Abstract
The effects of plant-bound zinc (Zn) and cadmium (Cd) on element uptake and their interactions in a parasite-host system were investigated in a model experiment. Male Wistar rats were divided into four groups (C, P, TC and TP). Groups TC and TP were infected with the rat tapeworm Hymenolepis diminuta. Groups C and TC were fed a standard rodent mixture (ST-1) and received 10.5 mg of Zn per week, while groups P and TP were fed a mixture supplemented with the Zn- and Cd-hyperaccumulating plant Arabidopsis halleri at a dosage of 236 mg Zn/week and 3.0 mg Cd/week. Rats were euthanized after 6 weeks, and Cd and Zn levels were determined in rat and tapeworm tissue. The results indicate that tapeworm presence did have an effect on Cd and Zn concentrations in the host tissue; the majority of tissues in infected rats had statistically significant lower Zn and Cd concentrations than did uninfected rats. Tapeworms accumulated more zinc and cadmium than did the majority of host tissues. This important finding confirms the ability of tapeworms to accumulate certain elements (heavy metals) from the host body to their own body tissues. Thus, tapeworms can decrease heavy metal concentrations in host tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Risk element contamination of the environment is a global problem (Brozova et al. 2015; Jankovska et al. 2014; Oprsal et al. 2015; Vaculik et al. 2015; Zarubova et al. 2015).
However, zinc, copper, chromium, manganese and cobalt are also essential trace elements. These are defined as elements contained in low concentrations (mg/kg or less) in plants and animals (Phipps 1981). They are essential for metabolic processes in animals, but at higher levels, they may be toxic. Zinc ensures proper development and an effective immune response. Zinc is the central atom of a wide range of metalloenzymes, and as a part of the insulin molecule, it interferes with the metabolism of sugars (Brown et al., 2001; Brody, 1998; Cuajungco and Lees, 1997; Frederickson et al., 2005; Sun et al. 2011). When zinc is taken at high doses for the long-term, it is absorbed at the expense of other metals, and symptoms of anaemia may develop (WHO 1996; FAO 2001; Hotz and Brown 2004).
Cadmium is a toxic element that is primarily acquired through food consumption. Intestinal absorption of Cd is proportional to its concentration in the diet; however, other factors also influence the rate of the intestinal absorption and organ retention of Cd. One significant problem is the interaction between Cd and other mineral nutrients that are antagonistic to Cd absorption (Reeves and Chaney 2008).
Biogenic (Cu, Cr, Co, Mn, Zn) and toxic (Pb, Cd, Hg) elements in soil can be released (under suitable physicochemical conditions) and become potentially available for plants in areas with elevated levels of these elements. Thus, soil-plant transfer is a possible way for these metals to become part of the food chain (Cadkova et al. 2013). Certain plants, including Arabidopsis halleri, can tolerate or even accumulate very high concentrations of cadmium and zinc. Such plants are known as hyperaccumulators and can considerably accelerate the introduction of soil-bound toxic elements into the food chain. Moreover, these species can also be used in phytoremediation and the monitoring of heavy metals in the environment (Zhenli et al., 2005). The majority of elements found in animals enter through the oral route and are subsequently absorbed in the digestive tract. This absorption process significantly interferes with gastrointestinal parasites, especially acanthocephalans (and tapeworms), which receive nutrients through the tegument, a metabolically active body surface (Sures et al., 2000a, Sures et al., 2000b, Sures et al., 2002; Eira et al. 2005; Kosik-Bogacka et al., 2010). Tapeworms are able to accumulate a considerable amount of metals and reduce their concentrations in host tissues (Jankovska et al. 2011; Cadkova et al. 2013).
The aim of the present study was to investigate the ability of the rat tapeworm Hymenolepis diminuta to not only accumulate cadmium and zinc derived from the hyperaccumulating plant A. halleri but also affect their concentrations in the tissues of a definitive host (Rattus norvegicus).
Material and methods
Breeding and infection of rats
Twenty-four male Wistar rats (R. norvegicus var. alba) each weighing 150 g, were immediately checked for the presence of the intestinal helminths through a faecal sampling examination.
