvia) in the Gulf of Annaba (Northeast Algeria). Mediterr Mar Sci 17:738–750. https://doi.org/10.12681/mms.1760
Hamdi S, Rousseau GM, Labrie SJ, Tremblay DM, Kourda RS, Slama KB, Moineau S (2017) Characterization of two polyvalent phages infecting Enterobacteriaceae. Sci Rep 7:1–12. https://doi.org/10.1038/srep40349
Hamid R, Ahmad A, Usup G (2016) Pathogenicity of Aeromonas hydrophila isolated from the Malaysian Sea against coral (Turbinaria sp.) and sea bass (Lates calcarifer). Environ Sci Pollut Res 23:17269–17276. https://doi.org/10.1007/s11356-016-6655-8
Hidouci S, Djebar AB, Amara R, Sahraoui EH (2014) Bacterial quality of coastal waters of Annaba (East Algeria). Eur J Sci Res 120:488–493
Inal A, Boulahdid M, Angelleti B, Radakovitch O (2018) Levels and ecological risk assessment of heavy metals in surface sediments of fishing grounds along Algerian coast. Mar Pollut Bull 136:322–333. https://doi.org/10.1016/j.marpolbul.2018.09.029
Intergovernmental Panel on Climate Change (IPCC) (2013) Climate change 2013: the physical science basis. Working group I contribution to the IPCC fifth assessment report. Cambridge University Press, Cambridge www.ipcc.ch/report/ar5/wg1
Islam MM, Hofstra N, Islam MA (2017) The impact of environmental variables on faecal indicator bacteria in the Betna river basin, Bangladesh. Environ Process 4:319–332. https://doi.org/10.1007/s40710-017-0239-6
Janda JM, Abbott SL (2002) Bacterial identification for publication: when is enough enough? J Clin Microbiol 40:1887–1891. https://doi.org/10.1128/FJCM.40.6.1887-1891.2002
Janda JM, Abbott SL (2007) 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol 45:2761–2764. https://doi.org/10.1128/JCM.01228-07
Jayme M, Silva M, Sales A, Nunes M, Freitas-Almeida A, Araújo FV (2016) Survey of pathogens isolated from mussels Perna Perna collected in rocky shore and fish market of Niterói, RJ, and Their Respective Resistance Profile to Antimicrobial Drugs. J Food Qual 39:383–390. https://doi.org/10.1111/jfq.12204
Jia Y, Wang L, Qu Z, Yang Z (2018) Distribution, contamination and accumulation of heavy metals in water, sediments, and freshwater shellfish from Liuyang River, Southern China. Environ Sci Pollut Res Int 25:7012–7020. https://doi.org/10.1007/s11356-017-1068-x
Jo JH, Kennedy EA, Kong HH (2016) Research techniques made simple: bacterial 16S ribosomal RNA gene sequencing in cutaneous research. J Invest Dermatol 136:e23–e27. https://doi.org/10.1016/j.jid.2016.01.005
JORA (1993) Official Journal of the Algerian Republic No. 46 Executive Decree No. 93-164 of 10/07/1993, pp 11 (in French)
JORA (2006) Official Journal of the Algerian Republic No. 26 Executive Decree No. 06-141 of 19 /04/2006, pp 4-9 (in French)
Joseph AV, Sasidharan RS, Nair HP, Bhat SG (2013) Occurrence of potential pathogenic Aeromonas species in tropical seafood, aquafarms and mangroves off Cochin coast in South India. Vet World 6:300–306. https://doi.org/10.5455/vetworld.2013.300-306
Kacar A, Omuzbuken B (2017) Assessing the seawater quality of a coastal city using fecal indicators and environmental variables (Eastern Aegean Sea). Mar Pollut Bull 123:400–403. https://doi.org/10.1016/j.marpolbul.2017.08.052
Kadri S, Dahel A, Djebbari N, Barour C, Bensouilah M (2015) Environmental parameters influence on the bacteriological water quality of the Algerian North East coast. Adv Environ Biol 9:180–189
