Abstract
Solanum nigrum L. is a hyperaccumulator and shows very high phytoremediation potential for Cd-contaminated soil. Fertilizer addition to soil is an effective pathway to improve Cd hyperaccumulation. This article compared the strengthening roles of commonly used four nitrogen fertilizers with three organic fertilizers on S. nigrum hyperaccumulating Cd at the same total nitrogen level. The results showed that Cd concentrations in roots and shoots of S. nigrum were not affected by the addition of inorganic nitrogen like NH4HCO3, NH4Cl, (NH4)2SO4, and CH4N2O compared with the control without nitrogen addition. However, Cd concentrations in S. nigrum roots and shoots were significantly decreased (p < 0.05) when the organic nitrogen was added in the form of chicken manure, pig manure, and commercial organic fertilizer (by 15.6% and 15.1%, 30.1% and 23.6%, 20.3% and 16.8%, respectively). On the other hand, of all nitrogen treatments, the addition of (NH4)2SO4 and CH4N2O to the soil enormously increased S. nigrum biomass, i.e., S. nigrum shoot biomass increased 2.0- and 2.1-fold compared with the control. Correspondingly, Cd loads in S. nigrum shoots were also the highest in former two treatments and amounted to 79.91 μg pot−1 and 80.17 μg pot−1, respectively. Compared with the control, the addition of three organic fertilizers significantly increased (p < 0.05) pH and decreased (p < 0.05) available Cd concentrations in the soil, which could be the main reasons for their negative effects on S. nigrum accumulating Cd. (NH4)2SO4 and CH4N2O significantly increased S. nigrum biomasses and exerted no effects on the available soil Cd concentration, which made them more better fertilizers in practice. In general, the same fertilizer may show different effects on different hyperaccumulators. The selection of fertilizer should be decided in accordance with the specific conditions in the phytoremediation practice of contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is becoming an increasingly significant problem in the world (Liu et al. 2015). According to the survey results of the Chinese Environmental Protection Bureau (2013), nearly one-fifth of the arable land (2 × 107 ha) in China is polluted by heavy metals, which directly caused the pollution of about 1.2 × 105 tons of crops every year (He et al. 2014). Among these heavy metal pollutants, cadmium (Cd) causes more serious pollution than other pollutants (Dai and Wei 2018). Usually, physical and chemical remediation methods are effective in the remediation of heavy metal–contaminated soil. However, these methods are difficult to use in practice due to their high costs and high possibility of secondary pollution (Bhargava et al. 2012; Lan et al. 2013). Thus, phytoremediation methods for removing heavy metals from contaminated soils by hyperaccumulator showed better prospects. Although there were dozens of Cd hyperaccumulators reported (Li et al. 2017), only a limited number of them demonstrated a higher phytoremediation potential. Among them, Sedum alfredii Hance (Pan et al. 2016), Solanum nigrum L. (Wang et al. 2015), Bidens pilosa L. (Dai et al. 2017), and Brassica juncea L. (Chowdhary et al. 2018), have been extensively so far.
Solanum nigrum has been identified as a Cd hyperaccumulator from among 54 weed plants of 20 families by pot and plot experiments (Wei et al. 2005). In a Pb and Zn mine field, S. nigrum was found to accumulate from 110 to 460 mg kg−1 Cd in its shoots when Cd concentrations in the soil ranged from 20 to 60 mg kg−1 (Wang et al. 2015). Particularly, S. nigrum showed a particularly strong ability to remove Cd from contaminated soil in the field experiment (Niu et al. 2015). Some effective and practical methods have been explored in order to increase Cd concentration and biomass using appropriate measures to maximize phytoremediation potential. Measures aimed at increasing the concentration of hyperaccumulators involved the enhancement of CO2 (Li et al. 2014), endophytic bacterium (Chen et al. 2015; Pan et al. 2016), some organic acids (Wei et al. 2016), plant growth–promoting bacteria (Pan et al. 2017), chelator and electric fields (Luo et al. 2018), and so on. The addition of fertilizer to soil is an effective way to increase plant biomass. Usually, the nitrogen-containing fertilizers performed better because phosphorus in polluted soil easily caused a decrease in heavy metal content in hyperaccumulators (Guo et al. 2017). The results showed that Cd extraction efficiency (μg pot−1) from rice plants significantly increased with the addition of urea and ammonium sulfate ((NH4)2SO4) (Jalloh et al. 2009). Amonium nitrate (NH4NO3) and (NH4)2SO4 also increased the biomass of sweet sorghum (Sorghum biocolor L.) and promoted lead enrichment (Zhuang et al. 2009). Some organic fertilizers such as pig manure, cattle manure, and compost also significantly increased plant biomass, but considerably reduced availability of heavy metal concentrations in the soil (Khan et al. 2018).