Rats were divided into four groups of six individuals and kept at a temperature of 21 ± 2 °C and a relative humidity of 75 ± 5 % for 3 weeks. This 3-week period is required for rats to acclimatize and for tapeworms to fully develop in the infected rats. During the acclimatization period (3 weeks), rats were given ad libitum access to both water and a standard ST-1 rodent feed, commercially available from Velaz Ltd. (Table 1).
Infection of rats was initiated with cysticercoids acquired from laboratory-bred beetles (Tribolium confusum), which were infected by ingesting tapeworm eggs collected from the excrements of previously infected rats. Cysticercoid development in beetles took place over a 20-day period. The cysticercoids were then collected, suspended in a solution of glucose and administered to the rats orally via micropipettes. Each rat was infected with three cysticercoids.
Experimental design
When tapeworm infection was verified, the rats were housed individually in metabolic cages with a controlled temperature of 21 ± 2 °C and relative humidity 75 ± 5 %. Mode light was set at a 12 h/12 h dark/light cycle. Rat group distribution is shown in Table 2. Over a period of 6 weeks, groups P and TP were given ST-1 (25 g/day) supplemented with dried and homogenized A. halleri containing 50.4 mg/kg of Cd and 3912 mg/kg of Zn. Therefore, both groups P and TP received weekly zinc and cadmium doses of 236 and 3.0 mg, respectively. Groups C and TC received only finely minced ST-1, which provided only 10.5 mg of zinc per week (we fed rats only 6 days a week; the seventh day was a fasting day, when rats were provided with water only). Animal body weight was monitored weekly. The EU Legislation limits Zn content in complete feed mixtures to 250 mg/kg (EU regulation 2316/98), i.e., 37.5 mg/kg/week.
Sampling and analytical determination of metals
Six weeks into the study, the rats were euthanized and tissues were taken from the following seven organs with Teflon tools: the liver, small intestine, kidneys, spleen, muscle, testes and bone tissue (marrow and osseous tissues). Furthermore, tapeworms were removed from the small intestines of the infected rats. All samples were immediately placed in a freezer at −20 °C and subsequently freeze-dried. The samples were then pulverized, and aliquots taking approximately from 400 to 500 mg were decomposed through microwave-assisted digestion using a mixture of 65 % HNO3 (8.0 ml) and 30 % H2O2 (2.0 ml) purchased from Analytica Ltd., Prague, Czech Republic by using the device Ethos 1 (MLS GmbH, Leutkirch, Germany) at 220 °C for 45 min. The digests were poured into 20-ml glass tubes and diluted to 20 ml with distilled water. Certified reference material BCR 185R bovine liver was added to the samples for quality assurance analysis.
Element contents in the digests were determined by inductively coupled plasma-atomic emission spectrometry (ICP-OES, Agilent 720, Agilent Technologies Inc., USA) equipped with a two-channel peristaltic pump, a Struman-Masters spray chamber and a V-groove pneumatic nebulizer made of inert material. To determine low Cd concentrations in the digests, we implemented electrothermal atomic absorption spectrometry (ETAAS) through the use of a VARIAN AA280Z (Varian, Australia) equipped with a GTA120 graphite tube atomizer.
Statistical analysis
Element concentrations and their statistical differences were compared between groups using the nonparametric Mann-Whitney U test. All computations were done using Statistica 10 software (Statsoft, USA).
Results and discussion
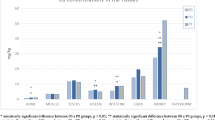
Our results indicated statistically significant differences between groups of rats infected with tapeworms and their non-infected counterparts (Figs. 1, 2, 3 and 4). The rat group affected by both the Cd- and Zn-hyperaccumulating plant and tapeworms (TP) had significantly lower (p < 0.01) concentrations of cadmium and zinc in the majority of their tissues when compared to the non-infected rat group (Figs. 2 and 4).