Kadri S, Belhaoues S, Touati H, Boufafa M, Djebbari N, Bensouilah M (2017) Environmental parameters and bacteriological quality of the Perna perna mussel (North East Algerian coast). Int J Biosci 11:151–165. https://doi.org/10.12692/ijb/11.5.151-165
Kara MH, Lacroix D, Rey-Valette H, Mathé S, Blancheton JP (2018) Dynamics of research in aquaculture in North Africa and support for sustainable development and innovation. Rev Fish Sci Aquac 26:309–318. https://doi.org/10.1080/23308249.2017.1410521
Kerdoussi A, Belhaouas S, Bensaad-Bendjedid L, Touati H, Bensouilah M (2017) Study of weight gain and reproduction in the Perna perna mussel using a standard animal. Int J Biosci 11:218–230. https://doi.org/10.12692/ijb/11.6.218-230
Kobayashi D, Saito M, Heike Y, Yokota K, Arioka H, Oshitani H (2019) The association between consuming bivalves, and acute gastroenteritis and norovirus in Tokyo, Japan. J Med Virol 91:986–996. https://doi.org/10.1002/jmv.25416
Koirala SR, Gentry RW, Perfect E, Schwartz JS, Sayler GS (2008) Temporal variation and persistence of bacteria in streams. J Environ Qual 37:1559–1566. https://doi.org/10.2134/jeq2007.0310
Krampah EA, Yankson K, Blay J (2020) Population dynamics of the Brown mussel Perna perna at a Rocky beach near Cape Coast, Ghana. Mar Ecol 41:e12575. https://doi.org/10.1111/maec.12575
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 3:1870–1874. https://doi.org/10.1093/molbev/msw054
Lacaze JC (1996) Degradation of the coastal environment, ecological consequences. Edition Masson, Paris
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Le Guyader FS, Bon F, DeMedici D et al (2006) Detection of multiple noroviruses associated with an international gastroenteritis outbreak linked to oyster consumption. J Clin Microbiol 44:3878–3882. https://doi.org/10.1128/JCM.01327-06
Letunic I, Bork P (2019) Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259. https://doi.org/10.1093/nar/gkz239
Levin-Edens E, Soge OO, No D, Stiffarm A, Meschke JS, Roberts MC (2012) Methicillin-resistant Staphylococcus aureus from Northwest marine and freshwater recreational beaches. FEMS Microbiol Ecol 79:412–420. https://doi.org/10.1111/j.1574-6941.2011.01229.x
Madeira F, Park YM, Lee J et al (2019) The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47:W636–W641. https://doi.org/10.1093/nar/gkz268
Maipa V, Alamanos Y, Bezirtzoglou E (2001) Seasonal fluctuation of bacterial indicators in coastal waters. Microb Ecol Health Dis 13:143–146. https://doi.org/10.1080/089106001750462687
Maravić A, Šamanić I, Šprung M, Fredotović Ž, Ilić N, Dragičević J, Puizina J (2018) Broad-spectrum resistance of Pseudomonas aeruginosa from shellfish: infrequent acquisition of novel resistance mechanisms. Environ Monit Assess 190:81. https://doi.org/10.1007/s10661-018-6471-3
Marion J, Lee J, Lemeshow S, Buckley TJ (2010) Association of gastrointestinal illness and recreational water exposure at an inland US beach. Water Res 44:4796–4804. https://doi.org/10.1016/j.watres.2010.07.065
Martinez DI, Oliveira AJFCD (2010) Faecal bacteria in Perna perna (Linnaeus, 1758) (Mollusca: Bivalvia) for biomonitoring coastal waters and seafood quality. Braz J Oceanogr 58:29–35. https://doi.org/10.1590/S1679-87592010000700005
Mebarki A (2000) Low water flows, effluents and protection of water resources in the Mediterranean basins of eastern Algeria. Geocarrefour 75:399-416. https://doi.org/10.3406/geoca.2000.2491
