Abstract
Phytoextraction has been considered an effective and environment-friendly method for removing heavy metals from contaminated soil. However, the efficiency, mechanism, and adaptability of phytoextraction by hyperaccumulators in Cd-polluted weakly alkaline soil have not been investigated in detail. In this study, pot experiments were conducted to evaluate the enhanced effects of S,S-ethylenediamine disuccinic acid (EDDS) on phytoextraction in alkaline soil by measuring the degradation kinetic characteristics of EDDS and Cd absorption dynamics of Tagetes patula L. (T. patula) and Phytolacca americana L. (P. americana) for a period of 55 days. Results showed that the half-life of EDDS varied from 4.20–7.07 days and 3.35–4.36 days for T. patula and P. americana, respectively. EDDS-activated Cd reached saturation at a low dosage (1 mM) and a single application of EDDS was found to be better than double applications. The activation of EDDS on Cd applied before 45 days of harvest was better than that before 15 days of harvest, and disappeared after a 35-day application. Correspondingly, the Cd concentration in P. americana and T. patula leaves increased significantly after 3 days of the EDDS application. However, T. patula had a biomass 2.57 times and Cd absorption capacity 10.06 times higher than P. americana. EDDS showed almost no influence on the stem and leaf biomass of T. patula; however, the root weight decreased by 9.44–71.77%. The Cd concentration in T. patula leaves of all the treatments was 1.00–1.81 times that of the control group. In comparison with other treatments, the EDDS application (3 mM) before 15 days of harvest extracted the highest amount of Cd (601.45 μg/pot) in T. patula shoots, reaching 1.40 times that in the control group. Therefore, T. patula might be a more suitable phytoremediator for Cd-polluted alkaline soil than P. americana; the most effective method was the EDDS application (3 mM) before 15 days of harvest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past three decades, China’s rapid industrialization and urbanization process have led to heavy metal pollution in farmland, which has attracted wide public attention at home and abroad (Zhao et al. 2015; Qu et al. 2016). Wheat is one of the three most important food crops in China, but it has been reported that wheat production in China is also facing the threat of heavy metal pollution (Chen et al. 2017; Li and Zhou 2019), of which Cd pollution is the most serious. In recent years, Cd pollution of wheat in some areas of the Henan province has attracted wide public attention (Guo et al. 2018; Li et al. 2019). Therefore, it is crucial to increase research on Cd pollution control technology in fields to ensure the safe production of wheat.
Several studies have investigated Cd pollution control technology in farmlands, especially in acidic rice fields in southern China. The existing methods include water management (Li and Xu 2015; Lei et al. 2018); foliar modulators (Saifullah et al. 2013; Chen et al. 2018); the application of biochar (Beesley et al. 2011; Ali et al. 2019), clays (Xu et al 2017; Liang et al. 2019), and nanoparticles (Rizwan et al. 2019); and phytoremediation (Ashraf et al. 2019). However, wheat-producing areas in China are mainly distributed in the north, and the soil in these areas is primarily weakly alkaline. In comparison with Cd-polluted acidic soils, there are few studies based on control technology for Cd-polluted weakly alkaline soils. To date, to the best of our knowledge, almost no effective technology has been proposed for controlling Cd pollution in weakly alkaline soils. Furthermore, since wheat is a dry land crop, it is difficult to reduce the available Cd concentration in the soil by adjusting the pH value of the alkaline soil through pH regulating amendments and water management.
In comparison with other remediation technologies, such as immobilization and agronomic measures, phytoremediation has attracted considerable attention because it can reduce the total amount of Cd in the soil. Currently, many studies are being conducted on techniques for the phytoremediation of Cd-contaminated acidic soils in southern China, including those related to the efficiency of phytoremediation (Liu et al. 2018; Ashraf et al. 2019); enhanced phytoremediation (Luo et al. 2005; Wang et al. 2009; Hseu et al. 2013; Li et al. 2018); physiological and molecular biological mechanisms of uptake, transport, and enrichment (Liu et al. 2017; Cao et al. 2019); detoxification mechanisms (Talebi et al. 2019); and effect of fertilization on phytoremediation efficiency (Guo et al. 2017; Yang et al. 2019). In recent years, phytoextraction of Cd-polluted alkaline soil has been performed. For example, Guo et al. (2017) reported that nitrogen, phosphorus, and potassium fertilizers can improve the phytoextraction of Cd-contaminated alkaline soil; the total amount of Cd removed by Sedum spectabile Boreau was approximately 80–90 μg/plant. Furthermore, Wang et al. (2019) reported that chelator complexes could enhance Amaranthus hypochondriacus L. and remove approximately 90–500 μg/pot of Cd from Cd-contaminated alkaline soil. However, the removal efficiency of heavy metals in weakly alkaline soil is relatively lower than that in acidic soil, and the remediation effect, mechanism, and adaptability of hyperaccumulators on weakly alkaline soil in northern China are not understood in detail. Therefore, it is important to further strengthen the phytoremediation of Cd-polluted weakly alkaline soil and provide control technologies for Cd-polluted wheat fields in northern China.
Tagetes patula L. (T. patula), commonly known as French marigold, is widely used for beautifying the environment and purifying soil (Wei et al. 2012; Liu et al. 2018). Phytolacca americana L. (P. americana) is an herbaceous perennial species that has been identified as a potential hyperaccumulator of trace metals, specifically for Mn and Cd (Peng et al. 2008). Their remarkable capabilities of Cd accumulation, fast growth, and high biomass make T. patula and P. americana potential plant species for Cd accumulation (Liu et al. 2010; Sun et al. 2011; Sun et al. 2018). Therefore, in the present study, T. patula and P. americana were selected as potential hyperaccumulators and S,S-ethylenediamine disuccinic acid (EDDS) was selected as a potential enhancing amendment. Pot experiments were conducted to investigate the phytoextraction efficiency after EDDS was applied, in relation to application dosage, period, and frequency by monitoring the degradation kinetic characteristics of EDDS and the Cd absorption dynamics of the plants. The results of this study will provide important scientific value for the feasibility of phytoremediation of Cd-polluted weakly alkaline soil in the wheat fields of northern China.