It was determined in the 1990s that several helminths (primarily acanthocephalants in fish) are able to accumulate considerable concentrations of heavy metals (Lafferty 1997; Sures et al. 1998; Sures 2001; Sures and Siddall 2001; Sures 2003; Thielen et al. 2004). Information regarding whether parasites in terrestrial vertebrates can serve as sentinels for heavy metal environmental pollution, as well as the benefits they provide to their hosts, remains inconsistent (Sures et al. 2002; Baruš et al. 2003; Torres et al. 2004, 2006; Jankovská et al., 2008, Jankovska et al., 2009, Jankovská et al., 2010). Since cestodes are more abundant in terrestrial mammals than are acanthocephalans and, thus, potentially more useful in passive as well as active biomonitoring; a very common animal (R. norvegicus) and its common tapeworm (H. diminuta) were selected for the present study. As Sures et al. (2002) reported in their lead biomonitoring study, this host-parasite model can be used both as a bioindicator to monitor environmental pollution (especially in urban areas) and as a means to reduce heavy metals in the organs and tissues. As Sures et al. (2002) did with their study dealing with lead concentrations, we compared Cd and Zn concentrations accumulated by the host and those in tapeworm tissues (bioconcentration factor BF = C (tapeworm)/C (host tissue).
With respect to Zn concentrations, tapeworms accumulated 160.34 (TC) and 200.26 (TP) mg kg−1. This translates to 1.9, 2.1, 2.0, 1.1, 2.2 and 4.0 times more Zn than that accumulated in the liver, spleen, kidneys, bone, small intestine and muscles, respectively, of the host from the TC group. Only testis tissue accumulated slightly more zinc than the tapeworms did (Table 3). This can be attributed to the protective effects of Zn against testicular damage caused by Cd (Bonda et al., 2004).
It is known that feeding high concentrations of zinc, iron and/or calcium to animals reduces the rate of absorption of cadmium from various food sources. When zinc is marginal in the diet, the delay of cadmium excretion is more pronounced (Reeves and Chaney, 2004). The rates of absorption and whole-body retention of dietary Cd increased 7- to 10-fold when experimental animals were fed diets containing marginal concentrations of Zn, Fe and/or Ca (Reeves and Chaney, 2001, 2002).
In A. halleri leaves, Zn is bound mainly to malate or other organic acids (Sarret et al. 2009); Cd is also bound to organic acids, cell wall components and, to a lesser extent, thiol-containing molecules (Huguet et al. 2012). Previously published papers have indicated that metals in plants are more easily absorbed than those in inorganic forms, which are artificially added to animal feed (Cadkova et al. 2013). To our knowledge, Válek et al. (2015) were the first to use A. halleri in a feeding study. Recent studies have suggested that using A. halleri in feed stresses the consumer organism due to its Cd content, rather than its Zn content. Cadmium (Cd) is an environmental pollutant that is ranked eighth among the top 20 most hazardous substances (Klaassen et al. 2009), and human activity has markedly increased its distribution in the global environment. Zinc is an essential element for all organisms. However, it is toxic when taken in excess (Johnson et al. 2007).
In Tables 3 and 4, we compared zinc and cadmium concentrations between organs of rats with or without parasites and with or without A. halleri diet supplementation. Cd concentrations were significantly higher in rats given Arabidopsis in their feed mixture (group P); this group (P) had Cd levels that were 329, 147, 87, 39, 10 and 3 times higher in the kidneys, liver, small intestine, testes, spleen and muscle, respectively, than in those of rats not given Arabidopsis (group C). Cadmium concentration differences between groups C and TC, as well as between P and TP, are presented in Table 4. There were only slight zinc concentration differences between groups C and TC, P and TP, as well as between P and C, ranging from ratios of 0.9 (testes) to 1.7 (small intestine tissue) as presented in Table 3.
The main site of zinc absorption in animals is the small intestine, where the distal duodenum and proximal jejunum play a key role. Zinc excretion is primarily through faeces (1–5 mg of Zn can be excreted by humans over a 24-h period). Zn levels are directly influenced by the content of zinc in the diet. Stools contain unabsorbed zinc from food, endogenous zinc secreted into the intestine from the pancreas and gallbladder and zinc from the intestinal epithelial cells (Krebs, 2000).
