Abstract
Investigating whether the same hyperaccumulator shows a high accumulation potential for different species of the same heavy metal in the soil has rarely been considered until now. In this experiment, Cd accumulation by a hyperaccumulator Bidens pilosa L. from soils spiked with 3 and 9 mg Cd kg−1 in the form of Cd(NO3)2, CdCl2, CdBr2, CdI2, CdSO4, CdF2, Cd(OH)2, CdCO3, Cd3(PO4)2, and CdS and effect of soil amendment with EDTA were determined. The results showed that the Cd concentrations in B. pilosa for high-solubility species were basically higher. But the enrichment factors (EFs) (shoot to soil Cd concentration ratio) and translocation factors (TFs) (shoot to root Cd concentration ratio) of low-solubility Cd species were all greater than 1, either indicating that there was a high Cd hyperaccumulative potentials of B. pilosa without considering on Cd species in soil. EDTA significantly improved B. pilosa Cd hyperaccumulation, especially for low-solubility Cd forms in soils. These results can perfectly explain the accumulation properties of one hyperaccumulator to different species of the same heavy metal. Phytoremediation may be applied for a wide scope for different Cd species–contaminated soil. Moreover, the total amount of Cd in soil was important when assessing the risk of Cd-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyperaccumulator is a general term for plants that take up and accumulate very high heavy metal concentrations from the soil. The accumulation properties of hyperaccumulators in comparison with non-hyperaccumulators were summarized and discussed, i.e., 4 criteria of a hyperaccumulator: threshold criteria, translocation factor (shoot to root concentration ratio, TF > 1), enrichment factor (shoot to soil concentration ratio, EF > 1), and tolerance criterion (biomass was not reduced). A plant characterized by simultaneous TF > 1, EF > 1, and the tolerance criterion was considered an accumulator (Wei and Zhou 2008; van der Ent et al. 2013). Based on these criteria, Bidens pilosa L. was confirmed as a Cd hyperaccumulator (Wei and Zhou 2008).
Although more than 100 species of plants have been registered in a new book titled Phytoremediation resources of heavy metal–contaminated soil (Xu et al. 2018), there were still very few hyperaccumulator or accumulator species described, including Noccaea caerulescens (van der Ent et al. 2013), Arabidopsis halleri subsp. gemmifera (Nouet et al. 2015; Jankovska et al. 2016), Solanum nigrum L. (Khan et al. 2015), Sedum alfredii Hance (Pan et al. 2017), or Noccaea spp. (Pavlík et al. 2018). These studies concerned the potential of various hyperaccumulators for the accumulation of different heavy metals, but essentially did not analyze the potential of a single hyperaccumulator for different species of the same heavy metal.
B. pilosa, collected in Shenyang city of Liaoning province of China, was firstly identified as a Cd hyperaccumulator by comparing 29 plant species of 18 families using a concentration gradient experiment (Wei and Zhou 2008). Later, wild B. pilosa collected from the Pb/Zn mine area showed very high Cd concentrations in the leaves or stems (from 112 to 213 mg kg−1) at Cd soil concentrations ranging from 50 to 85 mg kg−1. At the same time, B. pilosa EFs and TFs were all higher than 1, indicating that B. pilosa collected from natural polluted soil demonstrated all Cd hyperaccumulator characteristics, and could be confirmed as a Cd hyperaccumulator. B. pilosa collected from other places in China, such as B. pilosa from Beijing city (Li et al. 2009), Chongqing city (Chen et al. 2015), Hanzhong city of Shaanxi province (Dai et al. 2017), Sichuan province (Liu et al. 2017), and Xiangyang city of Hubei province (Zhang et al. 2017) also showed features of a Cd hyperaccumulator. B. pilosa collected from Xiangyang city of Hubei province exhibited particularly high phytoremediation potential in a Cd-contaminated field experiment with a 31.3% Cd removal rate (Zhang et al. 2017). Although different B. pilosa plants collected from different regions were demonstrated to have similar Cd hyperaccumulation properties, the accumulation potential of B. pilosa for different Cd species in the soil was rarely investigated. The aim of this article was to bridge the gap of knowledge by exploring the effect of Cd species in soil on its accumulation by B. pilosa and possible improvement role of EDTA on this plant. We hypothesized that (1) there might be significant differences in Cd accumulation by B. pilosa from species of different solubilities occurring in soil and (2) EDTA addition to soil may significantly improve Cd uptake by B. pilosa.