Materials and methods
Experimental materials
The soil utilized in the experiment was collected from the surface soil (0–20 cm) of Cd-contaminated agricultural farmlands in the Jiyuan, Henan province, China, which was polluted by the pollution discharge of non-ferrous smelters over an extended period of time. Analytical reagent grade EDDS was purchased from Tianjin, which was dissolved in NaOH and adjusted to 7.0 ± 0.1 by HCl. T. patula and P. americana seeds were purchased from Shenyang Agricultural University.
Pot experimental design
From March 26, 2019 to June 21, 2019, pot experiments were conducted in a greenhouse at the Agro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs of China. The soil was thoroughly mixed and sieved through a 2-mm sieve. The pot experiments were performed using plastic pots (20 cm in diameter and 18 cm in height) placed over plastic plates, and then 5 kg of dry soil was placed in each plastic pot. The soil in each pot was fertilized manually with ammonium bicarbonate, calcium superphosphate, and potassium sulfate at the doses of 0.112 g/kg N, 0.045 g/kg P2O5, and 0.067 g/kg K2O, respectively, and then equilibrated for 10 days.
To determine the effects of the application dosages, periods, and frequency of EDDS on the phytoextraction efficiency of plants, the treatments were divided into three groups: a single application of EDDS 45 days before harvest (5/06/2019, group A); a single application of EDDS 15 days before harvest (6/06/2019, group B); and two applications of EDDS, one 45 days and one 15 days before harvest (group C). Ten treatments were set as follows: (i) control (CK); (ii) 1 mmol/kg (mM) EDDS applied 45 days before harvest (1A); (iii) 3 mM EDDS applied 45 days before harvest (3A); (iv) 5 mM EDDS applied 45 days before harvest (5A); (v) 1 mM EDDS applied 15 days before harvest (1B); (vi) 3 mM EDDS applied 15 days before harvest (3B); (vii) 5 mM EDDS applied 15 days before harvest (5B); (viii) 1 mM EDDS applied twice, one 45 days and one 15 days before harvest (1C); (ix) 3 mM EDDS applied twice, one 45 days and one 15 days before harvest (3C); and (x) 5 mM EDDS applied twice, one 45 days and one 15 days before harvest (5C). At the abovementioned times, 500 mL of EDDS solution was homogeneously added to the pots at dusk. An equal volume of distilled water instead of EDDS solution was added to the control group. During the growth period, distilled water was added to each pot to maintain growth.
The absorption dynamics of Cd in T. patula and P. americana were measured before and after the application of EDDS at varying intervals. Each treatment was replicated twelve times, four of which were used for the final harvest, and the remaining were used for sampling at different growth periods. The pots were arranged in a randomized block every 2 weeks to obtain consistent lighting.
T. patula seeds were sterilized in 2% (v/v) H2O2 for 15 min, and P. americana seeds were sterilized in H2SO4 for 20 min. Then, they were washed with deionized water and sowed directly into vermiculite concretes in March 2019. Six T. patula seedlings with a similar size and four P. americana seedlings approximately 3 weeks old were transplanted into each pot on April 6, 2019. After 75 days of growth (June 21, 2019), T. patula and P. americana were harvested and separated into roots, stems, and leaves, then washed with tap water to remove the tightly bound soils, followed by washing with deionized water three times. Plant dry weights were recorded after oven drying at 75 °C to constant, then the plants were ground into powders for further analysis.
Soil solution analysis
The sampling devices (Rhizon MOM; length 20 cm, o.d. 2.5 mm, Rhizosphere Research Products, Wageningen, Netherlands) were buried at a 45° angle in the soil when transplanting the plants. Samples were collected from April 26 to June 19, 2019, and sampling time was based on the plant growth before and after the EDDS application with no set interval (April 24, 26, 28, May 5, 8, 13, 16, 22, 27, and June 5, 10, 14, and 19). The soil solutions were divided into two parts. One part was filtered through a 0.45-μm filter membrane immediately after collection, and 5% HNO3 was added to prevent iron oxide/hydroxide precipitation (Huang et al. 2018). The concentration of Cd in the soil solutions was determined utilizing inductively coupled plasma mass spectrometry (ICP-MS) (iCAP Q, Thermo Fisher Scientific, Waltham, MA, USA). The other part was used to measure the pH value using a pH electrode (PB-10, Sartorius, Germany).
Sample analysis
Vegetal sample analysis
Sampling time is based on the growth of plants before and after the EDDS application, including eight sampling times (April 26, May 6, 8, 13, 17, 22, and June 4 and 13). One pot was harvested at a time, and all the leaves were harvested, ground, and mixed for analysis. The remaining four pots were harvested at the final time and divided into roots, stems, and leaves for analysis. Plant tissue powders (0.2500 g) were digested with 8 mL HNO3 through a DigiBlock ED54 digestion system (ED54, LabTech, China), and then deionized water was added up to a volume of 50 mL in a 50-mL flask. The concentration of Cd in the digest solutions was determined using ICP-MS.
Soil sample analysis
Soil pH was determined using a pH meter in a 1:2.5 weight to volume (w/v) ratio of soil to deionized water. Electrical conductivity (EC) of the soil was determined using a conductivity meter (FE38, Mettler Toledo, China) in a 1:5 w/v ratio of soil to deionized water. Cation exchange capacity (CEC) was determined using a cation exchange meter (SKD-300, Peiou, China) that utilizes the ammonium acetate method, whereas total nitrogen was determined using an automatic zotometer (SKD-800, Peiou, China) that utilizes the Kieldahl boiling method. The concentrations of heavy metals in the soil samples (0.2500 g) were digested with 2 mL HF and 8 mL HNO3. The available Cd concentration in the soil was determined via diethylenetriaminepentaacetic acid (DTPA) extraction before the plants were transplanted in a 1:5 w/v ratio of soil to DTPA (0.005 M DTPA, 0.01 M CaCl2, and 0.1 M triethanolamine adjusted to pH 7.3 with HCl) (Norvell 1984).