Monticelli LS, Decembrini F, Bergamasco A, Caruso G (2019) Water quality assessment of transitional and coastal marine Sicilian waters (Italy): ecological and epidemiological significance of multiple antimicrobial resistant Enterococcus spp. Estuar Coast Shelf Sci 217:173–184. https://doi.org/10.1016/j.ecss.2018.11.021
Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D (2013) A review of ten years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals 6:1335–1346. https://doi.org/10.3390/ph6111335
Neogi SB, Yamasaki S, Alam M, Lara RJ (2014) The role of wetland microinvertebrates in spreading human diseases. Wetl Ecol Manag 22:469–491. https://doi.org/10.1007/s11273-014-9373-3
Neves RA, Santiago TC, Carvalho WF, dos Santos SE, da Silva PM, Nascimento SM (2019) Impacts of the toxic benthic dinoflagellate Prorocentrum lima on the brown mussel Perna perna: shell-valve closure response, immunology, and histopathology. Mar Environ Res 146:35–45. https://doi.org/10.1016/j.marenvres.2019.03.006
Nhung PH, Ohkusu K, Mishima N et al (2007) Phylogeny and species identification of the family Enterobacteriaceae based on dnaJ sequences. Diagn Microbiol Infect Dis 58:153–161. https://doi.org/10.1016/j.diagmicrobio.2006.12.019
Noble RT, Lee IM, Schiff KC (2004) Inactivation of indicator micro-organisms from various sources of fecal contamination in seawater and freshwater. J Appl Microbiol 96:464–472. https://doi.org/10.1111/j.1365-2672.2004.02155.x
O’Hara Z, Crossan C, Craft J, Scobie L (2018) First report of the presence of hepatitis E virus in Scottish-harvested shellfish purchased at retail level. Food Environ Virol 10:217–221. https://doi.org/10.1007/s12560-018-9337-5
Oliveira SSA, Sorgine MHF, Bianco K, Pinto LH, Barreto C, Albano RM et al (2016) Detection of human fecal contamination by nifH gene quantification of marine waters in the coastal beaches of Rio de Janeiro, Brazil. Environ Sci Pollut Res 23:25210–25217. https://doi.org/10.1007/s11356-016-7737-3
Ouali N, Belabed BE, Chenchouni H (2018) Modelling environment contamination with heavy metals in flathead grey mullet Mugil cephalus and upper sediments from north African coasts of the Mediterranean Sea. Sci Total Environ 639:156–174. https://doi.org/10.1016/j.scitotenv.2018.04.377
Ozkan D, Dagdeviren M, Katalay S, Guner A, Yavasoglu NU (2017) Multi-biomarker responses after exposure to pollution in the Mediterranean mussels (Mytilus galloprovincialis L.) in the aegean coast of Turkey. Bull Environ Contam Toxicol 98:46–52. https://doi.org/10.1007/s00128-016-1988-z
Patel JB (2001) 16S rRNA gene sequencing for bacterial pathogen identification in the clinical laboratory. Mol Diagn 6:313–321. https://doi.org/10.1054/modi.2001.29158
Perkins TL, Clements K, Baas JH, Jago CF, Jones DL, Malham SK, McDonald JE (2014) Sediment composition influences spatial variation in the abundance of human pathogen indicator bacteria within an estuarine environment. PLoS One 9:e112951. https://doi.org/10.1371/journal.pone.0112951
Plano LR, Shibata T, Garza AC et al (2013) Human-associated methicillin-resistant Staphylococcus aureus from a subtropical recreational marine beach. Microb Ecol 65:1039–1051. https://doi.org/10.1007/s00248-013-0216-1
Poharkar K, Doijad S, Kerkar S, Barbuddhe S (2017) Pathogenic bacteria of public health significance in estuarine mangrove ecosystem. In: Naik, Dubey (eds) Mar Poll Microb Remed. Springer, Sangapore, pp 239–253. https://doi.org/10.1007/978-981-10-1044-6_15
