Abstract
The fish hydrolyzed protein is the potential source of bioactive peptides. These peptides are inactive in the original structure of the protein molecule, but they can be activated after enzymatic hydrolysis. In this research, the effect of the half maximal inhibitory concentration (IC50) rainbow trout hydrolyzed proteins (< 3 kDa) was evaluated on enterotoxin A gene expression of Staphylococcus aureus ATCC 29,213 (the strain of enterotoxin A producer). After affecting IC50 of the fish hydrolyzed protein (< 3 kDa) and S. aureus 1.5 × 108 cfu/ml in BHI broth medium and incubating for the durations 8 and 12 h after exposure (logarithmic phase and stationary phase of S. aureus growth, respectively), the gene expression of enterotoxin A was investigated via Real-Time PCR. gyrA used as a housekeeping endogenous control. Notably only < 3 kDa fish hydrolyzed protein by Flavourzyme enzyme (HF3) showed an inhibitory effect on gene expression of S. aureus enterotoxin A in logarithmic phase. Therefore, fish hydrolyzed proteins can provide a new strategy as natural factors in preventing toxin production, due to the high quality and safety of sensitive foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enterotoxins are major virulence factors secreted by Staphylococcus aureus (S. aureus) strains (Qiu et al. 2010a, b). Staphylococcal enterotoxins (SEs) are the virulence factors responsible for staphylococcal gastroenteritis and are one cause of food poisoning in humans. S. aureus is a gram-positive and facultative anaerobic cocci that appear in the form of a cluster under the microscope. The bacterial colony is a golden yellow color, and when it grows on the agar plate, it creates hemolysis. S. aureus produces an enterotoxin, causing food poisoning (Ryan and Ray 2004). Staphylococcal food poisoning is one of the most important once, and in many countries, this bacterium is known to be the second or third leading cause of foodborne illness after Salmonella and Clostridium perfringens. A total of 24 million food poisonings reported in the US, 9 million are S. aureus, accounting for more than a third of all poisonings in the country (Ananou et al. 2005). Staphylococcal food poisoning is caused by swallowing of enterotoxin (poison) produced from some strains in food that causes swelling or inflammation of the gastrointestinal tract (Jørgensen et al. 2005a, b). 95% of the S. aureus strains induced enterotoxin toxicity was identified as types A, B, C, D and E, and 5% remained to other strains of this bacterium (Bergdoll 1983). Among all types, the most common poison in enterotoxin A is staphylococci. S. aureus has a wide range of infections develops such as simple skin infections, flushing, cork, scabies, eyelashes and acne to life-threatening diseases such as pneumonia, meningitis, osteomyelitis, endocarditis, toxic venipuncture syndrome. It is one of the five common causes of infectious diseases, especially post-surgical ulcers. Annually, 500,000 people in US hospitals are infected with S. aureus. Some studies have evaluated the inhibitory effects of natural compounds and essential oils (EOs) on growth, toxin production and gene expression of enterotoxins in S. aureus (Azizkhani et al. 2013; Qiu et al. 2010a, b).
The use of bioactive peptides is very effective in preventing diseases and improving human health. Biologically active peptides have a specific effect on amino acids in vitro and in vivo. Hence, they are introduced as functional food compounds (Kumar et al. 2011). In the spatial structure of the protein, peptide components exist in the form of various amino acids of 2 to 20 and molecular weight (MW) less than 10,000 Da, which inactive in the initial form of the protein. When the protein is exposed to biochemical hydrolysis, the peptides are evident depending on the MW, sequence and the number of amino acids that exhibit different functional properties (Guerard et al. 2002). Due to their bioactivity, these peptides have many physiological functions such as antihypertensive (Suetsuna et al. 2004), immune stimulation (Agyei and Danquah 2011), antimicrobial, antioxidant, and anti-cancer effects (Yaghoubzadeh et al. 2019). Inhibitors of protein synthesis, such as linezolid and clindamycin, significantly inhibit the production of virulence factors including α-hemolysin, Staphylococcal enterotoxin A (SEA), Staphylococcal enterotoxin B (SEB) and protein A in S. aureus at subinhibitory concentrations (Qiu et al. 2010a, b). In contrast, it has also been demonstrated that some plant compounds (e.g., oleuropein and epicatechin gallate) and EOs (e.g., oils of bay, cinnamon and clove) can influence the production of exotoxins when used at subinhibitory concentrations. Many studies have indicated that, in addition to drug resistance, S. aureus has the ability for virulence increased, which may lead to more severe and widespread diseases (Qiu et al. 2010a, b).
