Abstract
Several technologies and methods have been developed over the years to address the environmental pollution and nutritional losses associated with the dumping of fish processing waste and low-cost fish and by-products. Despite the continuous efforts put in this field, none of the developed technologies was successful in addressing the issues due to various technical problems. To solve the problems associated with the fish processing waste and low-value fish and by-products, a process called pH shift/acid and alkaline solubilization process was developed. In this process, proteins are first solubilized using acid and alkali followed by precipitating them at their isoelectric pH to recover functional and stable protein isolates from underutilized fish species and by-products. Many studies were conducted using pH shift process to recover proteins from fish and fish by-products and found to be most successful in recovering proteins with increased yields than conventional surimi (three cycle washing) process and with good functional properties. In this paper, problems associated with conventional processing, advantages and principle of pH shift processing, effect of pH shift process on the quality and storage stability of recovered isolates, applications protein isolates, etc. are discussed in detail for better understanding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been a gradual increase in exploitation of world fish stocks and no further increase in the fish catches can be expected in any near future. Majority of the catch is composed of small-sized fish that is rich in fat, bones, and dark muscle, which is unfit for human consumption and is generally used for cattle feeding purposes or discarded (FAO 2010). Fish processing generates large amounts of solid waste (30–85% of processed fish) depending upon the type of fishery (Chandra and Shamasundar 2011), which is generally discarded. This processing waste, low-cost fish, and by-products contain valuable nutrients which can be used for human edible purposes; otherwise, which is a serious loss to human kind. Utilization of these wastes not only addresses the environmental pollution problems but also contributes to food security.
Decrease in marine fish catch and increasing demand for fish and fish-based products which are inexpensive and nutritionally rich led to the production of protein ingredients from small underutilized fish species and by-products (Azadian et al. 2012). Several technologies and methods have been developed over the years to isolate proteins from fish muscle and by-products, many of which caused loss of functional properties of recovered proteins. One of such cases is production of surimi, for which the demand is very high but the traditional resources are limited. In order to meet the demand for surimi, other sources like low-value dark muscle species and their by-products have to be used. Using these raw materials and applying conventional surimi processing for recovery of proteins have not been successful and met with numerous technical problems (Hultin and Kelleher 2000a). To solve the problems associated with utilization of low-value fish and by-products, which are not suitable for surimi production, an alternate process was developed. This process, i.e., acid and alkali solubilization/pH shift process/isoelectric solubilization and precipitation, employs solubilization of the proteins using acid and alkali followed by precipitating them at their isoelectric pH to recover functional and stable protein isolates from underutilized fish species and by-products (Hultin and Kelleher 1999, 2000a, 2000b).
There are three main advantages with the acid-alkaline solubilization over traditional methods. Firstly, the process of mechanical deboning or separation of meat from skin and bones can be eliminated as crushed or minced raw materials can be used directly in this process. Secondly, the sarcoplasmic proteins are also recovered in this process that increases the protein yield, which is not possible in the case of surimi. Thirdly, the membrane lipids and neutral lipids can be efficiently removed in this process by minimizing the risk of oxidation of lipids (Nolsøe and Undeland 2009). Another advantage is that the wastewater produced from acid and alkaline solubilization process contains low solid content, nitrogen content, and chemical oxygen demand in comparison with wastes produced during conventional surimi processing (Park et al. 2003).
pH shift processing to recover fish proteins
Principle of pH shift processing
The process uses the principle that “the solubility of a solution containing protein is influenced by the pH of the mixture.” The changes that occur during the process are well explained by Nolsøe and Undeland (2009), Gehring et al. (2011), and Tahergorabi et al. (2012). In general, proteins in a solution are held together by weak protein–protein interactions (Undeland et al. 2003) and the side chains of the proteins can assume different charges with change in pH of the solution. During an increase or a decrease in pH of the protein solution, the side chains can assume strong positive or negative charges, resulting in strong repulsive forces between proteins that pull proteins apart from each other. These strong positive or negative charges will favor the protein–water interactions thus causing the solubility of proteins (Yasothai and Giriprasad 2015). Solubility of myofibrillar proteins that are bound in myofibrillar segments and bundles depends on electrostatic and hydrophobic interactions in the protein solution. When the hydrophobic reactions take over electrostatic interactions, the protein molecules get extracted (Zayas 1997). By creating the favorable and unfavorable conditions, the solubility of proteins can be turned off or on, respectively (Tahergorabi et al. 2012). Addition of acid will increase net positive charge on side chains of glutamyl and aspartyl residues through the addition of hydronium ions (H3O+), while addition of alkali will increase net negative charge on side chains of tyrosyl, tryptophenyl, cysteinyl, lysyl, arginyl, and histidinyl residues through the addition of hydroxyl ions (OH−) (Tahergorabi et al. 2012). In the same way, fish proteins get solubilized due to positive charges on aspartyl and glutamyl residues at acidic pH and negative charges on lysyl, tyrosyl, and cysteinyl residues at basic pH. When a protein solution reaches the stage of homeostasis due to the equilibrium in charges, the final status of a surface electrostatic charge of proteins at a given pH is termed as the net charge. The formation of net negative or net positive charges on protein surface results in protein–protein electrostatic repulsions and an increase in hydrodynamic volume due to swelling and expansion (Undeland et al. 2003; Kristinsson et al. 2005). As the charges on protein molecule become more positive or negative, they start interacting with water, which simultaneously decreases the protein–protein interactions. So when the protein assumes more polarity (charged molecule), more interaction will take place with water thus causing the solubilization.
Protein assumes zero net surface charge when the number of surface positive charges is equal to negative charges. The pH at which the net surface electric charge is equal to zero is called as the isoelectric point (pI) of that protein. The pI of the proteins is specific and isoelectric focusing technique is used to find the pI of proteins (Tahergorabi et al. 2015). When the protein reaches its pI, the surface net charges will diminish. It results in reduction of protein–water interaction causing reduction in protein solubility in water and low water holding capacity and poor gelling capacity. Simultaneously, hydrophobic protein–protein interactions will be favored when the pH moves towards pI, causing precipitation of proteins. This behavior of proteins at pI is used to switch on or switch off protein solubility. In general, protein solubility curves are used to determine the pI of particular protein. Many researchers have used the isoelectric behavior of fish proteins to recover functional proteins from different fishes, their by-products, and low-value species (Batista 1999; Cortes-Ruis et al. 2001; Undeland et al. 2002; Batista et al. 2003; Kristinsson and Demir 2003; Kristinsson and Hultin, 2002; Kristinsson et al. 2005; Nolsøe et al. 2007; Palafox et al. 2009; Nolsøe et al., 2011; Marmon 2012; Jafarpour et al. 2013; Shabanpour et al. 2015a) (Fig. 1).