Ammonium bicarbonate (NH4HCO3), ammonium chloride (NH4Cl), (NH4)2SO4, and urea (CH4N2O) and chicken manure (pure fermented chicken manure), pig manure (pure fermented pig manure), and commercial organic fertilizer are the four types of inorganic nitrogen fertilizers and the three types of organic nitrogen fertilizer, respectively, which are commonly used in agricultural production. Previous experiments showed that NH4HCO3 and NH4Cl not only increased Cd concentrations in the shoots of Rorippa globosa (Turcz.) Thell., but also increased its shoot biomass (Wei et al. 2015). The (NH4)2SO4 and CH4N2O increased shoot biomass but had no effect on Cd concentrations in R. globosa (Wei et al. 2015). Although chicken manure reduced the concentration of available Cd in the soil, it greatly increased the biomass of S. nigrum, and ultimately significantly improved its remediation ability (Wei et al. 2010). However, there is a question which one or two of the four common chemical nitrogen fertilizers and three organic fertilizers can more effectively promote the excessive accumulation of Cd in S. nigrum. We hypothesized that a chemical nitrogen fertilizer could be more efficient in promoting Cd accumulation than an organic nitrogen fertilizer, and the same fertilizer might have a different effect on various hyperaccumulators.
Materials and methods
Soil and its properties
Soil sample is meadow soil and its soil texture is middle loam soil with pH 6.94, organic material 15.18 mg kg−1, total N 0.85 g kg−1, available K 80.96 mg kg−1, CEC 22.9 cmol kg−1, clay 22.1%, silt 43.4%, and sand 34.5%, which was collected from the field of Shenyang ecological experimental station of the Chinese Academy of Sciences (41°31′ N and 123°41′ E). This soil belongs to neutral soils. The collected soil sample was very clean compared with the soil environmental quality risk control standard for soil contamination of agricultural land (SEQ 2018).
Experiment design
Eight treatments were designed and arranged according to the common fertilization amount added in agricultural production and Cd contamination status of about 2 mg kg−1 in the field (Huo et al. 2018). Details are listed in Table 1. The concentration of Cd treatment in the form of CdCl2 2.5H2O spiked to soil was 2 mg kg−1. The total amount of nitrogen added in all types of fertilizers was the same in order to make them comparable. Nitrogen fertilizers with total N concentration of 200 mg kg−1 were added into the soil together with Cd according to the experimental design (Table 1). After Cd and N fertilizers were added into the soils at spring, all treated soils were filled in pots (φ = 20 cm and H = 15 cm) with 2.5 kg of soil (dry weight) each, and were subsequently equilibrated for 2 months. Inorganic and organic fertilizers were all bought from local market. Cd, Pb, Cu, and Zn concentrations in these fertilizers were basically largely lower than that of in the collected soil (Table 1).
Soil pot experiments
The seeds of S. nigrum were collected from the field at the Shenyang ecological experimental station of the Chinese Academy of Sciences. The station is semi-moist continental climate with total annual radiation of the area of 520–544 kJ cm−2, average annual precipitation of 650–700 mm, and approximately 127–164 frostless days a year (Wei et al. 2010). The seedlings were grown on a seedling tray before experiment to ensure that the size of S. nigrum was the same in each treatment. When all seedlings of S. nigrum had four leaves and the same height, six seedlings were transplanted to each pot. Each treatment was repeated three times. All pots were randomly arranged under plastic cloth for shelter from rain in the Shenyang ecological experimental station of the Chinese Academy of Sciences. Tap water was replenished every day to maintain about 80% of soil water-holding capacity. All plants and their corresponding soil samples were harvested when plants reached maturity after 118 days of growth.