Materials and methods
Experimental design
Soil pot culture experiment was arranged and conducted in a greenhouse of Shenyang ecological experimental station of Chinese Academy of Sciences (123° 41′ E and 41° 31′ N). Soil type is meadow burozem and its soil texture is middle loam soil. Basic properties of top soil (0–20 cm) used in this experiment were with pH 6.56, organic material 16.73 g kg−1, total N 0.73 g kg−1, available P 10.33 mg kg−1, available K 89.05 mg kg−1, Cd 0.17 mg kg−1, Pb 14.3 mg kg−1, Cu 12.5 mg kg−1, and Zn 40.0 mg kg−1. Compared with the soil environmental quality risk control standard for soil contamination of agricultural land (SEQ 2018), it was not contaminated by heavy metal and very clean (Yang et al. 2019).
After complete mixing and sieving through a 1-mm nylon sieve, the soil sample was thoroughly stirred and mixed with different Cd species according to the experimental design (Table 1). The treatment without Cd addition was as controls, i.e., with or without EDTA addition only.
Different Cd chemicals were added to the soil according to a single Cd in 10 different formulas and soil dry weight per pot. Finally, 3 and 9 mg kg−1 (Cd3 and Cd9) Cd in soils were obtained and put into pots φ = 20 cm and H = 15 cm. All pots with different Cd species were equilibrated for 2 months.
Plant culture
B. pilosa was found around different plots and fields in the Shenyang ecological experimental station of the Chinese Academy of Sciences. B. pilosa seeds were collected and air dried when they reached the maturity phase in autumn. In spring, these seeds were put into the soil in a seedling tray for germination. When B. pilosa seedlings reached the same height of 5 cm, two healthy plants were transplanted to each pot (Table 1). When the transplanted plant grew well, approximately 10 days after transplantation, 0.1 mmol Kg−1 EDTA was added into the soil watering 3 times every 15 days (Cd3 + EDTA, Cd9 + EDTA) (Table 1). Each treatment with different Cd species in the soil was performed in three replications. All pots were randomly placed in the greenhouse, but received well-distributed light. The loss of water in each pot was replenished with tap water and maintained at about 80% of soil water-holding capacity every day (Yang et al. 2019). After 80 days of growth at original maturity, all plants in pots and corresponding soil samples were collected.
Sample analysis and data processing
Roots and shoots of all collected plants were rinsed with tap water and then with deionized water. EDTA was used to soak roots and removed Cd attached to its surface. Oven-dried plant sample (until constant weight) was powdered and filtered through a 2-mm sieve. Concentrated nitric acid and perchlorate (87% HNO3/13% HClO4) were used to digest plant and soil samples. Atomic absorption spectrophotometry (AAS, Hitachi 180) was used to determine Cd concentration in digested solutions. All measured values of Cd were verified (QA/QC) by using standard reference material GBW07405 (GSS-5). Extractable Cd concentrations in soils collected when plant samples were harvested were determined after extraction by 1 mol L−1 MgCl2. Soil pH was measured with a pH meter and electrode (PHS-3B) in soil slurries of a soil: water ratio of 1:2.5. Some physical and chemical characteristics of soil were analyzed by a routine method. Enrichment factor (EF) was calculated with the concentration ratio in plant shoot to soil. Translocation factor (TF) was the ratio of concentration in shoots to roots (Yang et al. 2019).
Data processing and calculations of standard errors were calculated using Microsoft Excel. Duncan’s multiple range tests of different treatments at p < 0.05 was calculated using the SPSS software (Yang et al. 2019).
Results
B. pilosa biomass and accumulation potential for 10 Cd species
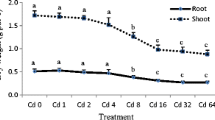
B. pilosa shoot and root biomasses in the controls without EDTA addition were 1.65 g plant−1 and 0.76 g plant−1, and 1.61 g plant−1 and 0.77 g plant−1 for the addition of EDTA, respectively. Basically, there were no significant differences (p < 0.05) among them and the other treatments. The data of the controls were now presented in Fig. 1 since it is hard to show.
The change trend of B. pilosa root biomass was similar to shoot biomass in all treatments (Fig. 1), indicating that the effects of different forms of Cd in the soil on B. pilosa biomass were weak. B. pilosa shoot biomass, affected by 10 different Cd species, in the treatments of 3 mg kg−1 and 9 mg kg−1 Cd, added with or without EDTA, ranged from 1.61 g plant−1 to 1.77 g plant−1. The differences in B. pilosa shoot biomass in 10 Cd species treatments (including Cd3, Cd3 + AS, Cd9, and Cd9 + AS) and the controls were not significant (p < 0.05).