A protein at its isoelectric point (pI) has a zero net electrostatic charge (adopted from Tahergorabi et al. 2015). a At its pI, protein–water interactions are at its minimum, while protein–protein interactions via weak hydrophobic bonds are at its maximum, causing protein precipitation. b Protein–water interactions prevail under acidic or basic conditions far from the pI, resulting in protein solubility. (Reprinted with permission from Tahergorabi et al. 2015)
Methodology to recover fish proteins
The raw material such as fish meat, by-product, or processing waste has to be initially kept at lower temperatures, preferably < 4 °C to avoid degradation or denaturation of proteins. The raw material has to be ground to fine paste using mortar grinder or chopper. Care must be taken to avoid the rise in raw material temperature during grinding, which can be controlled by doing the whole operation in cold room or in the presence of ice. Ground material has to be mixed with 1–10 volumes of cold deionized water (< 4 °C) and homogenized with a high speed homogenizer. The solubility pattern of a particular protein must be known to adjust the pH to its solubility range, which requires constructing a solubility curve. A protein solubility curve can be constructed by adjusting the pH of homogenate to different pH, generally ranging from 1.5 to 13.0, and measuring the solubility of protein at each pH. In general, proteins solubility will be increased towards alkaline and acidic side from the neutral pH.
pH with maximum and minimum solubility can be obtained from the solubility curve and homogenate will be adjusted to the pH of highest solubility using either acid (for acidic pH) or alkali (for alkaline pH). Hydrochloric acid and sodium hydroxide are most commonly used to adjust the pH of fish proteins as they are generally regarded as safe (GRAS). The pH-adjusted homogenate will be left for some time to aid solubilization of proteins followed by centrifugation at high speed using a refrigerated centrifuge. After the centrifugation, the supernatant will be filtered through a double-layered fine cloth to separate it from the insoluble bones, skin, scales, etc. The separated supernatant will be readjusted to the pH with lowest solubility (isoelectric point) using acid/alkali and left for some time to aid precipitation of proteins. It is followed by centrifugation at high speed in a refrigerated centrifuge and filtration of supernatant through double-layered fine cloth to separate it from the precipitated proteins. The recovered proteins need to be stored under frozen condition till further use (Fig. 2).
Characteristics of fish protein isolates
Solubility
The protein solubility is considered as a prerequisite for other functional properties like emulsification and gelation. Fish muscle protein solubility is often used as a criterion for the protein alterations (Zayas 1997). During isoelectric solubilization, high solubility is needed to separate them from impurities, whereas low solubility is needed during precipitation for the maximum recovery of solubilized proteins (Kristinsson et al. 2005). Solubility pattern of proteins from some fishes under pH shift processing is given in Table 1, and the reasons for the differences in protein solubility are discussed below for better understanding.
Different studies reported that the maximum solubility of fish proteins was found at pH 2.0 to 3.0 from acidic side and pH 11.0 to 13.0 from alkaline side, with minimum solubility at pH 5.0 to 6.0, which is the isoelectric point of most of the proteins. Some of the findings reported a sharp change in solubility between pH 3.5 and 4.0 and pH between 9.5 and 11.0. Some of the reasons explained for the differences in solubility of proteins from different fishes are discussed below. The differences in solubility between different species are related to the species and the prior treatments given to the raw material (Batista 1999). The minimum solubility of fish proteins at pH 5.0 to 5.5 might be due to myosin, which is a major myofibrillar protein with isoelectric pH between 5.0 and 5.5. Above and below this pH range, the proteins will get more positive or more negative charge, thus increasing the solubilization due to electrostatic repulsions (Hamm, 1994; Kelleher and Hultin 1994). The sudden increase in solubility between pH 2.5 and 7.0 compared to gradual increase in solubility between pH 7.0 and 11.0 might be because of more ionizable groups with pKa values between pH 2.5 and 7.0 (Undeland et al. 2002). Low protein solubility during pH shift process might be the result of denaturation of proteins during pH shift process (Rawdkuen et al. 2009). Protein denaturation results in a decrease in solubility and an increase in hydrophobic interactions thus causing the precipitation of proteins (Zayas 1997).
Some of the findings reported that solubility of proteins was more in alkaline-aided process than acid-aided process (Zayas 1997; Rawdkuen et al. 2009). Kristinsson and Liang (2006) stated that improper unfolding of proteins could reduce the solubility, which might be the reason behind reduced solubility during acid-aided solubilization. But Kristinsson and Hultin (2003) made it clear that acidic or alkaline unfolding of cod myosin did not show any effect on solubility, when it was refolded at pH 7.5. The solubility of proteins depends on hydrophobic or polar nature of the amino acids, pI-pH relationship, and structural status or denaturation of the proteins (Gehring et al. 2009).
Yield
The protein recovery or yield is an important parameter, which determines the economical feasibility of pH shift method. The protein yields obtained from different species and by-products using isoelectric solubilization method are given in Table 2. The differences in protein recoveries or yields may be because of the differences in methods used to determine protein concentration, fish species used, centrifugation force, and a relative concentration of sarcoplasmic proteins (Gehring et al. 2011).
The recoveries will be influenced by several factors like solubility at acid and alkaline pH, the size of the sediments formed during the centrifugation process, and solubility at precipitation or isoelectric pH. Among these, the solubility at acid and alkaline pH is the main factor that influences recoveries. Some of the studies indicated that pH shift processing results in higher protein recoveries compared to the conventional surimi processing (Kristinsson et al. 2005; Rawdkuen et al. 2009; Ibrahim 2015), while some reports showed that acid-aided process results in higher protein recoveries than alkaline-aided process (Kristinsson and Hultin, 2002; Rawdkuen et al. 2009) during pH shift processing. The reason for high protein recovery in pH shift process compared to protein recovery using conventional surimi processing was explained by Choi and Park (2002). Sarcoplasmic proteins in muscle are water soluble in nature, which will be removed during washing steps of surimi processing. Excess washing will result in solubilization and loss of the myofibrillar proteins thus by reducing the yields. As sarcoplasmic proteins will be retained during pH shift processing, maximum yields can be obtained compared to surimi processing (Choi and Park 2002).
The recoveries using acid and alkaline solubilization methods were reported to vary between 31 and 98%. The reasons for the differences in protein recoveries of various species during pH shift processing are attributed to the differences in raw material composition, differences in process variables, and the conditions. In some of the studies, acid solubilization process was found to have more protein yields (Undeland et al. 2002; Batista et al. 2003; Kristinsson et al. 2005; Kristinsson and Liang, 2006; Shabanpour et al., 2015b; Surasani et al., 2017a) compared to alkaline-aided processing, while the other studies indicated that the protein yield was maximum in alkaline-aided processing (Batista et al. 2003; Kristinsson and Ingadottir 2006; Palafox et al. 2009; Nolsøe et al., 2011; Shabanpour et al., 2015a; Surasani et al. 2018) than acid-aided processing. The main reasons for the differences in protein yields between acid and alkaline-aided processing are attributed to (i) denaturation of proteins during solubilization process, resulting in increased hydrophobic interactions and aggregation of the proteins (Zayas 1997). Thus, the aggregated proteins can simultaneously separate together with other bone, skin, and debris from the supernatant during the first centrifugation step. (ii) Different degree of denaturation of the proteins at acidic and alkaline pH results in different degree of refolding. A more extensively denatured protein has more hydrophobic areas exposed and is better able to form more and stronger protein–protein interactions thus precipitating more proteins during the precipitation process. (iii) The amount of lipids in the homogenate. The higher the lipid content in the homogenate, the high will be the lipid-protein interaction thus reducing the protein yield.
Protein pattern
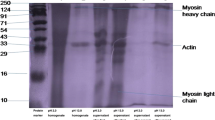
The knowledge on protein pattern or composition is important to select most appropriate and inexpensive method for scaling up the technology. The SDS pattern will be helpful in knowing the protein fractions and to compare the treatments or processes. Several researchers (Undeland et al. 2002; Kristinsson and Hultin, 2002; Palafox et al. 2009) reported that the protein from fish and squid had major fractions of myosin and actin followed by paramyosin and tropomyosin. A band just below the myosin, which might be the result of myosin hydrolysis, was also reported in various studies (Kelleher and Hultin 1994; Takeda and Seki 1996; Kristinsson and Hultin, 2003; Yeung and Jinx-Soo 2005). This might be due to the action of enzymes that got activated at pH 5.5 during precipitation process (Choi and Park 2002).