Smith et al. (2000) examined the rainbow trout antibacterial proteins that showed at least four antibacterial proteins in mucosal chromatography analysis included two proteins of ordinary lysosomes, the unusual and cationic peptide of 3 kDa was very hydrophobic (Smith et al. 2000). Cole et al. (2000) reported pleurocidin with a linear antimicrobial peptide (AMP) isolated from the skin of winter flounder (Pleuronectes americanus), was challenged with bacterial isolation showed extensive antimicrobial activity, could be involved in mucosal immunity intrinsic, useful as a therapeutic agent (Cole et al. 2000).
The goal of this study was to investigate the effect of half maximal inhibitory concentration (IC50) of rainbow trout (Oncorhynchus mykiss) skin hydrolysate using Alcalase (HA) and Flavourzyme (HF) enzymes on the expression of enterotoxin A (SEA) of S. aureus.
Materials and Methods
Materials
Fresh rainbow trout (O. mykiss) with an average weight of 530 ± 45 g were obtained from Borzo Aquaculture, Sari, Iran in 2018. The fish were transferred to ice in a ratio of 1:2 (w/w) and were transported within less than 1 h to the Laboratory of Caspian Sea Ecology Research Center (LCSERC), Sari, Iran. Fish skin were separated and spliced into small pieces, were stored at − 20 °C. Alcalase (extracted from Bacillus licheniformis) and Flavourzyme (extracted from Aspergillus oryzae) enzymes were provided by the Novosim Company (Denmark) through their agency in Iran and were stored at 4 °C. Bacterial strain of S. aureus (ATCC 29213), which has the ability to secrete SEA, was prepared as a lyophilized culture from Iranian Research Organization for Science and Technology (IROST), Tehran, Iran.
Hydrolyzed Protein of Rainbow Trout Skin Preparation
First, rainbow trout skins were thawed at ambient temperature, and then 50 g was chosen and transferred to 250 ml Erlenmeyer. 100 ml distilled water (weight ratio–volume 1 to 2) was added to each sample. In order to inactivate the internal enzymes of the skin, samples were heated for 20 min at 85 °C (Guerard et al. 2002). Firstly, the Alcalase enzyme with 1% protein content was added to the sample. Hydrolysis was done for 90 min at pH 8.5 and 58 °C, the slurry was cooled, after deactivating the enzyme at 90 °C for 10 min. The Flavourzyme enzyme with 1% protein content was added to the second Erlenmeyer, before adding the enzyme, pH and temperature were settled in the range of 7 and 50 °C, respectively for 90 min. Hydrolysis was terminated by heating the samples at 90 °C for 10 min in a water bath. After cooling, samples were centrifuged at 10,000 × g at 4 °C for 10 min (Ketnawa and Liceaga 2017). Supernatants were poured off, lyophilized (Dura-stop, NY, USA) and stored at − 20 °C for further analyses (Ketnawa and Liceaga 2017). The protein hydrolyzate powder was kept in polyethylene bag under vacuum at room temperature in a desiccator.
Ultrafiltration (UF)
Hydrolyzed protein powder was dissolved in 10% (w/v) and then, was centrifuged by 3 kDa Amicon filters (Amicon Ultra-15; Millipore Co., Billerica, MA, USA) at 25 °C for 30 min in a 12,000 × g (Sigma 2-16kl, Spain). F-I and F-III were represented the fractions with MWs distribution of > 30 and < 3 kDa, respectively. Hydrolyzed protein solution and fractions were recovered, lyophilized, then of each, the IC50 and the effect of fish hydrolyzed protein were determined on the expression of enterotoxin A gene of S. aureus (Pezeshk et al. 2018; Yaghoubzadeh et al. 2019).
Determination of Half Maximal Inhibitory Concentration (IC50)
The data conducted here was derived from our previous published work as Anticancer Activity of Rainbow Trout Protein Hydrolysate (RTPH) (Yaghoubzadeh et al. 2019).