The results showed that shoot and root Cd concentrations of B. pilosa in the controls without EDTA addition were 0.18 mg kg−1 and 0.78 mg kg−1, and 0.21 g plant−1 and 0.82 g plant−1 for the addition of EDTA, respectively. Basically, both treatments were the same (p < 0.05). As shown in Fig. 2A, Cd concentration (24.5 mg kg−1) in B. pilosa shoots in case of Cd(NO3)2 addition was significantly higher (p < 0.05) than that for CdSO4 (21.7 mg kg−1) when Cd addition level was 3 mg kg−1. However, shoot Cd concentrations were not significantly different (p < 0.05) in the treatments with Cd(NO3)2, CdCl2, CdBr2, and CdI2. The same changes were found between CdI2 and CdSO4 treatments. The differences in Cd shoot concentration between CdF2, Cd(OH)2, CdCO3, Cd3(PO4)2, and CdS additions were not significant (p < 0.05), but they were all significantly lower (p < 0.05) compared with CdSO4 addition at 3 mg kg−1 Cd. The average Cd concentration in the shoots for Cd(NO3)2, CdCl2, CdBr2, and CdI2 treatments was 23.8 mg kg−1. The average Cd concentration in shoots in the CdI2 and CdSO4 additions was 22.4 mg kg−1. The average Cd concentration in the shoots for CdF2, Cd(OH)2, Cd CO3, Cd3(PO4)2 and CdS treatments was 14.9 mg kg−1. The former two were 60.3% and 50.8% higher (p < 0.05) than the latter, respectively. Cd concentration in B. pilosa shoots was significantly increased (p < 0.05) by EDTA added in all Cd treatments compared with the treatments without this compound (Fig. 2A). Cd concentration was on average of 24.7% higher in the samples with Cd(NO3)2, CdCL2, CdBr2, and CdI2, and on average 24.4% higher in the treatments with CdI2 and CdSO4, and lastly, it was on average increased by 33.5% in the treatments with CdF2, Cd(OH)2, Cd CO3, Cd3(PO4)2, and CdS at 3 mg kg−1 Cd compared with the treatments without EDTA added.
Cd levels in B. pilosa shoots were significantly higher (p < 0.05), compared with the treatments with a Cd level at 3 mg kg−1, when Cd concentration added in the soil was increased to 9 mg kg−1 (Fig. 2A). There was the same tendency of changes observed in shoot Cd levels between the treatments with 3 mg kg−1 and 9 mg kg−1 concentrations for all 10 Cd species. The average shoot Cd concentration in the treatments with Cd(NO3)2, CdCl2, CdBr2, and CdI2 was 44.3 mg kg−1, while in cases of CdI2 and CdSO4, it was 39.8 mg kg−1. In turn, the average Cd concentration in the shoots treated with CdF2, Cd(OH)2, Cd CO3, Cd3(PO4)2, and CdS was 25.7 mg kg−1. The former two were 72.4% and 54.9% higher than the latter, respectively. EDTA addition also significantly increased (p < 0.05) Cd concentrations in B. pilosa shoots when 9 mg kg−1 Cd was added compared with the treatments without EDTA (Fig. 2A). They were on average increased by 17.5% in case of Cd(NO3)2, CdCl2, CdBr2, and CdI2 additions, by 16.8% for CdI2 and CdSO4 additions, and by 25.5% in case of CdF2, Cd(OH)2, CdCO3, Cd3(PO4)2, and CdS additions.
The differences in Cd accumulation in B. pilosa roots in response to 10 different Cd species (3 and 9 mg kg−1 additions) in the soil and the assisting role of EDTA were generally the same for the shoots (Fig. 2B).
Effects of 10 Cd species on soil pH and extractable Cd concentration in soil
In the treatments of controls, pH were 6.36 and 6.38 without or with EDTA addition, respectively. The soil pH in different treatments ranged from 6.35 to 6.39 (Fig. 3). Basically, these differences were not significant (p < 0.05), irrespective of Cd species added (Cd(NO3)2, CdCl2, CdBr2, CdI2, CdSO4, CdF2, Cd(OH)2, Cd CO3, Cd3(PO4)2, and CdS), concentration applied (3 mg kg−1 and 9 mg kg−1), and with or without EDTA addition.