Sample analysis and data processing
Plant samples were divided into two parts, i.e., roots and shoots, and then rinsed with EDTA to chelate and remove Cd attached to the roots. Subsequently, they were washed with deionized water. Firstly, these samples were dried in the oven at 105 °C for 5 min, secondly, at 70 ° overnight until completely dry. Lastly, these dried plant samples were ground and sieved through a 2-mm sieve. Plants and air-dried soil samples were digested with 87% guarantee reagent HNO3 and 13% guarantee reagent HClO4 (v/v) for detection and analysis. The available Cd concentration in the soil was extracted with 1 mol L−1 MgCl2. Heavy metal concentration was determined using an atomic absorption spectrophotometer (AAS, Hitachi 180). The wavelengths used for Cd, Pb, Cu, and Zn are 228.8, 283.3, 324.8, and 213.8 nm, respectively. The quality control sample certified reference materials were used for total Cd concentration determination, i.e., GBW10015 (GSB-6) for plant sample and GBW07405 (GSS-5) for soil sample, which have standard Cd values of 0.15 mg kg−1 and 0.45 mg kg−1 respectively. The measured values were 0.14 mg kg−1 and 0.46 mg kg−1, which were within expected standard ranges. Common methods were used to determine organic matter, nitrogen, and extractable P, i.e., potassium dichromate method, Kjeldahl, and sodium bicarbonate extraction method (Lu 2000). pH value was determined by a pH meter (PHS-3B), in which the ratio of soil to water was 1:2.5 (v/w) (Wei et al. 2015).
The average and standard deviation (SD) of three replicates for each treatment were performed using Microsoft Excel software, and the analysis of Duncan multiple comparison was performed at p < 0.05 using SPSS 17.0 software (Ma 1990).
Results
Effect of different fertilizers on Cd phytoextraction by S. nigrum
Cd concentrations in roots and shoots of S. nigrum in different nitrogen treatments were listed in Table 2. Cd extraction from shoots (Cd load in shoots, μg pot−1) calculated according to Cd concentration in shoots and shoot biomass was listed in Table 2 either. Compared with the control without N addition, Cd concentrations in the roots and shoots of S. nigrum did not change (p < 0.05). However, Cd concentrations in the roots and shoots of S. nigrum were significantly decreased (p < 0.05) in the samples treated with organic fertilizers (chicken manure, pig manure, and commercial organic fertilizer) by 15.6% and 15.1%, 30% and 23.6%, and 20.3% and 16.8%, respectively. The highest decrease was recorded in the pig manure treatment. The change in shoot Cd extraction (μg pot−1) in different treatments was not consistent with Cd concentration in S. nigrum shoots because Cd accumulation capacity (μg pot−1) in plant shoots is a product of concentration and biomass.
Shoot Cd extractions in NH4HCO3 and NH4Cl treatments were equal to 55.76 and 62.17 μg pot−1, respectively, and the latter was significantly higher (p < 0.05) than the former. However, the same Cd shoot extraction of 79.91 and 80.17 μg pot−1 was obtained in (NH4)2SO4 and CH4N2O treatments. Though the addition of chicken manure, pig manure, and commercial organic fertilizer similarly increased (p < 0.05) Cd extraction in shoots of S. nigrum with 45.78, 43.05, and 45.28 μg pot−1 compared with the control at 25.33 μg pot−1, these increases (p < 0.05) were not that high when compared with inorganic fertilizers, indicating the huge effects of shoot biomasses.
Root and shoot biomass of S. nigrum in different treatments
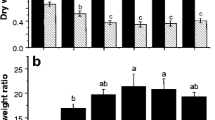
As shown in Fig. 1, root and shoot biomasses of S. nigrum in the control without N addition were 0.19 and 1.06 g pot−1, respectively. The addition of fertilizer significantly increased (p < 0.05) the biomass of S. nigrum. Solanum nigrum root and shoot biomasses in the treatments with chicken manure, pig manure, and commercial organic fertilizer increased 1.10 and 1.12, 1.19 and 1.19, 1.08 and 1.04 times, respectively compared with the control. However, inorganic fertilizer treatments resulted in higher biomass increases. The root and shoot biomasses of S. nigrum in NH4HCO3, NH4Cl, (NH4)2SO4, and CH4N2O treatments increased by 1.17 and 1.19, 2.24 and 1.48, 3.17 and 1.99, and 3.28 and 2.08 times, respectively, in relation to the control. Particularly, the best and similar effects were observed for (NH4)2SO4 and CH4N2O (p < 0.5). This result was obviously caused by different N availability in the soil between individual inorganic and organic fertilizers. Considering the effects of these fertilizers on Cd concentration in S. nigrum (Table 2), it is obvious that they exerted the greatest effect on plant biomasses (Fig. 1).