Calculation of potentially toxic metal distribution and EDDS degradation kinetics
The phytoextraction efficiency was evaluated by calculating the total Cd accumulation amount, bioaccumulation factor (BF), translocation factor (TF), and remediation factor (RF). BF represents the ability of a plant to uptake Cd from the soil into the aerial parts and root systems. TF represents the ability of a plant to transfer Cd from the roots to the aerial parts. RF refers to the percentage of Cd accumulation in aerial parts to that in soil (Sun et al. 2009). The calculation formulae are as follows:
where Caerial and Croot are the concentrations of Cd in the aerial portion and root of the plants (μg/g DW), respectively; Waerial is the plant dry biomass in the aerial portion (g/pot); available-Csoil and total-Csoil are the concentrations of available and total Cd in soil (μg/g), respectively, before the plants were transplanted; and Wsoil is the amount of soil in each pot (g/pot).
EDDS is the most frequently used biodegradable chelating agent, and its half-life effect is defined as the reduction in heavy metals activation through soil amendments over time (Meers et al. 2005). The degradation kinetics can be calculated as follows:
where C0 is the activated Cd concentration at initial activation (μg/L), Ct is the activated Cd concentration at time t (μg/L), k is the degradation constant of the chelating agent, and t is the treatment time in days. Considering the activation lag of EDDS, C0 was calculated utilizing the May 13, 2019, sampling point.
Quality control and statistical analysis
Standard spinach reference materials (SRM 1570a, National Institute of Standards and Technology, NIST) and soil (SRM 2586, National Institute of Standards and Technology, NIST) were used to correct the analytical values, and duplicate and blank samples were included in each batch experiment for quality control purposes.
Data were processed using Microsoft Excel 2010 (Microsoft Corporation, WA, USA), and all figures were made using Origin 9.0 software (OriginLab Corporation, MA, USA). One- and two-way variance analyses were performed utilizing least significant difference and Tukey’s post hoc test to statistically test the significant differences at the 95% confidence interval (P < 0.05) using IBM SPSS Statistics 23.0 software.
Results
The basic physical and chemical characteristics of soil are listed in Table 1. The total Cd concentration in the soil sample was 2.44 mg/kg, which is much higher than 0.6 mg/kg (pH > 7.5, dry land), i.e., the soil environment quality risk control standard for soil contamination of agricultural land published by the Ministry of Ecology and Environment of the People’s Republic of China (GB 15618–2018). The DTPA-extractable Cd concentration was 1.32 mg/kg, indicating that the soil was significantly contaminated by Cd.
Changes of Cd concentration in soil solutions
EDDS was applied to the pots on May 6 and June 6, 2019. To ensure consistency of the experiments, the same amount of distilled water was poured into all treatments the night before sampling. The concentration of Cd in the soil solutions of all treatments for the T. patula and P. americana growing periods is presented in Fig. 1, the concentration of Cd in the control ranged from 0.51 to 4.57 μg/L and 0.29 to 4.01 μg/L in T. patula and P. americana throughout the growth period. Before EDDS addition on May 6, 2019, the concentration of Cd in the soil solutions of all the treatments was below 5 μg/L; thereafter, those with EDDS treatments increased by an order of magnitude, which took approximately 7 days to reach the peak value and then gradually decreased with the degradation of EDDS. According to the results of group A, the activation effects of EDDS on Cd largely dissipated after a 35-day application.
Cd concentration in the soil solution before and after EDDS application in T. patula (left) and P. americana (right). CK = no chelate-treated control, 1A = 1 mM EDDS applied 45 days before harvest; 3A = 3 mM EDDS applied 45 days before harvest; 5A = 5 mM EDDS applied 45 days before harvest; 1B = 1 mM EDDS applied 15 d before harvest; 3B = 3 mM EDDS applied 15 days before harvest; 5B = 5 mM EDDS applied 15 days before harvest; 1C = 1 mM EDDS applied twice, one 45 days and one 15 days before harvest; 3C = 3 mM EDDS applied twice, one 45 days and one 15 days before harvest; and 5C = 5 mM EDDS applied twice, one 45 days and one 15 days before harvest. Values are means ± standard deviations (n = 3)
For group B, before EDDS application on June 6, 2019, the concentration of Cd in the soil solution was similar to that in the control group, whereas it increased rapidly after the EDDS application. At the end of the experiment, the Cd concentration in the group B soil solution was not removed completely. In P. americana, the Cd concentration in the 5A soil solution was higher than that in 1A and 3A soil solutions, whereas there were no differences between 3B and 5B soil solutions, indicating that the activation effects of EDDS on Cd concentration were related to the applied time period. The different trends on the graph among groups A, B, and C were related to the different EDDS application periods and sampling intervals.
For group C, the concentration of Cd after the second EDDS application did not increase significantly compared with the first application. The activation of Cd by EDDS might be related to the plant species. For T. patula, the maximum concentration of Cd in the soil solution was 8112.86 μg/L, which appeared in treatment 3B, while in P. americana the maximum concentration was 4957.39 μg/L and was observed in the treatment 5A. The concentration of Cd in the soil solution decreased with time, which might be due to the strong photodegradation, chemical decomposition, and biodegradation of EDDS in the soil.
As shown in Fig. 1, in group A, the Cd concentration in the soil solution of the two plants gradually declined to the same level as that in the control with the degradation of EDDS. It takes 7 days for EDDS to activate heavy metals fully. Table 2 shows the k-values, corresponding t1/2 values, and observed correlation coefficients of equation (R). The t1/2 value is the time taken to reduce the Cd concentration by half, i.e., the half-life, which is an important parameter when considering the enhancement of phytoextraction. In this study, t1/2 of the EDDS in T. patula and P. americana at different applied dosages were 4.20–7.07 and 3.35–4.36 days, respectively. These results are consistent with Tandy et al. (2006), who determined that EDDS degraded after a lag phase of 7–11 days with a half-life of 4.18–5.60 days. In addition, the t1/2 value of the different treatments of T. patula and P. americana showed a dosage-response effect. This finding is in line with Meers et al. (2005), who reported that the rate of EDDS degradation appeared to be dependent on the applied dose; this was attributed to potential additional metal toxicity at higher mobilization levels, causing slightly lower microbial activity, and therefore resulting in a lower biodegradation rate.