Several workers (Choi and Park 2002; Undeland et al. 2002; Kristinsson and Demir 2003) reported hydrolysis during pH shift processing especially during acid-aided processing, which might be the reason for the appearance of small bands in the isolates made through acid-aided processing. SDS PAGE analysis showed no difference between the proteins obtained using acid and alkaline process (Kristinsson and Hultin, 2002; Palafox et al. 2009). The presence of myosin, actin, and paramyosin at higher concentrations in supernatant after centrifugation and in isolates indicates that the proteins have excellent functional properties. The absence of these protein fractions in supernatant after second centrifugation indicates that these proteins got precipitated efficiently.
Amino acid content
The quality of protein is determined by the total number and quantity of the essential amino acids (EAA). A protein is considered good for consumption, when it has all the essential amino acids with a minimum quantity of 287 mg per gram of protein (Food and Nutrition Board of Institute of Medicine, 2005). Amino acid analysis gives a clear picture of composition of the recovered protein. Some of the researchers studied the amino acid profile of proteins recovered using pH shift process. Studies indicated that the isolates obtained through pH shift processing were rich in all the essential amino acids (Batista 1999; Chen et al. 2009; Lee et al. 2016). Batista (1999) during protein recovery from hake waste reported that alkaline-aided extraction did not affect the amino acid profile substantially. They found a slight reduction in proline and glycine contents which are the major amino acids of connective tissue collagen. This was attributed to the non-extractability of connective tissue during alkaline processing. They have also reported that the ratio of EAA to total amino acids increased in isolates suggesting the enrichment of essential amino acids. The similar findings were also reported by Chen et al. (2009) during their studies on krill proteins. They have reported that highest amino acid (EAA and non-EAA) content was found in proteins solubilized at pH 3.0 and 12.0 compared to other pH values. This might be due to the high pH-induced hydrolysis at other pH values, which was minimum at pH 3.0 and 12.0 causing lowest loss of hydrolyzed amino acids.
Lee et al. (2016) reported that yellow fin tuna roe isolates obtained through pH shift processing had high ratio of EAA and non-EAAs (1.08–1.17) than casein (0.92) but it was less than that of hemoglobin (1.33). They found that roe protein isolates were rich in lysine, leucine, glutamine, and asparagines. They have also reported that roe alkaline insolubles had high content of proline and glycine, which might be due to the non-extractability of connective tissues during alkaline process.
Lipid content
During the extraction of proteins through pH shift processing, considerable amounts of lipids also get extracted, which need to be reduced as they may contribute to rancidity if retained with the proteins (Gehring et al. 2011). This is the reason behind reduction of fat to less than 1% during surimi processing. The advantage of pH shift processing over surimi processing is higher reduction in lipid content in the form of surface layers and sediments during centrifugation (Marmon 2012). Lipid reduction in different processes employed for the recovery of proteins from fish and fish by-products is given in Table 3.
Reductions in lipid content sometimes more than 80% also have been reported in pH shift processing (Nolsøe and Undeland 2009). However, the exact reduction in lipid content depends on the composition of raw material used, fish species, and the type of the pH shift process used. Nolsøe et al., 2011 during their studies on blue whiting isolates found that replacing centrifugation with filtration during pH shift process could improve the protein yield and color but also increased the amount of lipids in the isolates. Cold washing and pH shift processing could reduce the lipid content of short-bodied mackerel to a greater extent. Alkaline-aided process could cause more lipid reduction compared to conventional surimi processing (Chaijan et al. 2010). Cortes-Ruis et al. (2001) studied the strategies for lipid removal during surimi production from Bristly sardine. The process involved four washing steps (1:5) with 0.5% sodium bicarbonate solution replacing water in the first wash. They found that the lipid reduction in surimi process was (67.4 ± 6.9%) less than that of pH shift process (88.3 ± 4.8%).
The reason for the differences in lipid reduction was explained as in conventional method during the washing process membrane lipids will be retained and a part of storage lipids co-aggregates with proteins. In pH shift method, proteins will be exposed to high and low pH conditions that cause their solubility thus separating them from storage and membrane lipids. These lipids will be later separated based on density and solubility differences using centrifugation (Kristinsson et al. 2005). There are several reasons explained in support of high lipid reductions in pH shift processing. Final lipid content depends on initial lipid content of raw material, viscosity of homogenate, and the speed of centrifugation that separates the lipids (Park et al. 2003). In conventional surimi processing, membrane lipids have close affinity with cell membrane and some of the storage lipids form complexes with proteins during the washing process resulting in higher lipid content. In pH shift process, proteins get easily separated from lipids which further will be removed based on density differences in centrifugation process Kristinsson et al. (2005). The high lipid reduction in pH shift process might be due to its high emulsification ability (Kristinsson and Hultin 2003).
Though lipids can be removed to a greater extent by pH shift process, the remaining lipids and pro-oxidants are sufficient enough to cause oxidation and quality deterioration in protein products. In order to prevent these changes, the lipid content has to be reduced to as minimum as possible. Pattaravivat et al. (2008) studied the strategies to remove lipids from escolar meat and reported that the temperature greatly affects the lipid removal and the temperature of < 1 °C during alkaline washing and a shorter washing time could greatly remove the lipids from escolar meat. When washed mince was washed again with 0.25% palmitic sucrose ester (P-1670), the lipid content was reduced further in the meat. They could find the better gelling ability of alkaline washed and P-1670-treated surimi compared to conventional surimi. More research is needed in this area to find alternate methods for the greater removal of lipids in proteins obtained through pH shift processing and to retard the lipid oxidation in the protein products.
Myoglobin, hemoglobin, and total pigment content
Chroma is one of the important characteristics of proteins that determine their food applications. The color of muscle foods is due to the presence of chromoproteins that contain a porphyrinic group conjugated with a transition metal. Caroteneproteins and carotene also exist alongside these proteins and play a major role in imparting muscle color (Perez-Alvarez and Fernandez-Lopez, 2006). Researchers have reported that pH shift processing can remove efficiently more myoglobin from the proteins compared to conventional processing (Chen 2003; Rawdkuen et al. 2009; Jafarpour et al. 2013) but the pigment removal ability of pH shift processing is less compared to conventional processing (Jafarpour et al. 2013). Whiteness of extracted proteins is directly related with the pigment removal. This might be the reason for reduced whiteness of isolates compared to conventionally processed surimi (Jafarpour et al. 2013).
Hemoglobin, which is a main catalyst in fish muscle oxidation, is highly pro-oxidative at low pH but it gets stabilized at high pH (Kristinsson and Hultin, 2003). High pH can remove heme proteins more actively than from surimi and can yield a more stable and whiter product (Kristinsson 2002). The higher hemoglobin removal by pH shift processing was explained as pH shift process causes higher degradation of muscle causing myofibrillar proteins come out of the intracellular structure of the muscle during their solubilization, whereas oxidation and insolubility of myoglobin cause lower extractability during conventional processing (Chen 2003; Rawdkuen et al. 2009). Study by Tian et al. (2016) reported that acid and alkaline processes significantly increased the L* and b* values of isolates, while it decreased their a* value resulting in the isolates whiter than fish muscle but less whiter than surimi. The reason behind this color improvement was considered to be the removal of pigments in pH shift processing (Marmon 2012). The decrease in a* values of isolates was explained as the extreme pH in acid and alkaline processing causes change in protein structure thus separating hemoglobin from proteins. Adjusting its pH to isoelectric point during precipitation will cause denaturation, unfolding, and oxidation of the heme proteins resulting in brown color associated with the increased yellowness (Kristinsson et al. 2005).
Abdollahi et al. (2016) reported that alkaline process could remove more hemoglobin from blood-rich fish muscle compared to acid process. The heme protein removal ability was highest during precipitation at pH 6.5, which was further increased with the addition of phytic acid and prewash. Panpipat and Chaijan (2016) during their work on protein isolates from big eye snapper revealed that greater removal of hemoglobin and melanin can be possible with acid-aided pH shift process. They have stated that acid treatment will dissociate the hemoglobin into colorless heme and globin thus increasing the whiteness. In acid-aided process, they observed the disappearance of a soret peak of myoglobin spectra which is an indication of highest hemoglobin. They could observe more whiteness of the isolates obtained by acid process compared to alkaline process. The isolates obtained using alkaline method had more redness compared to acid-processed isolates, which might be due to more heme proteins precipitation (Shabanpour and Etemadian 2016).