The pH value of the soil in different treatments
Figure 2 showed the soil pH in different treatments. After the growth of S. nigrum, soil pH was slightly decreased from 6.94 (original soil) to 6.85 (control) (Fig. 2). Soil pH values after S. nigrum growth did not changed significantly (p < 0.05) in four types of inorganic fertilizer treatments compared with the control (6.85). On the contrary, the addition of three types of organic fertilizers caused significant increases (p < 0.05) in soil pH values (by 4.28%, 4.23%, and 4.14%, respectively), which might be one of the reasons for Cd concentration decrease (Table 2).
The concentrations of extractable Cd in soils under different treatments
Figure 3 illustrates the lack of a significant increase (p < 0.05) of the extractable Cd in polluted soils after the addition of inorganic N fertilizers compared with the control without N addition. In contrast, organic fertilizer treatments (chicken manure, pig manure, commercial organic fertilizer) significantly descended (p < 0.05) the available Cd concentrations by 10.9%, 16.8%, and 12.4%, respectively, compared with the control, which was another main reason for lowered Cd concentration in roots and shoots of S. nigrum (Table 2).
Discussion
Sedum alfredii was reported to act as a Cd hyperaccumulator. In the soil pot culture experiment which used three types of nitrogen fertilizers ((NH4)2SO4, Ca(NO3)2, NH4NO3), Cd phytoextraction capacity (μg pot−1) of S. alfredii was the highest with (NH4)2SO4 addition due to the maximum increase of its biomass (Zhu et al. 2011). This conclusion was consistent with the results in the present experiment (Table 2, Figs. 1, 2, and 3). The effect of Ca(NO3)2 addition was very small (Zhu et al. 2011). However, another experiment concerning winter wheat accumulating Cd from soil showed that Ca(NO3)2 addition significantly increased (p < 0.05) Cd concentrations in the shoots and shoot biomasses (Wangstrand et al. 2007). In our previous experiment, Cd concentration in the shoots and shoot biomass of R. globose were significantly increased (p < 0.05) either (Wei et al. 2015). However, NH4HCO3 and NH4Cl addition in this experiment did not improve S. nigrum Cd accumulation from the soil (Table 2). In fact, root exudates of different hyperaccumulators vary considerably, which has a huge impact on the concentrations of extractable heavy metals in the rhizosphere (Zhou and Song 2004). These results may suggest that the effect of the same inorganic nitrogen fertilizer on Cd accumulation may vary in different plants. Furthermore, the amount of (NH4)2SO4 fertilizer added into the soil had a little effect on soil pH after a long period of hyperaccumulator growth due to the regulation role of root exudates (Zhou and Song 2004).
Wang et al. explored the effect of pig manure vermicompost on S. alfredii Cd hyperaccumulation from soil (Wang et al. 2012). The results showed that the biomass of S. alfredii was largely increased. Thus, the extraction of Cd (μg pot−1) by S. alfredii was significantly increased. However, the addition of pig manure vermicompost significantly decreased Cd concentration in S. alfredii and available Cd concentration in the soil. These results were consistent with the experimental results of the current study. In another experiment, when composted peat was added into the soil, Cd phytoextraction (μg pot−1) of Brassica oleracea and Vicia faba was significantly increased (p < 0.05) due to greatly increased of their biomasses, though Cd concentrations in shoots and available Cd concentrations in soils were slightly decreased (Pichtel and Bradway 2008). These results were similar to the results of this experiment (Table 2, Figs. 1 and 3).
There were many factors affecting the available Cd concentration in the soil, and pH is undoubtedly one of the most important ones (Barancíkova et al. 2004). Soil pH value and effective utilization of Cd and plant uptake are tightly associated (Tudoreanu and Phillips 2004). Within a certain range, the Cd available concentration in the soil was shown to decrease with increasing pH (Kim et al. 2009). The addition of organic nitrogen fertilizer also resulted in such trend in the present experiment (Fig. 2). In fact, the pH value of the soil polluted by heavy metals increased after adding bio-fertilizer, and the bioavailability and extraction ability (μg pot−1) of heavy metals in plants decreased due to organic matter’s immobilization (Houben et al. 2013). For example, when pig manure was added to the soil, the available Cd and Pb concentrations in the soil and Cd and Pb concentrations in Helianthus annuus were significantly decreased (Gunadasa et al. 2012), which might be the main reason why organic fertilizer reduced Cd concentration in S. nigrum and the available Cd concentration in the soil in this experiment (Figs. 2 and 3).