The liver, kidneys, bones and testes of group TC had significantly lower Zn concentrations (p < 0.01) than did those of group C (Fig. 1); statistically significant differences between these two groups were also found in the small intestine (p < 0.05).
The liver, bones, small intestine, testes and muscles of rats with tapeworms (TC) had significantly lower Cd concentrations (Fig. 3) than did those of group C (non-infected rats).
The liver, kidneys, muscles, bones, testes and spleen of rats infected with tapeworms and fed A. halleri (TP) had significantly (p < 0.01) lower Cd concentrations (Fig. 4) than did those of the non-infected rats (P). In the TP group, tapeworms accumulated Zn concentrations that were 1.8, 2.3, 1.9, 1.4, 1.7, 1.2 and 5.5 times higher than those accumulated by the liver, spleen, kidneys, bones, small intestine, testes and muscles, respectively (Table 3). Zn concentrations were lower in all host tissues than in the tapeworms. This supports the theory regarding the ability of tapeworms to accumulate heavy metals from the host.
Scheef et al. (2000) described the ability of the acanthocephalan parasite (Moniliformis moniliformis) to accumulate cadmium from its rat host (R. novergicus). The study lasted 3 weeks, and the rats were exposed to a solution of CdCl2. They found that the parasite accumulated significantly more of this element than did rat tissues. However, there was no indication that cadmium levels in the tissues of infected rats were significantly lower than those in non-infectected rats. Similar results were published by Sures et al. (2000b) in the case of lead, another risk element. They investigated the acanthocephalan parasite M. moniliformis, which parasitizes in rats, and found that it accumulated Pb from the host body. They determined that acanthocephalan females contained 25, 39, 2 and 9 times more Pb than did the host liver, small intestine, kidney cortex and kidney medulla, respectively. The ratio of acanthocephalan males was different (7; 11; 0.5 and 3). However, tapeworms are hermaphrodites, so our study could not provide such comparisons. It is evident from both experiments that acanthocephalans have the ability to accumulate higher concentrations of metals than do the host tissues.
In the case of cadmium, tapeworms accumulated 2.2 and 2.6 times higher levels than did the host testis and muscle tissue, respectively (group TC). The remaining host tissues contained higher Cd concentrations than did the tapeworms (Table 4); however, there were only trace quantities in this case since rats from the TC group were not affected by Cd in food.
Group TP rats were affected with cadmium through diet, and the tapeworm Cd concentrations for this group were 2.2, 32.0, 127.6, 2.7, 59.6 and 1551.6 times higher than those in the liver, spleen, bone, small intestine, testes and muscles of host, respectively (Table 4). The kidneys are major Cd-accumulating organs in mammals. This was confirmed by our study; Cd concentrations in kidneys reached 7.94 mg kg−1, which was 1.6 times higher than those in tapeworms (Table 4).
There is not sufficient scientific literature concerning the behaviour of the rat tapeworm H. diminuta in the presence of cadmium or zinc in a host. Sures et al. (2002) studied the effects of rat tapeworms (H. diminuta) on laboratory rats exposed to lead as Pb(CH3COO)2. After calculating the bioconcentration factor, they found lead concentrations in the tapeworms that were 17 times higher than those found in the rat kidneys.
Our study determined Zn concentrations in tapeworms that were 1.9 times higher than those in the host kidneys (Table 3, group TP). Contrarily, Cd concentrations in the kidneys of hosts from the same group (TP) were 2.85 mg kg−1 higher than those in the tapeworms (Table 4).
Nevertheless, our results showed that tapeworms have a significant effect on zinc and cadmium accumulation in host (rat) tissues. Even though Zn concentrations were similar in both groups (with or without Arabidopsis), Cd concentrations were significantly higher in rats given Arabidopsis in their feed mixture (group P); this group (P) exhibited Cd levels that were 329, 147, 87, 39, 10 and 3 times higher in the kidneys, liver, small intestine, testes, spleen and muscle, respectively, than in those of rats not given Arabidopsis in their feed mixture (group C). Tapeworms accumulated more zinc and cadmium than did the majority of host tissues. For example, tapeworms accumulated 5.5 times more Zn and 1542 times more Cd than did the host muscle tissue. Moreover, when we compared group TC (standard feed mixture and tapeworm infection) with group TP (feed mixture with added hyperaccumulating plants and tapeworm infection), we found that tapeworms from group TP accumulated 848 times more Cd than did tapeworms from group TC.