Absorption dynamics of Cd in T. patula and P. americana
The Cd absorption dynamics in plants can directly reflect the effect of EDDS application on soil and plants. Figure 2 shows the absorption dynamics of Cd in the leaves of T. patula and P. Americana before and after the application of EDDS. The sampling points were randomly selected after 20 days of growth for both the plants. For T. patula, the average Cd concentration in the leaves of all treatments before EDDS application on April 26 and May 6 increased from 13.44 ± 0.94 to 26.71 ± 2.78 mg/kg, respectively, and there was no significant difference between treatments (P > 0.05). This increase before EDDS application may be attributed to a decrease in the pH of the soil solution (Fig. S1), which increased the chemical activity of Cd in the soil, thereby resulting in increased Cd uptake by the plants (Luo et al. 2019). The Cd concentration in the leaves of groups A, B and C increased significantly after treatment with EDDS than that in control group (P < 0.05). However, for group C, which had two-time EDDS applications, the Cd concentration in the leaves of T. patula did not significantly enhance than that in groups A and B, which had one-time EDDS application.
Concentration of Cd in T. patula (left) and P. americana (right) leaves before and after the EDDS application. CK = no chelate-treated control, 1A = 1 mM EDDS applied 45 days before harvest; 3A = 3 mM EDDS applied 45 days before harvest; 5A = 5 mM EDDS applied 45 days before harvest; 1B = 1 mM EDDS applied 15 days before harvest; 3B = 3 mM EDDS applied 15 days before harvest; 5B = 5 mM EDDS applied 15 days before harvest; 1C = 1 mM EDDS applied twice, one 45 days and one 15 days before harvest; 3C = 3 mM EDDS applied twice, one 45 days and one 15 days before harvest; and 5C = 5 mM EDDS applied twice, one 45 days and one 15 days before harvest. Different letters in each bar are significantly different at P < 0.05
With the growth of P. americana, the concentration of Cd in the leaves of groups A and C increased significantly compared with the control (P < 0.05). For group B, although the concentration of Cd in the leaves also increased after adding EDDS, it was significantly lower than that in groups A and C. The twice applications of EDDS (group C) did not significantly enhance the Cd concentration in the P. americana leaves in comparison with group A (one-time application), but had a significant enhancing effect compared with group B (one-time application). EDDS showed a significant time-induced effect and the highest concentration of Cd in the P. americana leaves was 13.11 mg/kg.
Effects of EDDS on biomass production of T. patula and P. americana
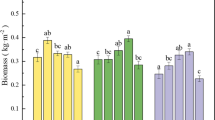
The biomass of the roots, stems, and leaves of T. patula and P. americana at the end of the experiment are shown in Fig. 3. The dry weight of T. patula was higher than that of P. americana in the control group. For T. patula, the dry weight of the roots in the treated group was significantly lower than that in the control group (P < 0.05), except for the 1A treatment, which showed a significant application method- and dosage-induced effects. However, the biomass of the stems and leaves showed no significant changes after EDDS addition, except for the 5C treatment (P > 0.05). The mean biomass of the T. patula stems and leaves were 15.87–20.65 and 12.04–19.61 g/pot, respectively, and the highest biomass of the stems and leaves appeared in the 3A and 1C treatments, which were 4.68 and 5.84 g higher than that in the control group, respectively.
Effects of EDDS application on the roots, stems, and leaves dry weights of T. patula (left) and P. americana (right). CK = no chelate-treated control, 1A = 1 mM EDDS applied 45 days before harvest; 3A = 3 mM EDDS applied 45 days before harvest; 5A = 5 mM EDDS applied 45 days before harvest; 1B = 1 mM EDDS applied 15 days before harvest; 3B = 3 mM EDDS applied 15 days before harvest; 5B = 5 mM EDDS applied 15 days before harvest; 1C = 1 mM EDDS applied twice, one 45 days and one 15 days before harvest; 3C = 3 mM EDDS applied twice, one 45 days and one 15 days before harvest; and 5C = 5 mM EDDS applied twice, one 45 days and one 15 days before harvest. Different letters in each bar are significantly different at P < 0.05. Values are means ± standard deviations (n = 4)
P. americana showed strong toxic symptoms for EDDS, such as yellow leaves, dysplasia, and necrosis, especially in the treatments with high doses of EDDS (treatments 5A and 5C). However, it was shown that the biomass of the stems could be promoted by applying EDDS in group B. For example, the dry weight of the P. americana stems in treatment 3B increased by 7.65 g, while the early addition of EDDS (treatment 3A) significantly inhibited plant growth, and the biomass of the roots and stems decreased by 44.34% and 41.07%, respectively. P. americana showed significant application method- and dosage-induced effects, while the interaction effect was weak and only demonstrated in the stems (P < 0.001).
Effects of EDDS on Cd absorption of T. patula and P. americana
Figure 4 shows the Cd concentration in the roots, stems, and leaves of T. patula and P. americana at the end of the experiment. For T. patula, in group B, the Cd concentration in the roots decreased significantly after the EDDS application than that in control group (P < 0.05), whereas that in group A was not significantly different (P > 0.05). However, the Cd concentration in the T. patula stems decreased gradually with an increase in the EDDS dose for all treatments. In contrast, the Cd concentration in the T. patula leaves harvested from the treated soil increased significantly (P < 0.05), i.e., ranging from 20.14 to 33.74 mg/kg, which was much higher than that in the control group (16.27 mg/kg). Meanwhile, the concentration of Cd in the T. patula leaves had method- and dosage-response effects with EDDS application (P < 0.05). Additionally, for the cases with two-time EDDS applications (group C), no significant increase in the Cd concentration in leaves was observed in comparison with the cases with one-time EDDS application (group B).
Effects of EDDS application on Cd concentration in the roots, stems, and leaves of T. patula (left) and P. americana (right). CK = no chelate-treated control, 1A = 1 mM EDDS applied 45 days before harvest; 3A = 3 mM EDDS applied 45 days before harvest; 5A = 5 mM EDDS applied 45 days before harvest; 1B = 1 mM EDDS applied 15 days before harvest; 3B = 3 mM EDDS applied 15 days before harvest; 5B = 5 mM EDDS applied 15 days before harvest; 1C = 1 mM EDDS applied twice, one 45 days and one 15 days before harvest; 3C = 3 mM EDDS applied twice, one 45 days and one 15 days before harvest; and 5C = 5 mM EDDS applied twice, one 45 days and one 15 days before harvest. Different letters in each bar are significantly different at P < 0.05. Values are means ± standard deviations (n = 4)
For P. americana, EDDS application period significantly influenced the concentration of Cd in the tissues. For example, the concentration of Cd in P. americana of group B was significantly lower than that in group A. Application of EDDS at earlier time points (groups A and C) significantly increased the concentration of Cd in P. americana, which showed the same changing trend in the roots, stems, and leaves. The maximum concentration of Cd in the leaves reached 17.14 mg/kg (treatment 3C), which was 12.30 times that of the control.