Some of the researchers reported that more whiteness of surimi could be due to more hemoglobin removal from the intracellular structures (Yongsawatdigul and Park 2004; Perez-Mateos and Lanier 2006; Jafarpour and Gorczyca 2008). High efficiency to remove more myoglobin with alkaline pH compared to conventional processing depends on type of species, type of fillet, condition of mince washing, preserving time, etc. (Chaijan et al. 2006). Isolates or surimi obtained by acid-aided processing would have low whiteness compared to alkali-aided isolates or surimi as hemoglobin will be denatured at lower pH and get precipitated along with some sarcoplasmic proteins after second centrifugation (Kristinsson and Hultin, 2003; Kristinsson and Liang 2006).
Color
Color is considered as one of the important parameters when comparing the proteins or gels obtained through different processes (Nolsøe and Undeland 2009). Whiteness is the critical and most important parameter while assessing the quality of restructured products like surimi (Gehring et al. 2011) and the market is interested in gels or proteins that are as white as possible (Choi and Kim 2005). The color of the isolates or gels can be affected by various factors like the amount of dark muscle and the presence of pigments and blood. Color is strongly affected by physical parameters like moisture of the samples or structure, which need to be kept in mind when considering color data from different isolates and gels (Nolsøe and Undeland 2009). The degree of whiteness of isolates obtained from different fishes by various researchers is given in Table 4. Various studies reported the whiteness values of isolates and gels ranging between 20 and 73, 20 and 74, and 35–76% for acid-aided process, alkaline-aided process, and conventional processing, respectively.
Nolsøe et al., 2011 reported that the blue whiting (fresh and frozen) isolates obtained through pH shift processing had higher whiteness than the starting material. Protein gels obtained from frozen fish had low whiteness than isolates but fresh fish gels had more whiteness than the isolates. It was also reported that acid-aided processing resulted in higher whiteness of isolates than in alkali-aided processing and centrifugation resulted in higher whiteness of isolates than filtration. Choi and Kim (2005) also reported that pH shift processed isolates and gels had lower whiteness compared to conventional processing and it was explained as hemoglobin and myoglobin remained in isolates that were recovered using pH shift process. Similar findings were also reported by Chaijan et al. (2010), Perez-Mateos and Lanier (2006), and Chaijan et al. (2006) where the highest whiteness was found in protein gels and isolates made by conventional processing than the gels and isolates obtained through pH shift processing. Some of the researchers (Kristinsson and Hultin, 2002; Yongsawatdigul and Park 2004; Nolsøe et al., 2011) reported that isolates made by acid-aided process had more whiteness than the isolates from alkaline-aided process, while the others (Undeland et al. 2002; Chaijan et al. 2006; Undeland et al. 2005) reported it as opposite. Color or whiteness of isolates can be affected by the amount of dark muscle in the raw material, presence of pigments and blood, processes like the use of prewash or exclusion of centrifugation (Nolsøe and Undeland 2009). The factors that influence the color and whiteness of isolates are the amount of connective tissue that gives lightness, lipid retention that causes yellowness, co-precipitation of heme proteins that causes redness and oxidation, and denaturation of hemoglobin that causes yellow-brownish color. High redness values of protein isolates may be due to the presence of heme proteins (Kristinsson et al. 2005).
Oxidative stability
Lipid oxidation will cause considerable loss in food quality and storage stability, which can be more severe in muscle foods. Application of fish protein isolates in food system depends on the efficacy of processing methods employed in stabilizing the residual lipids (Thorkelsson et al. 2008). Moreover, lipid oxidation can cause problems due to the reaction of the oxidation products of proteins (Kristinsson et al. 2005) and by reducing whiteness of the isolates thus further affecting their applications. Peroxide value (PV) and thiobarbituric reactive substance (TBARS) value can be used to measure lipid oxidation by determining the amount of peroxides and the amount of secondary oxidation products formed during oxidation (Frankel 1998). For oxidation of fish system, TBARS data correlates well with sensory data (Kristinsson et al. 2005). Reducing lipid content in protein isolates is very important as it can reduce the susceptibility to lipid oxidation (Nolsøe and Undeland 2009). Thorkelsson et al. (2008) reported that pH shift process can remove both membrane and neutral lipids to a greater extent, which can improve the oxidative stability of isolates. Although the amount of lipids and pro-oxidants can be reduced to a greater extent by pH shift method (Hultin et al. 2005), the leftover small amounts of lipids in the isolates are sufficient enough to cause considerable oxidation (Frankel 1998). Some findings showed that proteins obtained through pH shift processing did not possess good oxidative stability (Shaviklo et al. 2012; Shabanpour and Etemadian 2016), while a few studies reported that the isolates obtained by pH shift processing showed good oxidative stability (Kristinsson and Demir 2003; Petty and Kristinsson 2004; Panpipat and Chaijan 2016).
Shaviklo et al. (2012) reported that lipid oxidation in saithe protein isolate powder was more than that of fish mince powder, which was further increased during freeze-drying. Although the pH shift process has been reported to reduce lipids and active pro-oxidants from isolates to a greater extent (Hultin et al. 2005), a small amount of lipids in the product is enough to lead to significant oxidation. This oxidation might be due to the formation of secondary oxidation products during freezing and freeze-drying. Geirsdottir (2005) reported that drying increased the lipid oxidation of herring protein isolate resulting in a protein powder with rancid odor, dark color, and poor functionality. Shabanpour and Etemadian (2016) reported that recorded TBARS values were more for pH shift processed surimi than the conventional processed surimi of common carp during 5 months frozen storage, indicating low oxidative stability of surimi obtained through pH shift process.
Panpipat and Chaijan, 2016 during their work on protein isolates from big eye snapper head by-product found lower lipid oxidation in terms of TBARS value in isolates obtained by pH shift process compared to raw material, but PV values were comparable between protein isolates and initial head by-product. They explained that PV value represented the formation of peroxide, which is unstable compound degrading within a few seconds. Therefore, determination of PV at a specific time does not reflect indeed lipid oxidation status. Though the previous reports by Undeland et al. (2005) stated that acid processing can cause rapid lipid oxidation, no significant difference was observed in TBARS values of acid and alkaline processed isolates in this study. The oxidative stability of alkali processed isolates was more than the acid-processed isolates and sometimes more than surimi (Kristinsson and Demir 2003; Thorkelsson et al. 2008). The acid-aided processing causes heme protein denaturation and co-precipitation resulting in darker and less stable isolates (Kristinsson and Hultin 2003, 2002; Kristinsson et al. 2005). Tongnuanchan et al. (2013) reported that the oxidation (TBARS value) of films from red tilapia protein isolates was much lower compared to films made from unwashed mince during 40 days storage period at room temperature. They have related the high TBARS values of film from unwashed mince with the presence of pro-oxidants and high amount of lipids, which were reduced to a greater extent by washing and alkaline solubilization. From the study, it was also suggested that incorporation of antioxidants is an effective way to prevent oxidation of isolate products (Tongnuanchan et al. 2013).
Despite of greater removal of lipids and pro-oxidants in pH shift processing, the residual lipids and heme proteins can still cause considerable oxidation in protein isolates and products resulting in quality losses. The greatest defense against lipid oxidation is the use of antioxidants. Antioxidants occur exogenously and endogenously and can prevent initiation and propagation steps in the free radical mechanism. Antioxidants donate hydrogen to the peroxyl radical and eliminate it as a reactive substance in the radical mechanism (Erickson, 1999). Processing fish proteins through pH shift method followed by mixing with approved antioxidants such as α-tocopherol, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and propyl gallate (Storro and Mozuraityte 2013) to the food products before storage or application can be a better way to preserve the quality as well as functionality of fish proteins.