There were some field experiments concerning on S. nigrum phytoextraction of Cd after the addition of N fertilizers. In the plot experiment of monoculture and intercrop S. nigrum with low accumulation Welsh onion, the ratio of S. nigrum for Cd removal was about 7% in the intercrop plot when top soil (0–20 cm) Cd concentration was 0.45–0.62 mg kg−1 with combined N, P, and K fertilizers (2:1:1, weight ratio) added in soil before plant growth (Wang et al. 2015). In multiple crop experiment of S. nigrum with low accumulation Chinese cabbage, the average removal rate of S. nigrum to Cd was about 10% without assisted phytoextraction reagent addition for the top soil (0–20 cm) with Cd concentration at 0.53–0.97 mg kg−1 after its grew 90 days under the same condition of combine fertilizers of N, P, and K addition (Niu et al. 2015). Another field phytoremediation experiment was conducted in Huanjiang county of Guangxi province of China where Cd concentration in the soil was 0.71 mg kg−1. The results showed that Cd concentration in S. nigrum was up to 16.83 mg kg−1. The amount of Cd accumulated from the soil by S. nigrum was estimated to be more than 100 g ha−1 under the local normal cropping system and Cd extraction rate of more than 6%. It was assumed that only 9 years was sufficient to clean this contaminated soil. They used NaOH and organic fertilizer to regulate the acidified and Cd-contaminated soil (Zhao et al. 2015). A two-year in situ phytoremediation trial showed that Cd concentrations in the seven experimental plots with S. nigrum decreased from 2.75 to 1.53 mg kg−1, a decrease by 24.9% after 2-year phytoremediation by S. nigrum, and the fertilizer application was the same as in the local agricultural production (Ji et al. 2016). Obviously, it was not enough to draw attention to the strengthening roles of nitrogen fertilizers in these experiments. Therefore, the results of this experiment provided very good reference for further field phytoremediation experiment. However, the remediation potential is still limited compared with people’s expectations. Thus, we still need try to make every effort to improve its accumulation potential in the future.
Conclusion
The ability of Cd phytoextraction (μg pot−1) from polluted soil by S. nigrum itself is limited. The addition of four types of inorganic nitrogen fertilizer (NH4HCO3, NH4Cl, (NH4)2SO4, CH4N2O) and three types of organic fertilizer (chicken manure, pig manure, commercial organic fertilizer) significantly increased (p < 0.05) Cd extracting capacity (μg pot−1) of S. nigrum. Inorganic nitrogen fertilizer had little effect on Cd concentration in S. nigrum and mainly elevated Cd extraction amount (μg pot−1) by greatly increasing its biomass. Organic nitrogen fertilizer significantly reduced S. nigrum Cd concentration, but this decrease was low. When the biomass doubled, Cd extraction (μg pot−1) was eventually greatly increased. The main reason why organic nitrogen fertilizer can significantly reduce Cd concentration in S. nigrum may be the increased soil pH and decreased available Cd concentration in soil. The same fertilizer might play different roles in improving different hyperaccumulators accumulating the same heavy metal. In phytoremediation practice, specific problems need to be resolved and existing research results should not be simply copied.