The changes in trends of extractable Cd concentrations (Fig. 4) were basically the same as those for Cd concentrations in the shoots in all treatments with 3 mg kg−1 and 9 mg kg−1 Cd addition and with or without EDTA addition (Fig. 2A).
When 3 mg kg−1 Cd was added, the average extractable Cd concentration in the treatments with Cd(NO3)2, CdCl2, CdBr2, and CdI2 was 1.6 mg kg−1. Likewise, the average extractable Cd concentration in the treatments with CdI2 and CdSO4 was 1.6 mg kg−1, while for CdF2, Cd(OH)2, Cd CO3, Cd3(PO4)2, and CdS, it was 1.3. When EDTA was added, these averages were 29.2%, 28.1%, and 30.9% higher (p < 0.05) than the former three without EDTA addition, respectively (Fig. 4).
When 9 mg kg−1 Cd was added, the average extractable Cd concentration in the treatments with Cd(NO3)2, CdCl2, CdBr2, and CdI2 was 4.3 mg kg−1. The average extractable Cd concentration in the treatments with CdI2 and CdSO4 addition was 4.1 mg kg−1. The average extractable Cd concentration in the treatments with CdF2, Cd(OH)2, Cd CO3, Cd3(PO4)2, and CdS was 3.4 mg kg−1. The average extractable Cd concentrations when EDTA was added were 17.5%, 18.2%, and 20.5% higher (p < 0.05) than the former three without EDTA addition, respectively (Fig. 4).
Effects of 10 Cd species on EF and TF of B. pilosa
Various treatments with 10 different Cd species resulted in very similar B. pilosa TFs (p < 0.05) when Cd was added at 3 mg kg−1 and 9 mg kg−1 with or without EDTA (Fig. 5). However, TFs were significantly higher (p < 0.05) in the treatments with 3 mg kg−1 Cd addition than those with 9 mg kg−1 Cd, and the averages of the former and latter were 3.55, 3.52 (Cd3, Cd3 + EDTA) and 2.34, 2.37 (Cd9, Cd9 + EDTA), respectively. In this experiment, also another hyperaccumulation criterion was fulfilled. The enrichment factors EF (shoot-to-growing medium ratio of Cd concentrations) were all > 1 at all treatments. The changes in B. pilosa EF trends for 10 different Cd species (Fig. 3) were essentially the same (p < 0.05) as those for Cd concentrations in the shoots (Fig. 1A) and extractable Cd concentrations (Fig. 5) in all treatments with 3 mg kg−1 and 9 mg kg−1 Cd as well as with or without EDTA addition. The differences in detail data were also similar to those described above.
Discussion
One of the important factors reflecting phytoremediation efficiency and tolerance to trace metals in plants is biomass. Unaffected biomass represents tolerance to metal stress of the plant. Root growth inhibition under Cd stress can result from cell wall lignification, which limits cell expansion and nutrient uptake, and the response of shoot can result from photosynthetic reaction inhibition, which prevents organic accumulation (Baycu et al. 2017). In this experiment, B. pilosa biomass was not affected (p < 0.05) in the treatments with different Cd forms, indicating that B. pilosa showed very strong tolerance (Fig. 1). This result suggested that there may be a wide range for phytoremediating different Cd species–contaminated soil in terms of tolerance.
As shown in Table 1, the solubility of Cd in water in different forms is quite different. The solubilities of Cd(NO3)2, CdCl2, CdBr2, CdI2, and CdSO4 are high, in contrast, and CdF2, Cd(OH)2, CdCO3, Cd3(PO4)2, and CdS are low-solubility Cd species (Table 1). The trends of solubilities were generally consistent with the concentrations of Cd in B. pilosa when different Cd forms added in soils, and with the extractable Cd concentrations in different treatments, but not entirely consistent, especially for CdF2 and CdS (Table 1, Figs. 2 and 4). Basically, Cd concentrations of B. pilosa in the treatments with high solubilities of Cd forms (Cd(NO3)2, CdCl2, CdBr2, CdI2, and CdSO4) were higher than those in the treatments with low-solubility Cd species addition (CdF2, Cd(OH)2, CdCO3, Cd3(PO4)2, and CdS) (Fig. 2). But the ratio in B. pilosa Cd concentrations between high-solubility and low-solubility species was much lower than between the salt solubilities (Fig. 2), showing that the latter was not a unique factor of the differences. The results presented here (Fig. 4) confirmed that for the so-called low-solubility species of Cd, the extractable Cd concentrations were far from being low and not so different from that of the high-solubility Cd species, confirming that it is not the initial Cd form which mainly controls Cd availability, but its transformation through reactions in soil (Kabata-Pendias 2011). Using a model that simulates the transport by diffusion and convection, Lin et al. found that the most influential parameter was the initial Cd2+ concentration, followed by the soil buffer power for Cd2+, the soil water content, and impedance factor (Lin et al. 2016). Therefore, when assessing the risk of heavy metal–contaminated soils, we should not just consider the availability of heavy metals in soils, but the total amount of heavy metals in soils (Lin et al. 2016). This result also showed that phytoremediation was with wide usability for different Cd species in soil.