Evaluating the Effect of IC50 on the Gene Expression of S. aureus Enterotoxin A
S. aureus ATCC 29213 (the strain of enterotoxin A producer) was cultured in BHI broth medium (Merck, Germany) and incubated at 37 °C to achieved bacterial suspension in a concentration of 0.5 McFarland turbidity standard (equal 1.5 × 108 cfu/ml) in BHI broth medium. When turbidity of the tube was equal to 0.5 McFarland standard, the absorbance value should be between 0.08 and 0.1 in 625 nm wavelengths (Baron and Finegold 1990). Then, in a 5 ml Falcon tube, 1 ml of HA3 (hydrolysate using Alcalase) with the concentration of 249.5 µg/ml and 1 ml of suspension S. aureus were mixed and in another Falcon tube, 1 ml of HF3 (hydrolysate using Flavourzyme) with concentration of 727.4 µg/ml was added to 1 ml of suspension S. aureus. These tubes were prepared in two series: in 8 h after incubation (equal logarithmic phase of S. aureus growth) and in 12 h after incubation (equal stationary phase of S. aureus growth) at 37 °C. Control tubes of S. aureus with a concentration of 1.5 × 108 cfu/ml without any hydrolysis protein was prepared for 8 and 12 h after incubation at 37 °C (Qiu et al. 2010a, b; Misaghi et al. 2017). The samples were treated in triplicate. After incubation, the samples were immediately transferred to the temperature of 4 °C, and the effect of inhibitory concentration (IC50) hydrolyzed proteins on enterotoxin A gene expression of S. aureus was evaluated in the next step.
RNA Extraction and Purification
In this step, was evaluated enterotoxin A gene expression of S. aureus affected with IC50 of the fish hydrolyzed proteins. RNA was extracted from S. aureus for determination of enterotoxin A gene expression of S. aureus. RNA extraction of S. aureus was performed using RNX-Plus Kit (CinnaGen Co.), as follows.
The bacterial suspension was centrifuged at 9000 rpm for 10 min. The supernatant was removed from the isolated bacterial precipitate. 1 ml of cold Triazole solution was added to the bacterial precipitate in a 2 ml microtube for 15 s was vortexed, then incubated for 5 min. 0.2 ml of chloroform was added and put the samples at 4 °C for 10 min. Samples were then centrifuged at 12,000 × g at 4 °C for 15 min. The supernatant was transferred to a 1.5 ml RNase tube; the intermediate phase did not dissolve so that was added to the same isopropanol volume. The solution was gently mixed and incubated at − 20 °C for one night. The mixture was centrifuged at 12,000 × g at 4 °C for 15 min. The supernatant was dissolved and 1 ml of ethanol 75%, vortex shorter was added, centrifuged at 4 °C for 8 min at 7500 × g. The solution was discarded and allowed the resulting precipitate to dry at room temperature. The precipitate was dissolved in 30 μl DEPC treated water. To help the dissolve, the tube was placed at 65 °C for 10 min. Finally, the quality and quantity of extracted RNA was evaluated using electrophoresis and agarose gel 1.5% for qualitative evaluation and nanoDrop or bio photometers for quantitative one.
Genomic DNA Removal
DNA removal was performed by the DNase enzyme. 2 μg of extracting RNA was added to 2 μl of DNase and 2 μl of buffer and distilled water was delivered in a volume of 10 μl. It was then incubated for 30 min at 37 °C. To disable the DNase enzyme, 2 μl EDTA was used and incubated for 65 min at 10 °C.
cDNA Synthesis
Synthesis of cDNA was performed using the First strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). According to the Kit protocol, 8 μl of RNA was mixed with genomic DNA removal with 1 μl of Oligo dt and 1 μl of Random Hexamer and distilled in 12 μl of distilled water, incubated at 65 °C for 5 min. The master mix was prepared by the following materials and added to the mixture of primer and RNA. 4 μl of 5 × reaction buffer, 2 μl of dNTP Mix, 1 μl of RiboLock and 1 μl of RT enzyme. Then the samples were incubated at 25 °C for 5 min, and 42 °C for 1 h, and finally the samples were incubated at 85 °C for 10 min in order to disable the RT enzyme.
Real-Time PCR
To determine the amount of enterotoxin A gene expression in the extracted RNA, the Real-Time PCR of the SYBR Green bark was used. To perform this RealQ Plus 2 × Master Mix Green Kit (Ampliqon, Denmark) was applied. Reaction mixture for genomic amplification was prepared as described in Table 1. All phases were carried out on the ice. Each primer was applied twice and all reactions had a negative control. The surface of the solution was covered with 25 µl of mineral oil to prevent vaporization during the thermal cycles. Real Time PCR was achieved by an ABI Prism 7300 Applied Biosystems thermocycler. gyrA used as an housekeeping endogenous control for normalization. The primer pairs used for S. aureus enterotoxin A and gyrA (as the internal control or reference gene) are presented in Table 2. Applied thermal cycling conditions were showed in Table 3. For SYBR Green-based amplicon detection it is important to run a dissociation curve following real time PCR. This is because of the fact that SYBR Green will recognize any double-stranded DNA including primer dimers, contaminating DNA, and PCR products from misannealed primers. Therefore, the derivative plot of the melting curve of each gene in the reaction was measured by ΔΔCt method described in Applied Biosystems User Bulletin No. 2 was employed to determine the relative expression levels.