References
Barancíkova G, Madaras M, Rybar O (2004) Crop contamination by selected trace elements. J Soils Sediments 4:37–42
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manag 105:103–120
Chen B, Ma XX, Liu GQ, Xu XM, Pan FS, Zhang J, Tian SK, Feng Y, Yang XE (2015) An endophytic bacterium Acinetobacter calcoaceticus Sasm3-enhanced phytoremediation of nitrate-cadmium compound polluted soil by intercropping Sedum alfredii with oilseed rape. Environ Sci Pollut Res 22:17625–17635
Chowdhary P, Yadav A, Singh R, Chandra R, Singh DP, Raj A, Bharagava RN (2018) Stress response of Triticum aestivum L. and Brassica juncea L. against heavy metals growing at distillery and tannery wastewater contaminated site. Chemosphere 206:122–131
Dai HP, Wei SH (2018) Transformation process and mechanism of selenium and zinc in Soil-alfalfa. Science Press, Beijing
Dai HP, Wei SH, Twardowska I, Han R, Xu L (2017) Hyperaccumulating potential of Bidens pilosa L. for Cd and elucidation of its translocation behavior based on cell membrane permeability. Environ Sci Pollut Res 24:23161–23167
Gunadasa HKSG, Yapa PI, Nissanka SP, Perera SP (2012) Remediation of Pb/Cd contaminated forest soils by compost and mycorrhizae: will it be a solution to the forest dieback? In: International conference on future. Environ Energy 28:139–144
Guo JM, Lei M, Yang JX, Yang J, Wan XM, ChenTB ZXY, Gua SP, Guo GH (2017) Effect of fertilizers on the Cd uptake of two sedum species (Sedumspectabile Boreau and Sedum aizoon L.) as potential Cd accumulators. Ecol Eng 106:409–414
He S, Wu Q, He Z (2014) Synergetic effects of DA-6/GA3 with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere. 117:132–138
Houben D, Evrard L, Sonnet P (2013) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Huo WM, Zou R, Wang L, Guo W, Zhang DJ, Fan HL (2018) Effect of different forms of N fertilizers on the hyperaccumulator Solanum nigrum L. and maize in intercropping mode under Cd stress. RSC Adv 8:40210–40218
Jalloh MA, Chen J, Zhen F, Zhang G (2009) Effect of different N fertilizer forms on anti-oxidant capacity and grain yield of rice growing under Cd stress. J Hazard Mater 162:1081–1085
Ji PH, Song YF, Jiang YJ, Tang XW, Tong YA, Gao PC, Han WS (2016) A two-year field study of phytoremediation using Solanum nigrum L. in China. Inter J Phytoremediation 18:924–928
Khan MN, Mobin M, Abbas ZK, Alamri SA (2018) Fertilizers and their contaminants in soils, surface and groundwater. Encyclopedia Anthropocene 5:225–240
Kim KR, Owens G, Naidu R (2009) Heavy metal distribution, bioaccessibility and phytoavailability in long-term contaminated soils from Lake Macquarie. Aust J Soil Res 47:166–176
Lan JC, Zhang SR, Lin HC, Li T, Xu XX, Li Y, Jia YX, Gong DS (2013) Efficiency of biodegradable EDDS, NTA and APAM on enhancing the phytoextraction of cadmium by Siegesbeckia orientalis L. grown in Cd-contaminated soils. Chemosphere 91:1362–1367
Li TQ, Tao Q, Liang CF, Yang XE (2014) Elevated CO2 concentration increase the mobility of Cd and Zn in the rhizosphere of hyperaccumulator Sedum alfredii. Environ Sci Pollut Res 21:5899–5908
Li JT, Liang ZW, Jia P, Liu J, Xu YJ, Chen YJ, Shu HY, Kuang JL, Liao B, Shu WS (2017) Effects of a bacterial consortium from acid mine drainage on cadmium phytoextraction and indigenous soil microbial community. Plant Soil 415:347–358
Liu WT, Liang LC, Zhang X, Zhou QX (2015) Cultivar variations in cadmium and lead accumulation and distribution among 30 wheat (Triticum aestivum L.) cultivars. Environ Sci Pollut Res 22:8432–8441
Lu RK (2000) Analysis method of soil agricultural chemistry. Chinese Agricultural S & T Press, Beijing
Luo J, Cai LM, Qi SH, Wu J, Gu XWS (2018) The interactive effects between chelator and electric fields on the leaching risk of metals and the phytoremediation efficiency of Eucalyptus globulus. J Clean Prod 202:830–837
Ma YH (1990) Field experiment and statistic methods. Agriculture Press, Beijing