Since few comparative studies on heavy metal concentrations in tissues of infected and uninfected hosts are available, it remains unclear if conspicuous metal accumulation by parasitic worms affects metal levels in the tissues of the definitive host.
References
Baruš V, Tenora F, Šumbera R (2003) Relative concentrations of four heavy metals in the parasites Protospirura muricola (Nematoda) and Inermicapsifer arvicanthidis (Cestoda) in their definitive host silvery mole-rat (Heliophobius argenteocinereus: Rodentia). Helminthologia 40(4):227–232
Bonda E, Wlostowski T, Krasowska A (2004) Testicular toxicity induced by dietary cadmium is associated with decreased testicular zinc and increased hepatic and renal metallothionein and zinc in the bank vole (Clethrionomys glareolus). Biometals 17:615–624
Brody T (1998) Zinc and copper. Nutritional biochemistry. Academic Press, San Diego
Brown KH, Wuehler SE, Peerson JM (2001) The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food Nutr Bull 22:113–125. doi:10.1177/156482650102200201
Brozova A, Jankovska I, Miholova D, Schankova S, Truneckova J, Langrova I, Kudrnacova M, Vadlejch J (2015) Heavy metal concentrations in the small intestine of red fox (Vulpex vulpex) with and without Echinococcus multilocularis infection. Environ Sci Pollut Res 22:3175–3179. doi:10.1007/s11356-014-3733-7
Cadkova Z, Szakova J, Miholova D, Valek P, Pacakova Z, Vadlejch J, Langrova I, Jankovska I (2013) Faecal excretion dynamic during subacute oral exposure to different Pb species in Rattus norvegicus. Biol Trace Elem Res 152:225–232. doi:10.1007/s12011-013-9609-8
Cuajungco MP, Lees GJ (1997) Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol Dis 4:137–169. doi:10.1006/nbdi.1997.0163
Eira C, Torres J, Vingada J, Miquel J (2005) Concentration of some toxic elements in Oryctolagus cuniculus and in its intestinal cestode Mosgovoyia ctenoides, in Dunas de Mira (Portugal). Sci Total Environ 346:81–86. doi:10.1016/j.scitotenv.2004.11.014
FAO (2001) Human vitamin and mineral requirements. Food and agriculture organization of the United Nations. World Health Organization. Report of a joint FAO/WHO expert consultation Bangkok, Thailand
Frederickson CHJ, KohH JY, Bush AI (2005) The neurobiology of zinc in health and disease. Neuroscience 6:449–462. doi:10.1038/nrn1671
Hotz C, Brown KH (2004) Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25:91–202
Huguet S, Bert V, Laboudigue A, Barthes V, Isaure MP, Llorens I, Schat H, Sarret G (2012) Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ Exp Bot 82:54–65. doi:10.1016/j.envexpbot.2012.03.011
Jankovská I, Langrová I, Bejček V, Miholová D, Vadlejch J, Petrtýl M (2008) Heavy metal accumulation in small terrestrial rodents infected by cestodes or nematodes. Parasite 15(4):581–588
Jankovska I, Miholova D, Langrova I, Bejček V, Vadlejch J, Kolihova D, Sulc J (2009) Influence of parasitism on the use of small terrestrial rodents in environmental pollution monitoring. Environ Pollut 157:2584–2586
Jankovská I, Miholová D, Langrová I, Bejček V, Vadlejch J, Šulc M (2010) Influence of parasitism on trace element contents in tissues of red fox (Vulpes vulpes) and its parasites Mesocestoides spp. (Cestoda) and Toxascaris leonina (Nematoda). Arch Environ Contam Toxicol. doi:10.1007/s00244-009-9355-2
Jankovska I, Lukesova D, Szakova J, Langrova I, Vadlejch J, Cadkova Z, Valek P, Petrtyl M, Kudrnacova M (2011) Competition for minerals (Zn, Mn, Fe, Cu) and Cd between sheep tapeworm (Moniezia expansa) and its definitive host sheep (Ovis aries). Helminthologia 48:237–243. doi:10.2478/s11687-011-0033-3