Effects of EDDS on Cd accumulation in T. patula and P. americana
The amount of phytoextraction is determined by the concentration of heavy metals and the biomass of the plants, which is calculated according to Formula (1). The Cd accumulation amounts in the stems, leaves, and shoots of the two plants are listed in Table 3. In the control group, the accumulation of Cd in the T. patula shoots (428.64 μg/pot) was much higher than that in the shoots of P. americana (8.21 μg/pot). The maximum accumulation of Cd in the shoots after the EDDS application reached 601.45 μg/pot in treatment 3B (T. patula) and 74.28 μg/pot in treatment 3C (P. americana), which were 1.40 and 9.05 times higher than the control, respectively.
EDDS mainly increased the Cd accumulation in the T. patula leaves, and the accumulation in the stems was lower than in the leaves. EDDS application could not promote or even reduce the Cd accumulation in the stems, which were only increased in treatments 1A and 3A, while that in the P. americana stems was increased to varying degrees in all treatments. Although the biomass of the roots, stems, and leaves of T. patula and P. americana were inhibited to some extent, the addition of EDDS increased the removal of Cd in the soil. The results showed that the total Cd accumulation in the shoots was 3B > 3C> 1C > 5B > 3A > 1A > 1B > CK > 5A > 5C in T. patula, and 3C > 1A> 1C > 3A > 5C > 5A > 5B > 3B >1B > CK in P. americana.
Effects of EDDS on phytoextraction efficiency of T. patula and P. americana
In this study, BF, TF, and RF values of the plants were used to evaluate the effects of EDDS on the accumulation, translocation, and remediation of the two plants. As shown in Table 4, the BF, TF, and RF values of T. patula were greater than 1.0 in the control group and higher than that of P. americana. For T. patula, except for treatments 5A and 5C, BF, TF, and RF values increased significantly. The highest BF and TF values observed in treatment 5B were 12.75 and 12.56, respectively. The highest RF value observed in treatment 3B was 4.81%, which was 1.40 times that of the control. The RF values of T. patula followed the order: 3B > 3C > 1C > 5B > 3A > 1A > 1B > CK > 5A > 5C.
For P. americana, the RF value was much lower than that in T. patula; however, EDDS had a significant synergistic effect on P. americana, and the BF, TF, and RF values significantly increased after the EDDS application (P < 0.05). This enhancing effect showed a significant period-induced effect, and treatments with two-time EDDS applications resulted in higher phytoextraction effects. The maximum values of BF, TF, and RF appeared in treatment 3C, which were 10.25, 7.76, and 8.42 times that of the control, respectively.
Discussion
Degradation kinetics of EDDS in soil
Soil pore water is the critical medium for heavy metal migration and transformation in soil. Cd ion concentration and pH of the soil pore water directly affect the accumulation of Cd in the plants (Qian et al. 2019). In the present study, soil Cd in a pot experiment was activated by the addition of EDDS, and its content was monitored in the soil solution in relation to the growth of two plant species (T. patula and P. americana). The activation of Cd by EDDS was not related to the application dosage but was related to the application frequency. The activation of EDDS on Cd at the 1-mM dose was the same as that at the 3-mM dose. The degradation of EDDS is related to many factors, such as microbial activity, soil temperature, soil type, and degree of pollution (Meers et al. 2005, 2008). The different trends shown in groups A and B (Fig. 1) are attributed to the different EDDS application periods, the growth stage of the plants, the rhizosphere microenvironment, and the different sampling intervals.
In group C, the Cd enhancing effect of the second EDDS application was less than the first application. This may be caused by the ligand effect, which is defined as the amount of metal millimolar activated by chelating agents per amount of mmol applied to the soil (Meers et al. 2005), which was close to saturation after the first application. Guo et al. (2019) also found that in alkaline soil (pH = 8.39), the one-time application of EDTA and citric acid performed more effectively extracting metal from soil, as higher concentrations were determined in almost all treatments than in treatments with two-time applications. However, Hu et al. (2019) found that in uranium (U)-contaminated soil, the U concentration of the soil solution increased significantly with repeated applications of the chelates compared with a one-time application. These different results may be related to soil types, heavy metal elements, and detection methods.
Compared with the control, the addition of EDDS had little effect on the soil pH value of the two plants (Fig. S1), but in the early growth stage, the pH value with T. patula decreased slightly, possibly related to hyperaccumulators, which can acidify the rhizosphere and secrete carboxylate exudates (Li et al. 2011; Liu et al. 2017). Sun et al. (2018) reported that soil pH is an important chemical factor that influences Cd bioavailability and uptake by T. patula. These results provide a basis for the fact that the activation effects of EDDS on Cd reached a saturation state at a low dosage. A one-time application is better than two-time applications, which explains the results of the absorption and accumulation of Cd in the plants. However, the Cd in the soil solutions at the last sampling point in groups B and C were much higher than that in the control, which indicated potential leaching risks. A previous study also reported that long-term contact with EDDS might produce better phytoextraction results (Meers et al. 2005); therefore, application of EDDS at an earlier point before harvest may yield better results in terms of phytoextraction and the environment.
Absorption dynamics of Cd in T. patula and P. Americana
The concentration of Cd in plants depends on its concentration in the soil solutions. The concentration of Cd in the soil solution increased significantly after the EDDS application, and there was a significant increase in Cd concentration in the above ground parts of T. patula (Fig. 2). However, the concentration of Cd in the control decreased at some sampling points, which might be due to the dilution of the plant growth, resulting in a slight change in Cd concentration in the leaves.