Niu MF, Wei SH, Bai JY, Wang SQ, Ji DD (2015) Remediation and safe production of Cd contaminated soil via multiple cropping hyperaccumulator Solanum nigrum L. and low accumulation Chinese cabbage. Int J Phytoremediation 17:657–661
Pan F, Meng Q, Wang Q, Luo S, Chen B, Khan KY, Yang X, Feng Y (2016) Endophytic bacterium Sphingomonas SaMR12 promotes cadmium accumulation by increasing glutathione biosynthesis in Sedum alfredii Hance. Chemosphere 154:358–366
Pan FS, Meng Q, Luo S, Shen J, Chen B, Khan KY, Japenga J, Ma X, Yang X, Feng Y (2017) Enhanced Cd extraction of oilseed rape (Brassica napus) by plant growth promoting bacteria isolated from Cd hyperaccumulator Sedum alfredii Hance. Int J Phytoremediation 19(3):281–289
Pichtel J, Bradway DJ (2008) Conventional crops and organic amendments for Pb, Cd and Zn treatment at a severely contaminated site. Bioresour Technol 99:1242–1251
SEQ (Soil environmental quality) (2018) Soil environmental quality risk control standard for soil contamination of agricultural land. GB15618-2018. National standard of China
Tudoreanu L, Phillips CJC (2004) Modelling cadmium uptake and accumulation in plants. Adv Agron 84:121–157
Wang k, Zhang J, Zhu Z, Huang H, Li T, He Z, Yang X, Alva A (2012) Pig manure vermicompost (PMVC) can improve phytoremediation of Cd and PAHs cocontaminated soil by Sedum alfredii. J Soils Sediments 12:1089–1099
Wang SQ, Wei SH, Ji DD, Bai JY (2015) Co-planting Cd contaminated field using hyperaccumulator Solanum nigrum L. through interplant with low accumulation welsh onion. Int J Phytoremediation 17:879–884
Wangstrand H, Eriksso J, Oborn I (2007) Cadmium concentration in winter wheat affected by nitrogen fertilization. Eur J Agron 26:209–214
Wei SH, Zhou QX, Wang X, Zhang KS, Guo GL, Ma L (2005) A newly-discovered Cd-hyperaccumulator Solanum nigrum L. Chin Sci Bull 50:33–33
Wei SH, Li YM, Zhou QX, Srivastava M, Chiu SW, Zhan J, Wu ZJ, Sun TH (2010) Effect of fertilizer amendments on phytoremediation of Cd contaminated soil by a newly discovered hyperaccumulator Solanum nigrum L. J Hazard Mater 176:269–273
Wei SH, Ji DD, Twardowska I, Li YM, Zhu JG (2015) Effect of different nitrogenous nutrients on the cadmium hyperaccumulation efficiency of Rorippa globosa (Turcz.) Thell. Environ Sci Pollut Res 22:1999–2007
Wei SH, Bai JY, Yang CJ, Zhang QR, Knorr KH, Zhan J, Gao QH (2016) Compound amino acids added in media improved Solanum nigrum L. phytoremediating Cd-PAHs contaminated soil. Int J Phytoremediation 18:358–363
Zhao XM, Xie H, Wu K, Yu MH, Yang RG, Li XL (2015) Phytoremediation and health risk assessment of acidified and cadmium contaminated farmland. J Agro-Environ Sci 34:702–708
Zhou QX, Song YF (2004) Remediation of contaminated soils: principles and methods. Science Press, China, Beijing
Zhu E, Liu D, Li JG, Li TQ, Yang XE, He ZL, Stoffella PJ (2011) Effect of nitrogen fertilizer on growth and cadmium accumulation in Sedum alfredii Hance. J Plant Nutr 34:115–126
Zhuang P, Shu WH, Li Z, Liao B, Li JT, Shao JS (2009) Removal of metals by sorghum plants from contaminated land. J Environ Sci 21:1432–1437
Acknowledgments
All authors are grateful for the revision made by Dr. Lidia Skuza from the Department of Molecular Biology and Cytology, Institute for Research on Biodiversity, University of Szczecin, Szczecin 71-415, Poland.
Funding
This work was supported by the National Natural Science Foundation of China (41571300, 31870488, 31270540 and 31070455), the Special Plan in the Major Research & Development of the 13rd Five-Year Plan of China (2018YFC1800501, 2016YFD0800802), Key scientific research project of Shaanxi Provincial Education Department (17JS023, 2018SZS-27-07) and the project of Foreign Experts Bureau of Shaanxi province (GDT20186100430B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, W., Dai, H., Dou, X. et al. Effect and mechanism of commonly used four nitrogen fertilizers and three organic fertilizers on Solanum nigrum L. hyperaccumulating Cd. Environ Sci Pollut Res 26, 12940–12947 (2019). https://doi.org/10.1007/s11356-019-04848-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04848-1