Undeland et al. (2005) studied the effect of erythorbate (0.2%, 9.3 mM), sodium tripolyphosphate (STPP; 0.2%, 5.4 mM), ethylenediaminetetraacetic acid (EDTA; 0.044%, 1.5 mM), and milk proteins (4%) on lipid oxidation of herring protein isolates. They found that erythorbate alone, or in combination with STPP/EDTA, significantly (p < or = 0.05) reduced lipid oxidation during processing if added in the prewash or homogenization step. During ice storage, better stability was gained when antioxidants were added in both of these steps and when EDTA was used instead of STPP. Shaviklo (2008) also found that addition of salt, sucrose, and polyphosphate to cod proteins could decrease the oxidation during the frozen storage (Shaviklo 2008). According to Undeland et al. (2005), low molecular weight aqueous extracts, also known as “press juice,” from fish muscle are also effective in inhibiting lipid oxidation. Possible inhibitors in the “press juice” could include nucleotides and other reducing agents. The implementation of tocopherol and ascorbic acid at low pH significantly decreased levels of lipid oxidation in homogenates of Spanish mackerel (Petty 2007); however, antioxidants proved to be pro-oxidative with pH readjustment of homogenates from acidic and alkaline pH to neutral pH. Increases in lipid oxidation in the readjusted homogenates with antioxidants were observed. Readjustment alone had no effect on the levels of lipid oxidation in homogenates. Successful implementation of antioxidants into the acid and alkali-aided protein isolation process requires further investigation of appropriate antioxidants and their effectiveness at various pH values and holding times.
Gel quality
The temperature during processing and the pre-process history of raw materials are among the important factors that affect the gel quality of isolates and surimi (Nolsøe and Undeland 2009). The temperature of fish muscle increases by 5−10 °C after death because of continuing muscle metabolism (Lanier et al. 2005), which may cause more protein denaturation. This can be reduced by keeping the fish at possible lower temperatures before processing. Choi and Kim (2005) reported that breaking forces of protein gels from alkali-aided processing were dependant on species. They have also reported that fish proteins were more sensitive to acidic pH and reduction in myosin heavy chain and actin was high in acidic conditions resulting in reduced breaking force of heat-induced gels from acidic processing. Gels from alkaline processing showed better gel-forming ability than acid-processed gels, which was also reported by other workers (Ninomiya et al. 1990; Yongsawatdigul and Park 2004).
Nolsøe et al., 2011 during their studies on blue whiting protein isolates reported that large variations in the breaking forces could be due to variations in raw material quality. They have also reported that replacement of centrifugation with filtration during pH shift processing resulted in lower breaking force and deformation of isolates, which might be due to higher lipid content of the isolates obtained using filtration. The higher lipid content in the isolates obtained through filtration resulted in a lower protein content of gels thus by reducing the gel strength Nolsøe et al., 2011). Kristinsson and Liang (2006) reported that isolates obtained through pH shift processing had a higher G´ compared to surimi after heating to 80 °C as well as after cooling to 5 °C, which might be because of the stronger gel network formed by more protein–protein interactions. These reports were supported by the findings of Kristinsson and Hultin (2003). They have stated that acid- and alkali-treated cod myosin had high G´ value than surimi, which might be due to the partially denaturation of myosin head group causing initiation of gel network and strengthening of the network upon cooling.
Rawdkuen et al. (2009) studied the microstructure of modori and kamaboko gels made of surimi (conventional washing) and protein isolates (pH shift processing) and reported that there was no significant difference found between the microstructures of the gels. They compared the sizes of holes in gel matrix and reported that sizes of holes were smallest for surimi gels compared to gels from protein isolates. The gels of surimi had regularly ordered fine fibrillar structures, which might be the reason behind higher breaking force and deformation (Fig. 3). While studying the microstructures of raw mince and protein isolate obtained by pH shift processing of herring, Marmon (2012) reported that alkaline processing induced large differences in microstructure of herring proteins resulting in loosely formed protein network structure with no remaining myofibrillar structure. The gel strength of proteins precipitated at pH 6.8 was found to be more compared to other proteins obtained at their pI. During the microstructure studies, they found that protein precipitated at pH 6.8 had smaller pores and finer strands resulting in more homogenous protein network compared to the protein isolated at pH 5.5. This improved network was the reason explained for increased gel strength of the proteins precipitated at pH 6.8 compared to pH 5.5. The microstructure studies revealed that pH shift processing causes changes in microstructures of proteins thus by forming larger holes and poor gel networking.
Electron microscopic image of surimi gels prepared by using different conditions (magnification ×10,000, EHT 10 kV). Con, conventional method; Acid, acid-aided process; and Alk, alkaline-aided process (reprinted with permission from Rawdkuen et al. 2009)
Various researchers also found that alkali-aided isolates had highest G´ than the acid-aided isolates and surimi (Kristinsson and Demir 2003; Davenport and Kristinsson 2003, 2004, 2005; Kristinsson and Hultin, 2002). Many studies reported that the gel quality of isolates made using alkaline-aided processing was superior to surimi. The quality of gels from acid-aided process seems to be species dependant (Kristinsson and Liang 2006). Several researchers reported that acid-aided process gave gels of poor gel quality, which might be due to enzymatic action at solubilization and precipitation pH (Choi and Park 2002; Kristinsson and Demir 2003; Yongsawatdigul and Park 2004).
Functionality of isolates
Functionality is a sum of functional attributes, which influence the application of proteins and protein products into foods. Functional properties such as solubility, gelling, emulsification, water holding, and foaming and oil holding are more commonly used to measure the functionality of proteins. Fish and fish processing waste has been gaining importance in food applications as they are considered to be highly nutritious, protein rich, functional, and safe. Functionality of proteins is affected by various factors like protein characteristics, source, and process employed. Several studies reported that the proteins obtained through pH shift processing had better functional properties and gelation properties compared to proteins obtained through conventional processing (Hultin and Kelleher 2000a; Undeland et al. 2002; Kristinsson and Demir 2003). The functionality of fish protein isolates obtained using pH shift process is discussed here for better understanding.
Tian et al. (2016) during their studies on common carp proteins reported that the maximum solubility was observed for fresh mince followed by surimi, acid-processed isolates, and alkali-processed isolates. The reason for lower solubility of isolates was attributed to denaturation of proteins during the process. They have also explained that the functional properties of protein isolates are weaken at their precipitation pH (Isoelectric point), which necessitates the adjustment of pH to 7.0 for better functional properties. Tian et al. (2016) reported that the isolates obtained from common carp by pH shift process showed better digestibility than the fresh mince. They explained that pH shift processing causes some degeneration of proteins, increasing the sensitivity to proteases, which increases their digestibility. This is the reason that producing isolates, peptides, and fermented products has more advantage than direct extraction of fresh fish muscle.
Kristinsson and Liang (2006) during their work on Atlantic croaker found that the pH shift processed isolates (both fresh and frozen) had significantly more stronger gel network (G′—storage modulus) than the surimi after heating to 80 °C indicating protein–protein interactions in protein isolates. These differences in gel-forming ability of isolates and surimi might be due to partial denaturation during pH shift processing (Kristinsson and Hultin 2003). It was found that cooling from 80 to 50 °C caused firmer gel network of isolates (high G′) compared to surimi, particularly in frozen cryoprotected isolates. Alkali-processed isolates had more G′ followed by acid-processed isolates and surimi. They have further reported that addition of cryoprotectants and freezing could increase the G′ of the protein isolates significantly, which was much lower in case of surimi.