Pomykała R, Michalski M, Jóźwik A, Osek J (2012) Microbiological and marine biotoxins contamination of raw bivalve molluscs commercially available in Poland. Bull Vet Inst Pulawy 56:563–568. https://doi.org/10.2478/v10213-012-0099-9
Rincé A, Balière C, Hervio-Heath D, Cozien J, Lozach S, Parnaudeau S, le Guyader FS, le Hello S, Giard JC, Sauvageot N, Benachour A, Strubbia S, Gourmelon M (2018) Occurrence of bacterial pathogens and human noroviruses in shellfish-harvesting areas and their catchments in France. Front Microbiol 9:2443. https://doi.org/10.3389/fmicb.2018.02443
Rodier J, Legube B, Merlet N (2009) Water analysis. Dunod, Paris (in French)
Russell SM, Cox NA, Bailey JS, Fung DYC (1997) Miniaturized biochemical procedures for identification of bacteria. J Rapid Methods Autom Microbiol 5:169–177
Sabat AJ, Budimir A, Nashev D, Sá-Leão R, Van Dijl JM, Laurent F et al (2013) Overview of molecular typing methods for outbreak detection and epidemiological surveillance. Eurosurveillance 18:20380
Sánchez-Vargas FM, Abu-El-Haija MA, Gómez-Duarte OG (2011) Salmonella infections: an update on epidemiology, management, and prevention. Travel Med Infect Di 9:263–277. https://doi.org/10.1016/j.tmaid.2011.11.001
Santhiya G, Lakshumanan C, Selvin J, Asha D (2011) Microbiological analysis of seawater and sediments in urban shorelines: occurrence of heavy metals resistance bacteria on Chennai beaches, Bay of Bengal. Microchem J 99:197–202. https://doi.org/10.1016/j.microc.2011.05.004
Schets FM, van den Berg HH, Lynch G, de Rijk S, de Roda Husman AM, Schijven JF (2020) Evaluation of water quality guidelines for public swimming ponds. Environ Int 137:105516. https://doi.org/10.1016/j.envint.2020.105516
Shapiro SS, Francia RS (1972) An approximate analysis of variance test for normality. J Am Stat Assoc 67:215–216
Shoults DC, Ashbolt NJ (2018) Total staphylococci as performance surrogate for greywater treatment. Environ Sci Pollut Res 25:32894–32900. https://doi.org/10.1007/s11356-017-9050-1
Sokolowski A, Bawazir AS, Wolowicz M (2004) Trace metals in the brown mussel Perna perna from the coastal waters off Yemen (Gulf of Aden): how concentrations are affected by weight, sex, and seasonal cycle. Arch Environ Contam Toxicol 46:67–80. https://doi.org/10.1007/s00244-003-2164-0
Soltani N, Amira A, Sifi K, Beldi H (2012) Environmental monitoring of the Annaba gulf (Algeria): measurement of biomarkers in Donax trunculus and metallic pollution. Bull Soc Zool Fr 137:51–60
Stabili L, Acquaviva MI, Cavallo RA (2005) Mytilus galloprovincialis filter feeding on the bacterial community in a Mediterranean coastal area (Northern Ionian Sea, Italy). Water Res 39:469–477. https://doi.org/10.1016/j.watres.2004.10.010
Streeter K, Katouli M (2016) Pseudomonas aeruginosa: a review of their pathogenesis and prevalence in clinical settings and the environment. Infect Epidemiol Med 2:25–32. https://doi.org/10.7508/iem.2016.01.008
Telailia S (2014) Study of marine and coastal birds of Northeast Algeria: ecology and biology of reproduction and environmental impact on breeding species. Dissertation, University of El Tarf, El Taref, Algeria
Thapaliya D, Hellwig EJ, Kadariya J, Grenier D, Jefferson AJ, Dalman M, Kennedy K, DiPerna M, Orihill A, Taha M, Smith TC (2017) Prevalence and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus on public recreational beaches in northeast Ohio. GeoHealth 1:320–332. https://doi.org/10.1002/2017GH000106