Statistical Analysis
All experiments were performed in triplicate and each experiment had three replicates. The results were expressed as mean ± SD. Data analysis was conducted by using the SPSS version 18. Statistical differences were evaluated using one-way analysis of variance (ANOVA). The p-value less than 0.05 were considered to be statistically significant. For drawing curves fitting used Prism version 5.
Results
Chemical Composition of Rainbow Trout Skin and Protein Hydrolysates
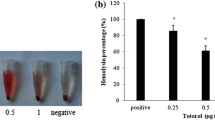
The results of chemical composition in the rainbow trout skin and protein hydrolysates were shown in Table 4. Data were determined based on wet skin weight. Accordingly, the approximate compounds of wet raw material were measured on moisture, fat, ash and protein, respectively. Rainbow trout skin moisture was the largest combination with the highest rate of 71.58%. Hydrolyzed proteins HA and HF showed the highest protein with 73.46% and 69.48%, respectively. The protein hydrolysates powders obtained by Alcalase and Flavourzyme enzymes were shown in Fig. 1.
IC50 Data
The results of IC50 in fractions of fish skin protein hydrolysates were shown in Table 5. Details of these results explained in our previous published paper (Yaghoubzadeh et al. 2019).
Quantitative Analysis Gene Expression of S. aureus Enterotoxin A
The results of quantitative PCR analysis in the gene expression of S. aureus enterotoxin A after affecting by HA3 and HF3 of the fish hydrolyzed protein (< 3 kDa) were shown in Fig. 2 and Table 6. The mean values of Ct in logarithmic and stationary (8 and 12 h) growth phases were shown for enterotoxin A and reference genes in Fig. 2.
The data obtained from these curves represents the value of the cycle Ct of the qPCR. a The proliferation curve of the control gene (gyrA) of S. aureus in logarithmic phase. b The proliferation curve of the enterotoxin A gene of S. aureus in logarithmic phase. c From left to right, first curve gyrA, second curve expression of enterotoxin A treated with HA3, third curve expression of enterotoxin A treated with HF3
The mean value of Ct (Table 6) of each pipe is calculated by the following formula.
Results showed the expression of enterotoxin A gene in one treatment was inhibited by a < 3 kDa fish hydrolyzed protein. It was found that the inhibitory concentration (IC50) of fish hydrolyzed protein of HF3 had only an inhibitory effect on gene expression of enterotoxin A in the logarithmic phase (expression ratio < 1).
Discussion
The numerous virulence factors produced by S. aureus play an important role in the pathogenesis of infection (Bernardo et al. 2004). Staphylococcal gastroenteritis and food poisoning do not result from the ingestion of S. aureus itself but rather from enterotoxins that are performed within the food (Qiu et al. 2010a, b). Fish protein hydrolyzed (bioactive peptide) extracted from wasted skin apply as functional properties. The most important benefits of peptides as drugs once have high biological activity and impose less costly treatments on the patient. Normally, such drugs do not have side effects and any complications for the patient due to low toxicity (Yaghoubzadeh et al. 2019). It has long been known that fish hydrolyzed proteins have antimicrobial activities. In view of the increasing incidence of antibiotic resistance, there is also a continuous need to find more and improved antimicrobial agents in the food industry. SEs preformed within the food could cause serious food-borne diseases, including staphylococcal gastroenteritis and food poisoning. Furthermore, SEs are a family of major serological types of heat-stable enterotoxins (Balaban and Rasooly 2000). Consequently, the ability of fish protein hydrolysis to decrease the production of SEA adds impetus to its potential as a novel food preservative. The expression of most virulence factors by S. aureus is regulated by a network of interacting regulators, such as agr, sar and sae (Goerke et al. 2001). AMPs have different mechanisms of action, including interaction with bacterial membrane, membrane disruption and intracellular targets in bacterial cells and immunomodulatory activities (Mahlapuu et al. 2016). The main mechanism of AMPs action generally involves destabilization of the target microbial membrane, and can also include action on multiple non-membranous targets (e.g., intracellular enzymes, proteins), altogether making AMPs less prone to induce resistance relative to conventional, single target-directed antibiotics (Casciaro et al. 2019).