Usually, EDTA is the commonly used chelator to improve plant accumulating Cd in soil (Eissa 2016; Tananonchai et al. 2019). The application of EDTA in the present experiment played an important supporting role by improving extractable Cd species in soil, in particular of those low-solubility species (Fig. 4), and thus enhanced Cd accumulation of B. pilosa (Fig. 2, Eissa 2016; Tananonchai et al. 2019).
EFs and TFs in all treatments were higher than 1 (Fig. 5), indicating B. pilosa exhibited characteristics of translocation factor (TF > 1) and enrichment factor (EF > 1) even for the treatments with low-solubility Cd species addition (Wei and Zhou 2008; van der Ent et al. 2013). Based on all of the above results, B. pilosa showed all Cd hyperaccumulator characteristics even for low-solubility Cd species added in soils, indicating a strong Cd accumulation capacity.
Although there were many studies concerned on plants hyperaccumulating Cd, only a few so far that concerning Cd species and most of using high solubility Cd species such as CdCl2 to explore the effects of endophytic bacterium SaMR12 on S. alfredii metal ion uptake (Pan et al. 2017), CdSO4 in the functional analysis of three HMA4 copies of a metal hyperaccumulator, A. halleri (Nouet et al. 2015), and Cd(NO3)2 to show the regulation of odd-numbered fatty acid content in the metabolism of Noccaea spp. hyperaccumulator adapted to oxidative stress (Pavlík et al. 2018). The hyperaccumulator characteristics of Cd in these plants were often questioned because high-solubility Cd species were used in these experiments. The results of the present study could perfectly explain the hyperaccumulative properties of a hyperaccumulator to different species of the same heavy metal.
Conclusions
There were significant differences in Cd accumulation by B. pilosa from species of different solubilities occurring in soil. The accumulation for high-solubility species was basically high. But the accumulation of B. pilosa to low-solubility species also showed all Cd hyperaccumulation characteristics, i.e., with high accumulation potential either. Thus, phytoremediation showed a wide application for different Cd species-contaminated soil based on the results of biomass and Cd accumulation in this experiment. EDTA addition to soil significantly improved different species of Cd uptake by B. pilosa, especially for low-solubility species. Furthermore, the total amount of Cd in soil should be considered together with the extractable concentration when assessing the risk of Cd-contaminated soils.