For P. americana, the concentration of Cd in plants increased rapidly for 3 days after the EDDS application and lasted for 30 days, which could be related to the higher Cd concentration in the soil solution. McBride and Zhou (2019) found that the concentration of Cd in the P. Americana shoots in the field and greenhouse experiments was less than 10% of that in a hydroponic experiment. The results indicated that the concentration of Cd in P. americana is highly dependent on the concentration of Cd in the soil solution. Contact time between EDDS application and plant harvest also played an important role for P. americana. The same result was reported by Meers et al. (2005), who found that the accumulation level of heavy metals in Helianthus annuus L. (H. annuus) shoots increased significantly after 3 weeks of EDDS application. In addition, transpiration might be the driving force of heavy metal uptake and transport by plants (Salt et al. 1995). The increase in transpiration usually leads to an increase in Cd concentration in the shoots. In this study, the daily water demand of P. americana was lower than that of T. patula. This indirectly indicated that the transpiration of T. patula was higher than that of P. americana, which further led to an increase in Cd concentration in the T. patula leaves. Liu et al. (2010) also showed that transpiration plays an important role in Cd accumulation in the P. americana shoots. Therefore, higher Cd concentrations in the soil solution, sufficient exposure time, and higher transpiration between treatment and harvest are necessary conditions to promote Cd absorption in P. americana.
There were significant differences in Cd uptake between the two species; however, for both plants, the Cd-enhancing effects of a one-time EDDS application in the plants was equal to a two-time application. This finding is consistent with the results of Cd activation in the soil solution that a one-time application was more cost-effective than a two-time application.
Dry biomass of T. patula and P. americana
In this study, T. patula have fast growth and high biomass characteristics; however, EDDS decreased the root biomass of T. patula and P. americana significantly. The activation of EDDS on heavy metals resulted in an increase in the potential toxic metal concentration in the soil, which exceeded the ability of plants to activate defense systems and inhibited growth (Li et al. 2015). Xu et al. (2007) found that the phytotoxicity of the chelator is larger than that of the chelator-metal complex, which might be because chelators can extract the necessary cations from the plasma membrane of root cells, leading to the damage of root cells, and allowing free metals and chelated metals to enter the plants. The EDDS-Cd complex or Cd ion can enter the root and be transported rapidly to bud through the endothelial layer. The toxic effects of Cd and EDDS lead to the reduction in the plant’s biomass (Fu et al. 2015).
In this study, a two-time application of 5 mM EDDS (treatment 5C) reduced the dry weight of the T. patula leaves to 68.64% of the control. This finding is consistent with previous studies (Luo et al. 2005; Wang et al. 2019), which reported that EDDS could inhibit the growth of H. annuus and amaranth plants. However, under 1- and 3-mM EDDS applications, there were no significant effects on the dry weight of T. patula stems and leaves. The stem dry weights of P. americana under the 3B and 5B treatments were 1.95 and 1.82 times that of the control, respectively. Li et al. (2015) also reported the same results, with the exception of the 10 mM EDDS application where the biomass of Macleaya cordata did not decrease significantly compared with the control. A previous study reported that the chelate application near the harvest period could avoid detrimental growth depressions (Meers et al. 2005). The results of this study showed that proper EDDS application could promote the growth of plants. These different results may be attributed to the use of different chelators, application period, dosage, and plant species. In addition, the degradation of EDDS can be used as a carbon source by many microorganisms and enhance microbial activity, directly or indirectly, improving plant growth (Yang et al. 2013).
Uptake and accumulation of Cd in soil by T. patula and P. Americana
The Cd uptake enhancing effects of EDDS showed a period-induced effect in the present study; however, it was different between T. patula and P. americana. For T. patula, Cd uptake was greater with an EDDS application 15 days before harvest than 45 days before harvest, which is the complete opposite for P. Americana. Therefore, it is necessary to choose suitable plants and enhancing methods for Cd phytoextraction in alkaline soil.
In this study, the total Cd accumulation in both plants treated with 5 mM EDDS was lower than that for the 3 mM EDDS treatment. Guo et al. (2019) also found the same result that low doses of EDDS produced better phytoextraction efficiency in potherb mustard, this might be related to the toxicity of EDDS on plants. The maximum Cd accumulation in T. patula was 601.45 μg/pot in treatment 3A. Therefore, this study suggests that T. patula is more suitable for the remediation of Cd-contaminated alkaline soil and the application of EDDS at a concentration of 3 mM 15 days before harvest might produce better phytoextraction efficiency. However, the Cd accumulation in T. patula in acidic soil (pH = 5.1, Cd = 20 mg/kg) was 1392 μg/plant in a previous study (Wei et al. 2012), which was relatively higher than 71.44 μg/plant in this study.
Phytoextraction efficiency of Cd by T. patula and P. americana
The phytoextraction efficiency is highly dependent on the soil condition, available Cd content, and hyperaccumulator plant species, and it is determined by BF, TF, and RF values, which were used to assess the accumulation, translocation, and remediation efficiency of plants (Sun et al. 2009; Moslehi et al. 2019). In the present study, the TF value of T. patula was significantly higher than 1.0 in all treatments and exhibited the strong ability to transport Cd, which is consistent with Sun et al. (2018). It should be noted that the TF, BF, and RF values in P. americana were far lower than those of T. patula. These values for P. americana amplified significantly after the EDDS application, however, and the maximum TF, BF, and RF values appeared in treatment 3C, which showed that a two-time EDDS application at a concentration of 3 mM produced better results. However, by increasing from 3 to 5 mM EDDS in groups A, B, and C, the RF value for both plants decreased, indicating that a higher EDDS concentration weakens the efficiency of phytoextraction. Wang et al. (2019) also illustrated that for two alkaline soils with different Cd pollution levels, the biomass and Cd concentration of hypochondriacus L. shoots with a 5-mM EDDS treatment were decreased when compared with a 3-mM EDDS treatment, causing the decrease of Cd accumulation amount, BF, TF, and RF values.