In the present study, the results showed the enterotoxin A of S. aureus was inhibited in the presence of fish hydrolyzed proteins < 3 kDa. Fish hydrolyzed proteins (HF3) with IC50 727.4 µg/ml reduced the gene expression of enterotoxin A of S. aureus in the logarithmic phase. The findings in our study showed fish hydrolyzed proteins could substantially inhibit the production of enterotoxin A at IC50 level without an effect on bacterial growth that may increase the likelihood as a novel natural food preservative. Indeed, many genes encoding virulence factors are coordinately regulated in response to a variety of intracellular and extracellular signals (Qiu et al. 2010a, b). Misaghi et al. (2017) reported that probiotics such as Lactobacillus officinalis, Lactobacillus acidophilus and Lactobacillus paracasei had an inhibitory effect on the growth and production of enterotoxin A of S. aureus, in addition to the importance of enterotoxins, their expression has not yet been documented. They also stated that the metabolism of proteins and bacteria in the expression of the enterotoxin gene of S. aureus need further study afterward (Misaghi et al. 2017). We proposed that fish hydrolysis could potentially be used in the pharmaceutical and food industries due to the inhibitory effect on gene expression enterotoxin A of S. aureus.
Conclusion
Gene expression analysis indicated that the IC50 level of fish hydrolysis protein (HF3) remarkably repressed the transcription of the SEA gene. According to these results, fish hydrolysis protein (HF3) has the potential to be rationally applied to food products as a novel food antimicrobial agent to inhibit the production of enterotoxin A by S. aureus. This results showed proteins hydrolysis as natural factors could prevent toxin production, due to the high quality in safety of sensitive foods. Additionally, fish hydrolysis protein may be useful for treatment of S. aureus infections when used in combination with antibiotics, which could induce the expression of enterotoxin A at the IC50 level.
References
Agyei D, Danquah MK (2011) Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnology Adv 29(3):272–277. https://doi.org/10.1016/j.biotechadv.2011.01.001
Ananou S, Maqueda M, Martínez-Bueno M, Gálvez A, Valdivia E (2005) Control of Staphylococcus aureus in sausages by enterocin AS-48. Meat Sci 71(3):549–556. https://doi.org/10.1016/j.meatsci.2005.04.039
Azizkhani M, Misaghi A, Basti AA, Gandomi H, Hosseini H (2013) Effects of Zataria multiflora Boiss essential oil on growth and gene expression of enterotoxins A, C and E in Staphylococcus aureus ATCC 29213. Int J Food Microbiol 163(2–3):159–165. https://doi.org/10.1016/j.ijfoodmicro.2013.02.020
Balaban N, Rasooly A (2000) Staphylococcal enterotoxins. Int J Food Microbiol 61:1–10. https://doi.org/10.1016/S0168-1605(00)00377-9
Baron EG, Finegold SM (1990) Diagnostic microbiology, 8th edn. The C. V. Mosby Company, St. Louis
Bergdoll MS (1983) Enterotoxins. In: Easton CSF, Adlam C (eds) Staphylococci and staphylococcal infections. Academic, London, pp 559–598
Bernardo K, Pakulat N, Fleer S, Schnaith A, Utermöhlen O, Krut O, Müller S, Krönke M (2004) Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother 48(2):546–555. https://doi.org/10.1128/AAC.48.2.546-555.2004
Casciaro B, Lin Q, Afonin S, Loffredo MR, de Turris V, Middel V, Ulrich AS, Di YP, Mangoni ML (2019) Inhibition of Pseudomonas aeruginosa biofilm formation and expression of virulence genes by selective epimerization in the peptide Esculentin-1a(1–21)NH2. FEBS J 286(19):3874–3891. https://doi.org/10.1111/febs.14940
Cole AM, Darouiche RO, Legarda D, Connell N, Diamond G (2000) Characterization of a fish antimicrobial peptide: gene expression, subcellular localization and spectrum of activity. Antimicrob Agents Chemother 44:2039–2045. https://doi.org/10.1128/AAC.44.8.2039-2045.2000