Vareltzis et al. (2012) during their studies on functionality of sardine protein isolates reported that emulsification activity index (EAI) and relative overrun (index of foaming capacity) were higher in proteins from the conventional surimi process compared to proteins from pH shift process. No significant difference was observed in liquid drainage. They have also reported that the alkaline-processed isolates had high gel-forming ability than acid-processed isolates which might be due to the differences in formation of bonds, particularly greater disulfide bond formation in protein pool during alkaline process (Vareltzis et al. 2012). It was suggested that the application of microbial transglutaminase (Pérez-Mateos et al. 2004) and control of protein exposure to extracting medium could improve the gelling properties of the isolates (Undeland et al. 2002).
Water holding capacity of protein isolates from Argentine anchovy residue was higher (at pH 3.0 and 11.0) after extraction by acid process compared to alkaline process (Freitas et al. 2011). The similar findings were also observed by Ferreira et al. (2013) during their studies on whitemouth croaker by-product. They have reported that the isolates from white mouth croaker had highest solubility and water holding capacity at pH 11. It was found that the isolate proteins also had good oil holding capacity (13.71 mL/g protein) and the digestibility (in vitro was 91.32 ± 0.15%). The low water holding capacity of isolates obtained by pH shift method indicated the changes in protein structure. However, the protein structure change might be due to intermolecular charge repulsions (Hultin and Kelleher 1999). Oil holding and emulsifying capacity of alkaline processed Argentine anchovy residue isolates were more compared to acid-processed isolates, which might be due to the lipid content in the initial sample. Most hydrophobic region of the pH shift processed isolates could be used as gelling or emulsifying agents in foods (Freitas et al. 2011). Fu et al. (2012) during their studies on silver carp protein isolates found that alkali-processed isolates had the best solubility at all the salt concentrations used. These isolates had highest water holding capacity (WHC) and gel strength at 0 and 1% salt concentrations, which was equal to the properties of surimi at 2% salt concentration. Based on the results, they have also suggested that alkaline processing can be used to produce functional low salt fish products from silver carp (Fu et al. 2012).
Shaviklo (2006) determined the quality of fish protein isolates obtained from cod, saithe, and Arctic charr fillets based on the Codex Code of Practice for frozen surimi (FAO/WHO 2005) and compared them with the quality characteristics of surimi. They have stated that though the quality attributes such as gel-forming ability, whiteness, and gel strength were different to surimi or protein isolates, fish protein isolates are still good source to produce ready-to-eat products. They reported that the sensorial quality of the products made of isolates was acceptable and can be improved further by adjusting different ingredients as per the demand (Shaviklo 2006).
Combination of methods
A few studies reported that combining other methods can improve the yields or quality of isolates obtained using pH shift processing. In a review, Nolsøe and Undeland (2009) reported the average protein yields of 56% and 67–70% in surimi processing and pH shift processing, respectively. They have also reported that running the pH shift process without the first centrifugation step resulted in an increase of average protein yields from 67 and 70% to 84 and 86%. These low protein yields in pH shift processing with centrifugation were attributed to the large gel-like sediment formation during the first centrifugation step, which trapped large amount of soluble proteins (Undeland et al. 2003).
Taskaya and Jaczynski (2009) combined flocculation with pH shift processing to separate protein precipitate from rainbow trout process water. During the study, they have found that a high molecular weight anionic flocculent at 100 mg/L at 10 min reaction time gave highest separation and they have suggested that this flocculent can be added to a bioreactor that precipitates proteins at pH 5.5 in a continuous pH shift processing. To increase the protein yields during pH shift processing, Nolsøe et al. (2011) combined filtration with pH shift processing. They have replaced first centrifugation during pH shift processing of blue whiting with filtration and found a substantial increase in protein yield (38 to 62%) but the isolates had low gel strength, high lipid content, and low whiteness values compared to the isolates from centrifugation process.
Tian et al. (2015) combined high intensity ultrasound (HIU) technique with alkaline pH shift process to recover proteins from tilapia. They found that HIU increased the protein solubility at less extreme pH (10.5) and decreased the consistency of the homogenate and sediment ratio. They reported that with HIU the protein yield increased from 47.0 to 62.6%, at pH 10, which is almost equivalent to the recovery at pH 11.5, suggesting a 40% reduction in alkali and acid volume. HIU caused the changes in thin filament of sarcomere resulting in an increased solubility. Combining HIU with alkaline process also elevated the gel strength of tilapia protein isolate (Tian et al. 2015). Abdollahi et al. (2016) studied the efficacy of pH shift process to remove hemoglobin from blood-rich cod muscle and reported that adding phytic acid to the first supernatant of alkaline process could improve the hemoglobin removal from 77 to 93%. They have also found that combining prewash with phytic acid at precipitation pH 5.5 followed by pH shift processing could improve the hemoglobin removal to 96 and 92% in alkaline- and acid-aided processing (Abdollahi et al. 2016).
Food applications of fish protein isolates
The success of any technology depends on the applicability and utilization of its outcome. From the studies, it was reported that the protein isolates recovered from fish, fish portions, and by-products could be successfully incorporated into food products (Leon et al. 1990; Shaviklo 2008; Tahergorabi et al. 2012, 2015; Ibrahim 2015). Some of the work done in this field so far is discussed below for better understanding. Leon et al. (1990) produced fish protein isolates and hydrolysates through alkaline process from mullet followed by powdering them to use in Mexican food dishes. From the study, they have reported that the powders with 90% protein could give 20–35% of protein concentration of final dishes and the prepared dishes were accepted by 70% of the panel.
Shaviklo (2008) prepared mince balls using the proteins recovered from haddock by pH shift process. During the study, he found that the cook loss of mince balls incorporated with haddock protein isolates was similar to pure mince balls and he also suggested that the haddock proteins can be used for the development of mince-based food products. Ibrahim (2015) prepared meat and fish balls using the protein isolates recovered from small Nile bolti fish through pH shift process. During their study, they have found that incorporation of fish protein isolates improved the protein content in fish and meat balls but reduced the content of fat and carbohydrates. When the pure meat cutlets were incorporated with fish protein isolate (FPI) (25%) amino acid content, value was increased from 94.37 to 96.78% with a good biological value and protein efficiency ratio, while a reduction was found in fish mince balls containing 50% fish protein isolates. From the studies, they have concluded that fish protein isolates obtained through pH shift process can be used to develop low-energy acceptable meat cutlets.
Hydrolysates from protein isolates
Many studies have reported the important roles of protein hydrolysates from fish and fish by-products such as hypertension control (Kawasaki et al. 2000), glucose homeostasis (Lavigne et al. 2001; Lavigne et al. 2003), immunomodulation (Bowgald et al. 1996; Gildberg et al. 1996), lowering cholesterol (Bergeron et al. 1992), antiproliferative and reparative properties (Picot et al., 2006), radical scavenging and reducing ability (Raghavan et al. 2008), and antioxidant activity (Wu et al. 2003). Various factors can affect the functionality of these hydrolysates, among which nature and quality of the raw material used for hydrolysis will greatly affect the functionality and their antioxidative ability (Kristinsson et al. 2005).