For T. patula, the maximum TF, BF, and RF values were observed in different treatments. Sun et al. (2009) demonstrated the same phenomenon for Sedum alfredii Hance (S. alfredii) that addition of 8 mM EDTA to the soil solution increased the BF and TF values but decreased the RF value. In this study, the highest RF value in T. patula (4.81%) was 8.15 times higher than that in P. americana (0.59%), indicating that T. patula has promising remediation potential, and one-time application of 3 mM EDDS to the soil 15 days before harvest could enhance phytoextraction capabilities for Cd-contaminated alkaline soil. T. patula showed a relatively higher RF value (2.48–4.81%) in alkaline soil than previous reports. For example, Moslehi et al. (2019) showed that the RF value of H. annuus in alkaline soil (pH = 7.6, Cd = 1.1 mg/kg) ranged from 1.20 to 1.45%, which was lower than that of S. alfredii (8.97%) in acidic soil (pH = 5.78, Cd = 3.03 mg/kg) (Sun et al. 2009).
Conclusion
The application of EDDS is an effective and environment-friendly method to enhance phytoextraction; however, the phytoextraction efficiency of plants in alkaline soil was relatively low. The results showed that the activation effect of EDDS on Cd in the soil solution was better for a one-time application than a two-time application, and an earlier time application was better than later. Cd in the soil solution significantly increased in 7 days after the EDDS application, then decreased rapidly with EDDS degradation, and disappeared after 35 days of application. Cd absorption dynamics in T. patula and P. americana leaves increased significantly after the EDDS application, which was largely dependent on the Cd in the soil solution and the application methods. T. patula showed higher translocation, accumulation, and remediation capacity for Cd than P. americana. There was no significant inhibition of T. patula growth, and the strengthening role of EDDS on T. patula accumulating Cd was mainly due to the activation of Cd in the soil solution and the increase of plant dry weight. In this experiment, application of 3 mM EDDS 15 days before harvest showed the greatest application potential for Cd phytoextraction in alkaline soil by T. patula.
References
Ali A, Guo D, Arockiam Jeyasundar PGS, Li YM, Xiao R, Du J, Li RH, Zhang ZQ (2019) Application of wood biochar in polluted soils stabilized the toxic metals and enhanced wheat (Triticum aestivum) growth and soil enzymatic activity. Ecotoxicol Environ Saf 184:109635
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol Environ Saf 174:714–727
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282
Cao Y, Feng HY, Sun D, Xu GH, Rathinasabapathi B, Chen YS, Ma LQ (2019) Heterologous expression of Pteris vittata phosphate transporter PvPht1;3 enhances arsenic translocation to and accumulation in tobacco shoots. Environ Sci Technol 53:10636–10644
Chen YR, Zhang QF, Fu BS, Cai SB, Wu JZ, Chen YH (2017) Differences of lead, cadmium and zinc accumulation among Chinese wheat mini-core collections germplasms and screening for low Pb, Cd and Zn accumulative cultivars in grains. J Nanjing Agric Univ 40:393–399 (in Chinese)
Chen R, Zhang CB, Zhao YL, Huang YC, Liu ZQ (2018) Foliar application with nano-silicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ Sci Pollut Res 25:2361–2368
Fu YZ, Lei WR, Shen ZG, Luo CL (2015) Permeability of plant young root endodermis to Cu ions and Cu-citrate complexes in corn and soybean. Int J Phytoremediation 17:822–834
Guo JM, Lei M, Yang JX, Yang J, Wan XM, Chen TB, Zhou XY, Gu SP, Guo GH (2017) Effect of fertilizers on the Cd uptake of two sedum species (Sedum spectabile Boreau and Sedum aizoon L.) as potential Cd accumulators. Ecol Eng 106:409–414
Guo GH, Lei M, Wang YW, Song B, Yang J (2018) Accumulation of As, Cd, and Pb in sixteen wheat cultivars grown in contaminated soils and associated health risk assessment. Int J Environ Res Public Health 15:2601
Guo D, Ali A, Ren CY, Du J, Li RH, Lahori AH, Xiao R, Zhang ZY, Zhang ZQ (2019) EDTA and organic acids assisted phytoextraction of Cd and Zn from a smelter contaminated soil by potherb mustard (Brassica juncea, Coss) and evaluation of its bioindicators. Ecotoxicol Environ Saf 167:396–403
Hseu ZY, Jien SH, Wang SH, Deng HW (2013) Using EDDS and NTA for enhanced phytoextraction of Cd by water spinach. J Environ Manag 117:58–64
Hu N, Lang T, Ding DX, Hu JS, Li CW, Zhang H, Li GY (2019) Enhancement of repeated applications of chelates on phytoremediation of uranium contaminated soil by Macleaya cordata. J Environ Radioact 199-200:58–65
Huang QQ, Xu YM, Liu YY, Qin X, Huang R, Liang XF (2018) Selenium application alters soil cadmium bioavailability and reduces its accumulation in rice grown in Cd-contaminated soil. Environ Sci Pollut Res 25:31175–31182
Lei L, Lv H, Yu Y, Hu RG, Wang RG, Xu YM, Ding YZ, Feng RW, Fan ZL (2018) Performances of water management, foliage dressing, and variation screening in controlling the accumulation of As and Cd and maintaining the concentrations of essential elements in the grains of rice plant. Front Environ Sci 6:3
Li JR, Xu YM (2015) Immobilization of Cd in a paddy soil using moisture management and amendment. Chemosphere 122:131–136
Li XF, Zhou DM (2019) A meta-analysis on phenotypic variation in cadmium accumulation of rice and wheat: implications for food cadmium risk control. Pedosphere 29:545–553
Li TQ, Di ZZ, Islam E, Jiang H, Yang XE (2011) Rhizosphere characteristics of zinc hyperaccumulator Sedum alfredii involved in zinc accumulation. J Hazard Mater 185:818–823
Li CW, Hu N, Ding DX, Hu JS, Li GY, Wang YD (2015) Phytoextraction of uranium from contaminated soil by Macleaya cordata before and after application of EDDS and CA. Environ Sci Pollut Res 22:6155–6163
Li ZW, Zhang RS, Zhang HM (2018) Effects of plant growth regulators (DA-6 and 6-BA) and EDDS chelator on phytoextraction and detoxification of cadmium by Amaranthus hybridus Linn. Int J Phytoremediation 20:1121–1128