Goerke C, Fluckiger U, Steinhuber A, ZimmerliW WC (2001) Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of alphatoxin during device-related infection resolved by direct quantitative transcript analysis. Mol Microbiol 40:1439–1447. https://doi.org/10.1046/j.1365-2958.2001.02494.x
Guerard F, Guimas L, Binet A (2002) Production of tuna waste hydrolysates by a commercial neutral protease preparation. J Mol Catal B 19:489–498. https://doi.org/10.1016/S1381-1177(02)00203-5
Jørgensen HJ, Mørk T, Rørvik LM (2005a) The occurrence of Staphylococcus aureus on a farm with small-scale production of raw milk cheese. J Dairy Sci 88(11):3810–3817. https://doi.org/10.3168/jds.S0022-0302(05)73066-6
Jørgensen HJ, Mørk T, Høgåsen HR, Rørvik LM (2005b) Enterotoxigenic Staphylococcus aureus in bulk milk in Norway. J Appl Microbiol 99(1):158–166. https://doi.org/10.1111/j.1365-2672.2005.02569.x
Kaatz GW, Seo SM (1997) Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother 41(12):2733–2737
Ketnawa S, Liceaga AM (2017) Effect of microwave treatments on antioxidant activity and antigenicity of fish frame protein hydrolysates. Food Bioprocess Technol 10(3):582–591. https://doi.org/10.1007/s11947-016-1841-8
Kumar NS, Nazeer RA, Jaiganesh R (2011) Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 32(7):1496–1501. https://doi.org/10.1016/j.peptides.2011.05.020
Mahlapuu M, Håkansson J, Ringstad L, Björn C (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194. https://doi.org/10.3389/fcimb.2016.00194
Misaghi A, Parsaeimehr M, Akhondzadeh A, Gandomi H, Azizkhani M (2017) The inhibitory effects of Lactobacillus fermentum, Lactobacillus acidophilus and Lactobacillus paracasei isolated from yoghurt on the growth and enterotoxin A gene expression of Staphylococcus aureus. Iran J Vet Med 11(2):191–201
Pezeshk S, Ojagh SM, Rezaei M, Shabanpour B (2018) Fractionation of protein hydrolysates of fish waste using membrane ultrafiltration: investigation of antibacterial and antioxidant activities. Probiotics Antimicrob Prot 1–8: https://doi.org/10.1007/s12602-018-9483-y
Qiu J, Feng H, Lu J, Xiang H, Wang D, Dong J, Wang J, Wang X, Liu J, Deng X (2010a) Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl Environ Microbiol 76(17):5846–5851
Qiu J, Wang D, Xiang H, Feng H, Jiang Y, Xia L, Dong J, Lu J, Yu L, Deng X (2010b) Subinhibitory concentrations of thymol reduce enterotoxins A and B and α-hemolysin production in Staphylococcus aureus isolates. PLoS ONE 5(3):e9736
Ryan K, Ray C (2004) Medical microbiology, 4th edn. McGraw Hill, New York
Smith VJ, Fernandes JM, Jones SJ, Kemp GD, Tatner MF (2000) Antibacterial proteins in rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol 10(3):243–260. https://doi.org/10.1006/fsim.1999.0254
Suetsuna K, Maekawa K, Chen JR (2004) Antihypertensive effects of Undaria pinnatifida (Wakame) peptide on blood pressure in spontaneously hypertensive rats. J Nutr Biochem 15(5):267–272
Yaghoubzadeh Z, Peyravii Ghadikolaii F, Kaboosi H, Safari R, Fattahi E (2019) Antioxidant activity and anticancer effect of bioactive peptides from rainbow trout (Oncorhynchus mykiss) skin hydrolysate. Int J Pept Res Ther 1–8: https://doi.org/10.1007/s10989-019-09869-5
Acknowledgements
We appreciate the sincere collaboration of the Microbiology Department, Ayatollah Amoli Branch of Islamic Azad University for assistance in the conduct of the study.
Funding
This study was supported by the Ayatollah Amoli Branch, Islamic Azad University, Amol, Mazandaran Province, Iran. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest relevant to this study.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed and this article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yaghoubzadeh, Z., Kaboosi, H., Peyravii Ghadikolaii, F. et al. The Half Maximal Inhibitory Concentration (IC50) Effect of Protein Hydrolysates from Rainbow Trout (Oncorhynchus mykiss) Skin on Enterotoxin A Gene Expression in Staphylococcus aureus. Int J Pept Res Ther 26, 2411–2418 (2020). https://doi.org/10.1007/s10989-020-10036-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10036-4