Jankovska I, Miholova D, Romocusky S, Petrtyl M, Langrova I, Kalous L, Sloup V, Valek P, Vadlejch J, Lukesova D (2014) Importance of fish gender as a factor in environmental monitoring of mercury. Environ Sci Pollut Res 21:6239–6242. doi:10.1007/s11356-013-2459-2
Johnson AR, Munoz A, Gottlieb JL, Jarrard DF (2007) High dose zinc increases hospital admissions due to genitourinary complications. J Urol 177:639–643. doi:10.1016/j.juro.2006.09.047
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238(3):215–220. doi:10.1016/j.taap.2009.03.026
Kosik-Bogacka DI, Baranowska-Bosiacka I, Salamatin R (2010) Hymenolepis diminuta: effect of infection on ion transport in colon and blood picture of rats. Exp Parasitol 124:285–294. doi:10.1016/j.exppara.2009.10.014
Krebs NF (2000) Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr 130:1374–1377
Lafferty KD (1997) Environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol Today 13(7):251–255
Oprsal J, Blaha L, Pouzar M, Knotek P, Vlcek M, Hrda K (2015) Assessment of silver nanoparticle toxicity for common carp (Cyprinus carpio) fish embryos using a novel method controlling the agglomeration in the aquatic media. Environ Sci Pollut Res 22:19124–19132. doi:10.1007/s11356-015-5120-4
Phipps DA (1981) Chemistry and biochemistry of trace metals in biological systems. Effect of heavy metal pollution on plants. Online ISBN:978–94–011-7339-1
Reeves PG, Chaney RL (2008) Bioavailability as an issue in risk assessment and management of food cadmium: a review. Sci Total Environ 398:13–19. doi:10.1016/j.scitotenv.2008.03.009
Reeves PG, Chaney RL (2001) Mineral status of female rats affects the absorption and organ distribution of dietary cadmium derived from edible sunflower kernels (Helianthus annuus L.). Environ Res 85:215–225
Reeves PG, Chaney RL (2002) Nutritional status affects the absorption and whole-body and organ retention of cadmium in rats fed rice-based diets. Environ Sci Technol 36:1632–1684
Reeves PG, Chaney RL (2004) Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets. Environ Res 96:311–322
Sarret G, Willems G, Isaure MP, Marcus MA, Fakra SC, Frérot H, Pairis S, Geoffroy N, Manceau A, Saumitou-Laprade P (2009) Zinc distribution and speciation in Arabidopsis halleri × Arabidopsis lyrata progenies presenting various zinc accumulation capacities. New Phytol 184:581–595
Scheef G, Sures B, Taraschewski H (2000) Cadmium accumulation in Moniliformis moniliformis (Acanthocephala) from experimentally infected rats. Parasitol Res 86:688–691. doi:10.1007/PL00008553
Sun JY, Wang JF, Zi NT, Jing MY, Weng XY (2011) Effects of zinc supplementation and deficiency on bone metabolism and related gene expression in rat. Biol Trace Elem Res 143:394–402. doi:10.1007/s12011-010-8869-9
Sures B (2001) The use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: a review. Aquat Ecol 35:245–255
Sures B (2003) Accumulation of heavy metals by intestinal helminths in fish: an overview and perspective. Parasitology 126:S53–S60
Sures B, Franken M, Taraschewski H (2000a) Element concentrations in the archiacanthocephalan Macracanthorhynchus hirudinaceus compared with those in the porcine definitive host from a slaughterhouse in La Paz, Bolivia. Int J Parasitol 30:1071–1076. doi:10.1016/S0020-7519(00)00094-1
Sures B, Grube K, Taraschewski H (2002) Experimental studies on the lead accumulation in the cestode Hymenolepis diminuta and its final host, Rattus norvegicus. Ecotoxicology 11:365–368. doi:10.1023/A:1020561406624