One major problem associated with the preparation of hydrolysates from muscle portions of fish is the presence of pro-oxidants, which can greatly decrease the stability of hydrolysates and limit their use. This led researchers to use fish protein isolates obtained through pH shift processing for producing hydrolysates and the results are satisfactory. Some of the findings reported during the study on fish protein isolate hydrolysates are discussed below. Theodore and Kristinsson (2007) prepared fish protein hydrolysates from alkaline-aided channel catfish protein isolates to study its angiotensin-converting enzyme inhibition (ACE) activity at different degree of hydrolysis (5, 15, and 30%). They found that hydrolysates and their soluble fractions had high ACE inhibition activity (70.0–90.6%) based on degree of hydrolysis and fraction. They have reported that the maximum ACE activity was performed by the soluble fractions of hydrolysates containing smallest peptides and they can be used as bioactive ingredients. Hamaguchi et al. (2008) studied the bioactivity of hydrolysates from saithe protein isolates. They have found that isolates and hydrolysates exhibited good oxidative stability. A significant increase in antihypertensive activity was found with a decrease in molecular weight of peptides.
Theodore et al. (2008) during their studies on protein hydrolysates prepared from alkaline-processed channel catfish isolates reported that with an increase in degree of hydrolysis, oxygen radical absorbance capacity (ORAC), metal chelating ability, and TBARS inhibiting ability of hydrolysates were increased, while 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging ability and ferric reducing antioxidant (FRAP) power were reduced. They have found that low molecular peptides showed high ORAC values and metal chelating ability, while high molecular peptides had high FRAP values. They have also reported that the supernatants had higher metal chelating ability and the hydrolysates had higher DPPH radical scavenging ability and Fe3+-reducing ability.
Martins et al. (2014) used fungi to produce hydrolysates from insoluble whitemouth croaker protein residue formed during pH shift processing. They have reported that Trichoderma sp. and Penicillium sp. showed highest proteolytic activity on alkaline and acidic substrates. Highest protein solubilization was showed by Fusarium sp., whereas highest amino acid solubilization was reported for hydrolysis by Penicillium sp. From the studies, they have suggested that the amino acids, peptides, and proteins recovered during the process can be used in traditional processes of protein concentrations to increase yield. Chai et al. (2015) studied the functionality of hydrolysates produced using alcalase, papain, and trypsin from blue-spotted stingray isolates. They have reported that 3-h alcalase hydrolysates had the lowest half maximal effective concentration and highest peptide content for copper chelating, iron chelating activity, hydroxyl radical scavenging, and ABTS radical scavenging activity. They have observed that the fractions of 3-h alcalase hydrolysis namely < 3, 3–10, and > 10 kDa exhibited highest iron chelating activity, ABTS radical scavenging activity, and copper chelating activity, respectively. From the study, they have concluded that alcalase is the best protease for producing hydrolysates from stingray proteins.
Frozen storage stability of isolates
Storage stability is one of the important properties any food component should possess. Many deteriorative changes that occur during storage including physical, chemical, and biological changes will affect the product quality, reduce the acceptability, and limit their applications. Some studies were conducted on frozen storage stability of fish protein isolates and the food products incorporated with fish protein isolates. Thawornchinsombut and Park (2006) studied the frozen storage stability of Pacific whiting proteins under various storage conditions. They have found that the gel texture was maximum when proteins were added with cryoprotectants and stored at pH 5.5 and 7.0 without freeze/thaw cycles, while minimum for isolates frozen and thawed without cryoprotectants. Proteins stored at pH 5.5 and 7.0 showed high hydrophobicity and low sulfhydryl content indicating the more disulfide bonds and hydrophobic interactions resulting in more protein aggregation. Alkali-treated isolates kept at pH 5.5 showed low stability compared to isolates kept at pH 7.0. From the studies, they have suggested that alkali-treated isolates should be added with cryoprotectants for extended shelf-life and frozen stability. Shaviklo (2008) studied the functional properties and rheological behavior of haddock protein isolates with 20% protein and added with salt, polyphosphate, and sucrose under storage at + 2, − 18, and − 24 °C. He has reported that time of storage and amount of additives significantly changed the attributes of haddock protein isolates. From the results, they have suggested that the proteins obtained through pH shift processing also need to be added with cryoprotectants to protect them from denaturation during frozen storage. Haddock protein isolates should be added with salt (1.3%) and sucrose (5%) to prevent denaturation during storage.
Shaviklo et al. (2010) during their studies on haddock mince balls reported that when the fish balls were added with 0, 25, and 50% haddock cutoff protein isolates followed by frozen storage, the sensorial quality (odor, flavor, texture, etc.) was significantly affected during frozen storage. They have found the most negative attributes with haddock mince balls containing 50% mince and 50% isolates. Tongnuanchan et al. (2013) developed protein-based films from tilapia protein isolates (FPIT) and studied the storage stability at room temperature after incorporating antioxidants (Trolox). They have compared the properties of FPIT film during storage with film made from unwashed mince film (UWM). They have found that elongation at break (EAB) and tensile strength (TS) and degradation temperature (Td) were more for FPIT film, while UWM film had high water vapor permeability (WVP). During the storage of 40 days, both the films showed an increase in TS and Td values, whereas EAB, WVP, and protein solubility were decreased. It was found that FPIT film had low lipid content with the low amplitude of amide B bond than UWM film. UWM film had non-disulfide covalent bonds in network, occurred due to interaction between proteins and lipids through Maillard reaction, which might be due to high amount of lipids and further oxidation. It was found that FPI film added with cryoprotectants had improved properties without discoloration. Shabanpour and Etemadian (2016) studied the chemical changes and shelf-life of common carp protein isolates and surimi during frozen storage for a period of 5 months. They have reported that the protein solubility of surimi prepared using pH shift process was more compared to conventional surimi during storage in freezer. The hydrolyzation of myosin light chain was high in the case of conventional surimi during storage. The lightness values were highest for conventional surimi followed by surimi obtained at pH 11.0. They have explained that the surimi prepared by pH shift method had low lipid levels than conventional surimi giving increased shelf-life and health of the product during storage.
Conclusion
Acid- and alkaline-aided processing has both advantages and disadvantages. Higher yields, higher reductions in lipids, and improved gel-forming ability of proteins are the advantages, whereas lower whiteness and protein denaturation during the processing are the disadvantages. Though a lot of work has been done on recovery of proteins from fish and by-products, utilization and application of recovered proteins are lacking still. Only a few reports are available on application of isolates for developing edible food products, suggesting the need of further research in this area. As low protein yields are the major drawback of the technology, combination of other methods should be used with pH shift process, which can reduce the loss of proteins during processing. Some of the studies reported the use of filtration, flocculation, and HIU technique in combination with pH shift process and could get successful outcome. More work is needed in this area to find an appropriate technology, which can improve the protein yields without affecting the functionality. Further studies are needed to find suitable applications of recovered proteins in order to utilize the resources efficiently.