Tiefenthaler LL, Stein ED, Lyon GS (2009) Fecal indicator bacteria (FIB) levels during dry weather from Southern California reference streams. Environ Monit Assess 155:477–492. https://doi.org/10.1007/s10661-008-0450-z
Torres-Bejarano F, González-Márquez LC, Díaz-Solano B, Torregroza-Espinosa AC, Cantero-Rodelo R (2018) Effects of beach tourists on bathing water and sand quality at Puerto Velero, Colombia. Environ Dev Sustain 20:255–269. https://doi.org/10.1007/s10668-016-9880-x
USEPA (2010) Sampling and consideration of variability (temporal and spatial) form monitoring of recreational waters, Rep. EPA-823-R-10-005, US Environmental Protection Agency, Office of Water
Vincy MV, Brilliant R, Pradeepkumar AP (2017) Prevalence of indicator and pathogenic bacteria in a tropical river of Western Ghats, India. Appl Water Sci 7:833–844. https://doi.org/10.1007/s13201-015-0296-9
Walters E, Graml M, Behle C, Müller E, Horn H (2014) Influence of particle association and suspended solids on UV inactivation of fecal indicator bacteria in an urban river. Water Air Soil Pollut 225:1822. https://doi.org/10.1007/s11270-013-1822-8
Wilkes G, Edge TA, Gannon VP et al (2011) Associations among pathogenic bacteria, parasites, and environmental and land use factors in multiple mixed-use watersheds. Water Res 45:5807–5825. https://doi.org/10.1016/j.watres.2011.06.021
Yaghoubzadeh Z, Kaboosi H, Ghadikolaii FP, Safari R, Fattahi E (2020) The half maximal inhibitory concentration (IC 50) effect of protein hydrolysates from rainbow trout (Oncorhynchus mykiss) skin on enterotoxin A gene expression in Staphylococcus aureus. Int J Pept Res Ther 26:1–8. https://doi.org/10.1007/s10989-020-10036-4
Yang SC, Lin CH, Aljuffali IA, Fang J (2017) Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch Microbiol 199:811–825. https://doi.org/10.1007/s00203-017-1393-y
Zannella C, Mosca F, Mariani F, Franci G, Folliero V, Galdiero M, Tiscar PG, Galdiero M (2017) Microbial diseases of bivalve mollusks: infections, immunology and antimicrobial defense. Mar Drugs 15:182. https://doi.org/10.3390/md15060182
Zegmout M, Basraoui Y, Meziane M, Chahlaouia A, Demnati S, Chafi A (2011) Bacteriological pollution of the Saidia/Moulouya coastal zone (eastern region of Morocco). Rev Microbiol Ind San Environn 5:71–78
Acknowledgements
The authors would like to thank Dr. Hassen Touati and Dr. Fatma Guellati Bouyema for their assistance in the statistical analysis of data.
Funding
This work was supported by the General Directorate for Scientific Research and Technological Development (DGRSDT), Algeria. Paul Sabatier University, Laboratoire de Microbiologie et Génétique Moléculaires (UMR5100) and Centre de Biologie Intégrative.
Author information
Authors and Affiliations
Contributions
MBo performed the experiments. MBo, SK, and MBe analyzed the environmental sampling data. PR analyzed the MLST data. MBo, SK, PR, and MBe wrote the paper.
Corresponding authors
Ethics declarations
No ethical approval was required for this study.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 406 kb)
Rights and permissions
About this article
Cite this article
Boufafa, M., Kadri, S., Redder, P. et al. Occurrence and distribution of fecal indicators and pathogenic bacteria in seawater and Perna perna mussel in the Gulf of Annaba (Southern Mediterranean). Environ Sci Pollut Res 28, 46035–46052 (2021). https://doi.org/10.1007/s11356-021-13978-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13978-4