References
Baycu G, Gevrek-Kurum N, Moustaka J, Csatari I, Rognes SE, Moustakas M (2017) Cadmium-zinc accumulation and photosystem II responses of Noccaea caerulescens to Cd and Zn exposure. Environ Sci Pollut Res 24:2840–2850
Chen JW, Sun YM, Wang FY, Zhang XQ, Yang ZN, Liu YH, Sun M (2015) Induction and accumulation of cadmium and lead by hairy root of Bidens pilosa. Acta Sci Circumst 35:1596–1602
Dai HP, Wei SH, Twardowska I, Han R, Xu L (2017) Hyperaccumulating potential of Bidens pilosa L. for Cd and elucidation of its translocation behavior based on cell membrane permeability. Environ Sci Pollut Res 24:23161–23167
Eissa MA (2016) Effect of sugarcane vinasse and EDTA on cadmium phytoextraction by two saltbush plants. Environ Sci Pollut Res 23:10247–10254
Jankovska I, Sloup V, Szakova J, Langrova I, Sloup S (2016) How the tapeworm Hymenolepis diminuta affects zinc and cadmium accumulation in a host fed a hyperaccumulating plant (Arabidopsis halleri). Environ Sci Pollut Res 23:19126–19133
Kabata-Pendias A (2011) Trace elements in soil and plants, 4th edn. CRC Press, Boca Raton
Khan AR, Ullah I, Khan AL, Park GS, Waqas M, Hong SJ, Jung BK, Kwak Y, Lee IJ, Shin JH (2015) Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp RSC-14 inoculation. Environ Sci Pollut Res 22:14032–14042
Li Y, Cui YS, Chen XC, Hu DS (2009) Effect of different types of sulphur fertilizer on oilseed rape and railway beggarticks herb uptake of lead and cadmium in lead-cadmium contaminated soil. J Grad Sch Chin Acad Sci 26:621–626
Lin Z, Schneider A, Sterckeman T, Nguyen C (2016) Ranking of mechanisms governing the phytoavailability of cadmium in agricultural soils using a mechanistic model. Plant Soil 399:89–107
Liu L, Ma QQ, Lin LJ, Tang Y, Wang J, Lv XL, Liao MA, Xia H, Chen SX, Li JH, Wang X, Lai YS, Liang D (2017) Effects of exogenous abscisic acid on cadmium accumulation in two ecotypes of hyperaccumulator Bidens Pilosa. Environ Prog Sustain 36:1643–1649
Ma XH, Li ZL, Zhou GQ (2014) Inorganic and analytical chemistry, 2nd edn. Chemical Industry Press, Beijing
Nouet C, Charlier JB, Carnol M, Bosman B, Farnir F, Motte P, Hanikenne M (2015) Functional analysis of the three HMA4 copies of the metal hyperaccumulator Arabidopsis halleri. J Exp Bot 66:5783–5795
Pan FS, Luo S, Shen J, Wang Q, Ye JY, Meng Q, Wu YJ, Chen B, Cao XR, Yang XE, Feng Y (2017) The effects of endophytic bacterium SaMR12 on Sedum alfredii Hance metal ion uptake and the expression of three transporter family genes after cadmium exposure. Environ Sci Pollut Res 24:9350–9360
Pavlík M, Zemanová V, Pavlíková D, Kyjaková P, Hlavsa T (2018) Regulation of odd-numbered fatty acid content plays an important part in the metabolism of the hyperaccumulator Noccaea spp. adapted to oxidative stress. J Plant Physiol 208:94–101
SEQ (Soil environmental quality) (2018) Soil environmental quality risk control standard for soil contamination of agricultural land. GB15618-2018. National standard of China
Song TY, Xu JN, Cheng GZ (2015) Inorganic chemistry (3ird edition). Higher Education Press, Beijing
Tananonchai A, Sampanpanish P, Chanpiwat P, Tancharakorn S, Sukkha U (2019) Effect of EDTA and NTA on cadmium distribution and translocation in Pennisetum purpureum Schum cv. Mott. Environ Sci Pollut Res 26:9851–9860
van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Wei SH, Zhou QX (2008) Screen of Chinese weed species for cadmium tolerance and accumulation characteristics. Int J Phytoremediat 10:584–597
Xu ZH, XU JM, Zhu YW (2018) Plant resources for phytoremediation of heavy metal contaminated soils. Science Press, Beijing
Yang W, Dai HP, Dou XK, Zhang QR, Wei SH (2019) Effect and mechanism of commonly used four nitrogen fertilizers and three organic fertilizers on Solanum nigrum L. hyperaccumulating cd. Environ Sci Pollut Res 26:12940–12947
Zhang YP, Wu Y, Shi Z (2017) Study on Cd and Pb pollution of soil in Xiangyang, Hubei province. Resour Environ Engine 31:713–716
Zhong XH, Xu SL, Lu YY (2011) Inorganic chemistry series (sixth volume). Science Press, Beijing
Funding
This work was financially supported by the Natural Science Foundation of China (41571300, 31870488, 31270540 and 31070455), the Special Plan in the Major Research & Development of the 13th Five-Year Plan of China (2018YFC1800501, 2016YFD0800802), Projects of Shaanxi Province of China (2019JM-413, 17JS023, 2018SZS-27-07), and the project of Foreign Experts Bureau of Shaanxi province of China (GDT20186100430B).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dou, X., Dai, H., Skuza, L. et al. Bidens pilosa L. hyperaccumulating Cd with different species in soil and the role of EDTA on the hyperaccumulation. Environ Sci Pollut Res 26, 25668–25675 (2019). https://doi.org/10.1007/s11356-019-05831-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05831-6