Li J, Zhang PY, Ye JP, Zhang GM, Cai YJ (2019) Simultaneous in-situ remediation and fertilization of Cd-contaminated weak-alkaline farmland for wheat production. J Environ Manag 250:109528
Liang XF, Li N, He LZ, Xu YM, Huang QQ, Xie ZL, Yang F (2019) Inhibition of Cd accumulation in winter wheat (Triticum aestivum L.) grown in alkaline soil using mercapto-modified attapulgite. Sci Total Environ 688:818–826
Liu XQ, Peng KJ, Wang AG, Lian CL, Shen ZG (2010) Cadmium accumulation and distribution in populations of Phytolacca americana L. and the role of transpiration. Chemosphere 78:1136–1141
Liu X, Fu JW, Da Silva E, Shi XX, Cao Y, Rathinasabapathi B, Ma LQ (2017) Microbial siderophores and root exudates enhanced goethite dissolution and Fe/As uptake by As-hyperaccumulator Pteris vittata. Environ Pollut 223:230–237
Liu ZL, Chen W, He XY (2018) Evaluation of hyperaccumulation potentials to cadmium (Cd) in six ornamental species (compositae). Int J Phytoremediation 20:1464–1469
Luo CL, Shen ZG, Li XD (2005) Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59:1–11
Luo JP, Liu YY, Tao Q, Hou Q, Wu KR, Song YC, Liu YK, Guo XY, Li JX, Hashmi M, Liang YC, Li TQ (2019) Successive phytoextraction alters ammonia oxidation and associated microbial communities in heavy metal contaminated agricultural soils. Sci Total Environ 664:616–625
McBride MB, Zhou YT (2019) Cadmium and zinc bioaccumulation by Phytolacca americana from hydroponic media and contaminated soils. Int J Phytoremediation 21:1215–1224
Meers E, Ruttens A, Hopgood MJ, Samson D, Tack FMG (2005) Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58:1011–1122
Meers E, Tack FMG, Verloo MG (2008) Degradability of ethylenediaminedisuccinic acid (EDDS) in metal contaminated soils: implications for its use soil remediation. Chemosphere 70:358–363
Moslehi A, Feizian M, Higueras P, Eisvand HR (2019) Assessment of EDDS and vermicompost for the phytoextraction of Cd and Pb by sunflower (Helianthus annuus L.). Int J Phytoremediation 21:191–199
Norvell WA (1984) Comparison of chelating agents as extractants for metals in diverse soil materials. Soil Sci Soc Am J 48:1285–1292
Peng KJ, Luo CL, You WX, Lian CL, Li XD, Shen ZG (2008) Manganese uptake and interactions with cadmium in the hyperaccumulator-Phytolacca Americana L. J Hazard Mater 154:674–681
Qian TT, Wu P, Qin QY, Huang YN, Wang YJ, Zhou DM (2019) Screening of wheat straw biochars for the remediation of soils polluted with Zn (II) and Cd (II). J Hazard Mater 362:311–317
Qu CD, Shi W, Guo J, Fang BB, Wang S, Giesy JP, Holm PE (2016) China’s soil pollution control: choices and challenges. Environ Sci Technol 50:13181–13183
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Zia Ur Rehman M, Waris AA (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Saifullah SN, Bibi S, Ahmad M, Sik Ok Y (2013) Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum Aestivum L.). Environ Earth Sci 71:1663–1672
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Sun YB, Zhou QX, An J, Liu WT, Liu R (2009) Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma 150:106–112
Sun YB, Zhou QX, Xu YM, Wang L, Liang XF (2011) Phytoremediation for co-contaminated soils of benzo [a] pyrene (B [a]P) and heavy metals using ornamental plant Tagetes patula. J Hazard Mater 186:2075–2082
Sun RL, Sun QQ, Wang RQ, Cao LD (2018) Cadmium accumulation and main rhizosphere characteristics of seven French marigold (Tagetes patula L.) cultivars. Int J Phytoremediation 20:1171–1178
Talebi M, Tabatabaei BES, Akbarzadeh H (2019) Hyperaccumulation of Cu, Zn, Ni, and Cd in Azolla species inducing expression of methallothionein and phytochelatin synthase genes. Chemosphere 230:488–497
Tandy S, Schulin R, Nowack AB (2006) Uptake of metals during chelant-assisted phytoextraction with EDDS related to the solubilized metal concentration. Environ Sci Technol 40:2753–2758
Wang X, Wang Y, Mahmood Q, Islam E, Jin XF, Li TQ, Yang XE, Liu D (2009) The effect of EDDS addition on the phytoextraction efficiency from Pb contaminated soil by Sedum alfredii Hance. J Hazard Mater 168:530–535
Wang K, Liu YH, Song ZG, Wang D, Qiu WW (2019) Chelator complexes enhanced Amaranthus hypochondriacus L. phytoremediation efficiency in Cd-contaminated soils. Chemosphere 237:124480–124488
Wei JL, Lai HY, Chen ZS (2012) Chelator effects on bioconcentration and translocation of cadmium by hyperaccumulators, Tagetes patula and Impatiens walleriana. Ecotoxicol Environ Saf 84:173–178
Xu Y, Yamaji N, Shen RF, Ma JF (2007) Sorghum roots are inefficient in uptake of EDTA-chelated lead. Ann Bot 99:869–875
Xu Y, Liang XF, Xu YM, Qin X, Huang QQ, Wang L, Sun YB (2017) Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 27:193–204
Yang L, Wang GP, Cheng ZN, Liu Y, Shen ZG, Luo CL (2013) Influence of the application of chelant EDDS on soil enzymatic activity and microbial community structure. J Hazard Mater 262:561–570
Yang W, Dai HP, Skuza L, Wei SH (2019) Strengthening role and the mechanism of optimum nitrogen addition in relation to Solanum nigrum L. Cd hyperaccumulation in soil. Ecotoxicol Environ Saf 182:109444–109450
Zhao FJ, Ma Y, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759
Funding
This work was supported by the Science and Technology Innovation Project from the Chinese Academy of Agricultural Sciences (No. CAASXTCX-xym-2018), and the China Agriculture Research System (CARS-03-25).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 77 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Xu, Y., Qin, X. et al. Effects of EDDS on the Cd uptake and growth of Tagetes patula L. and Phytolacca americana L. in Cd-contaminated alkaline soil in northern China. Environ Sci Pollut Res 27, 25248–25260 (2020). https://doi.org/10.1007/s11356-020-08877-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08877-z