Sures B, Jürges G, Taraschewski H (2000b) Accumulation and distribution of lead in the archiacanthocephalan Moniliformis moniliformis from experimentally infected rats. Parasitology 121:427–433. doi:10.1017/S003118209900654X
Sures B, Jürges G, Taraschewski H (1998) Relative concentrations of heavy metals in the parasites Ascaris suum (Nematoda) and Fasciola hepatica (Digenea) and their respective porcine and bovine definitive hosts. Int J Parasitol 28:1173–1178. doi:10.1016/S0020-7519(98)00105-2
Sures B, Siddall R (2001) Comparison between lead accumulation of Pomphorhynchus laevis (Palaeacanthocephala) in the intestine of chub (Leuciscus cephalus) and in the body cavity of goldfish (Carassius auratus auratus). Int J Parasitol 31:669–673
Thielen F, Zimmermann S, Baska F, Taraschewski H, Sures B (2004) The intestinal parasite Pomphorhynchus laevis (Acanthocephala) from barbel as a bioindicator for metal pollution in the Danube river near Budapest, Hungary. Environ Pollut 129(3):421–429
Torres J, de Lapuente J, Eira C, Nadal J (2004) Cadmium and lead concentrations in Gallegoides arfaai (Cestoda: Anoplocephalidae) and Apodemus sylvaticus (Rodentia: Muridae) from Spain. Parasitol Res 94(6):468–470
Torres J, Peig J, Eira C, Borrás M (2006) Cadmium and lead concentrations in Skrjabinotaenia lobata (Cestoda: Catenotaeniidae) and in its host, Apodemus sylvaticus (Rodentia: Muridae) in the urban dumping site of Garraf (Spain). Environ Pollut 143(1):4–8
Vaculik M, Mrazova A, Alexander L (2015) Antimony (SbIII) reduces growth, declines photosynthesis, and modifies Lea tissue anatomy in sunflower (Helianthus annuus L.). Environ Sci Pollut Res 22:18699–18706
Válek P, Sloup V, Jankovská I, Langrová I, Száková J, Miholová J, Horáková B, Křivská D (2015) Can the hyperaccumulating plant Arabidopsis halleri in feed influence a given consumer organism (Rattus norvegicus var. alba)? Bull Environ Contam Toxicol 95:116–121
WHO (1996) Trace elements in human nutrition and health. World Health Organization Geneva. ISBN 92 4 156173 4
Zarubova P, Hejcman M, Vondrackova S, Mrnka L, Szakova J, Tlustos P (2015) Distribution of P, K, Ca, Mg, Cd, Cu, Fe, Mn, Pb and Zn in wood and bark age classes of willows and poplars used for phytoextraction on soils contamined by risk elements. Environ Sci Pollut Res 22:18801–18813. doi:10.1007/s11356-015-5043-0
Zhenli LH, Xiaoe EY, Peter JS (2005) Trace elements in agroecosystems and impacts on the enviroment. J Trace Elem Med Biol 19:125–140. doi:10.1016/j.jtemb.2005.02.010
Acknowledgments
This study was supported by the University-wide internal grant agency of the Czech University of Life Sciences Prague (CIGA), project No 20142053 and the Grant Agency of Czech Republic (GACR), project No. 13-18154S. The authors also gratefully acknowledge Brian Kavalir (Toronto, Canada) for his proofreading services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments with laboratory animals were conducted in compliance with the current laws of the Czech Republic Act No. 246/1992 coll. on the Protection of Animals against Cruelty and EC Directive 86/609/EEC.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Jankovská, I., Sloup, V., Száková, J. et al. How the tapeworm Hymenolepis diminuta affects zinc and cadmium accumulation in a host fed a hyperaccumulating plant (Arabidopsis halleri). Environ Sci Pollut Res 23, 19126–19133 (2016). https://doi.org/10.1007/s11356-016-7123-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7123-1