References
Abdollahi M, Marmon S, Chaijan M, Undeland I (2016) Tuning the pH-shift protein isolation method for maximum hemoglobin removal from blood rich fish muscle. Food Chem 1:212–213
Azadian M, Nasab MM, Abedi E (2012) Comparison of functional properties and SDS-PAGE patterns between fish protein isolate and surimi produced from silver carp. Eur Food Res Technol 235:83–90. https://doi.org/10.1007/s00217-012-1721-z
Batista I (1999) Recovery of proteins from fish waste products by alkaline extraction. Eur Food Res Technol 210:84–89
Batista I, Mendes R, Nelhas R, Pires C (2003) Proteins from sardine and blue whiting recovered by new extraction techniques: solubility and gelation properties. In: Proceedings of the 1st Trans Atlantic Fisheries Technology Conference, Reykjavik, Iceland: Icelandic Fisheries Laboratories: 276–278
Batista I, Pires C, Nelhas R (2007) Extraction of Sardine Proteins by Acidic and Alkaline Solubilisation. Food Sci Technol Int 13(3):189–194
Bergeron N, Deshaies Y, Jacques H (1992) Dietary fish-protein modulates high-density-lipoprotein cholesterol and lipoprotein-lipase activity in rabbits. J Nutr 122(8):1731–1737
Bowgald J, Dalmo R, Leifson R, Stenberg E, Gildberg A (1996) The stimulatory effect of a muscle protein hydrolysate from Atlantic cod, Gadua morhua L., on Atlantic salmon, Salmo salar L., head kidney leucocytes. Fish Shellfish Immunol 23:3–16
Chai TT, Tong SR, Law YC, Ismail NIM, Manan FA, Wong FC (2015) Anti-oxidative, metal chelating and radical scavenging effects of protein hydrolysates from blue-spotted stingray. Trop J Pharm Res 14(8):1349–1355
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2006) Physicochemical properties, gel-forming ability and myoglobin content of sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) surimi produced by conventional method and alkaline solubilisation process. J Eur Food Res Technol 222:58–63
Chaijan M, Panpipat W, Benjakul S (2010) Physicochemical and gelling properties of short-bodied mackerel (Rastrelliger brachysoma) protein isolate prepared using alkaline-aided process. Food Bioprod Process 88:174–180
Chandra MV, Shamasundar BA (2011) Fish processing waste management. In: Joshi VK, Sharma SK (eds) Food processing waste management—treatment and utilization technology. 1st new India Publishing Agency, New Delhi, pp 161–194
Chen HH (2003) Effect of cold storage on the stability of chub and horse mackerel myoglobins. J Food Sci 68:1416–1419
Chen YC, Tou JC, Jaczynski J (2007) Protein recovery from rainbow trout (Oncorhynchus mykiss) processing byproducts via isoelectric Solubilization/precipitation and its gelation properties as affected by functional additives. J Agric Food Chem 55(22):9079–9088
Chen YC, Tou JC, Jaczynski J (2009) Amino acid and mineral composition of protein and other components and their recovery yields from whole Antarctic krill (Euphausia superba) using isoelectric solubilization/precipitation. J Food Sci 74(2):31–39
Choi JY, Kim JS (2005) Fish protein recovered using pH shifting method and its physicochemical properties. J Ocean Univ China 4(3):224–228
Choi YJ, Park JW (2002) Acid aided protein recovery from enzyme-rich Pacific whiting. J Food Sci 67:2962–2967
Chomnawang C, Yongsawatdigul J (2013) Protein recovery of tilapia frame by-products by pH-shift method. J Aquat Food Prod Technol 22(2):112–120
Cortes-Ruis J, Pachero-Aguilar R, Garcia-Sanchez G, Lugo-Sanches ME (2001) Functional characterization of a protein concentrate from Bristly sardine made under acidic conditions. J Aquat Food Prod Technol 10:5–23
Davenport M, Kristinsson HG (2003) Low and high pH induce a molten globular structure in myosin which improves its gelation properties. IFT Annual Meeting Conference Proceeding, Chicago, IL, Abstract 42–9
Davenport M, Kristinsson HG (2004) Effects of different acid and alkali treatments on the molecular and functional properties of catfish muscle proteins. IFT Annual Meeting Conference Proceeding, Las Vegas, Abstract 42–9
Davenport M, Kristinsson HG (2005) Effect of cold storage and freezing of ground catfish muscle prior to acid or alkali-aided isolation of muscle proteins. IFT Annual Meeting Conference Proceeding, New Orleans, LA, Abstract 89B-33
Erickson MC (1999) Lipid oxidation of muscle food. In: Quality attributes of muscle foods. Kluwer academic, Boston, pp 365–368
FAO (2010) The state of world fisheries and aquaculture, Food and Agriculture Organization, Rome, 2010
FAO/WHO (2005) Codex code for frozen surimi. In: Park JW (ed) Surimi and Surimi Sea Food. Taylor and Francis Group, Boca Raton, p 869–885
Fatin NS, Huda N, David W (2015) Physicochemical properties of Japanese scad (Decapterus maruadsi) surimi prepared using the acid and alkaline solubilization methods. Int J Sci Eng Res 6(4):141–147
Ferreira FA, Freire BP, Andreghetto de Souza JT, Cortez-Vega WR, Prentice C (2013) Evaluation of physicochemical and functional properties of protein recovered obtaining from whitemouth croaker (Micropogonias furnieri) byproducts. Food Nutr Sci 4:580–585
Food and Nutrition Board of Institute of Medicine (2005) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids, [1], page 691 National Academies Press
Frankel EN (1998) Methods to determine extent of oxidation. In: Lipid oxidation. Glasgow, Bell and Bain Ltd, pp 79–98
Freitas GV, Gautério DG, Rios Prentice C (2011) Functionality of protein isolates from Argentine anchovy (Engraulis anchoita) residue obtained using ph shift processing. J Food Sci Eng 1:374–378
Freitas IR, Cortez-Vega WR, Prentice C (2015) Evaluation of properties of protein recovered from fish muscles by acid solubilization process. Int Food Res J 22(3):1067–1073
Fu XJ, Wu Y, Li ZH (2012) Using pH shifting process to recover proteins for low salt gel products from silver carp. Adv Mater Res 554- 556:1285–1288
Gehring CK, Jaczynski J, Moritz JS (2009) Improvement of pellet quality with proteins recovered from whole fish using iso-electric solubilization/precipitation. J Appl Poult Res 18:418–431
Gehring CK, Gigliotti JC, Moritz JS, Tou JC, Jaczynsky J (2011) Functional and nutritional characteristics of protein and lipid recovered by isoelectric processing of fish by-products and low-value fish: a review. Food Chem 124(2):422–431
Geirsdottir M (2005) Protein isolation from herring. Nordic Innovation Centre, Project No 00075: 102–103
Gildberg A, Bowgald J, Johansen A, Stenberg E (1996) Isolation of acid peptide fractions from a fish protein hydrolysates with strong stimulatory effect on Atlantic salmon (Salmo saalr) head kidney leucocytes. Comp Biochem Physiol Part B: Biochem Mol Biol 114(1):97–101
Hamaguchi PY, Bergsson AB, Halldorsdottir SM, Thorkelsson G, Kristinsson HG, Johansson R (2008) Bioactivity of saithe (Pollachius virens L.) protein hydrolysates. 5th World Fisheries Congress, Yokohama, Japan, 1–2
Hamm R (1994) The influence of pH on the protein net charge in the myofibrillar system. Reciprocal Meat Conference Proceedings 47:5–9
Hultin HO, Kelleher SD (1999) Inventors, Process for isolating a protein composition from a muscle source and protein composition. Patent US6005073
Hultin HO, Kelleher SD (2000a) Surimi processing from dark muscle fish. In: Park JW (ed) Surimi and surimi seafood. Marcel Dekker, New York, pp 59–77
Hultin HO, Kelleher SD (2000b) Inventors, High efficiency alkaline protein extraction. Patent US6136959
Hultin HO, Kristinsson HG, Lanier TC, Park JW (2005) Process for recovery of functional proteins by pH shifts. In: Park JW (ed) Surimi and surimi seafood. Taylor and Francis Group, Boca Raton, pp 107–139
Ibrahim HMI (2015) Chemical composition, minerals content, amino acids bioavailability and sensory properties of meat and fish balls containing fish protein isolate. Int J Curr Microbiol Appl Sci 4(4):917–933
Jafarpour A, Gorczyca EM (2008) Alternative techniques for producing a quality surimi and kamaboko from common carp (Cyprinus carpio). J Food Sci 73(9):E415–E424
Jafarpour SA, Shabanpour B, Filabadi SS (2013) Biochemical properties of fish protein isolate (FPI) from silver carp (Hypophthalmichthys molitrix) by application of acid-alkali processes compared to traditional prepared surimi. Ecopersia 1(3):315–327
Kawasaki T, Seki E, Osajima K, Yoshida M, Asada K, Matsui T, Osajima Y (2000) Antihypertensive effect of Valyl-Tyrosine, a short chain peptide derived from sardine muscle hydrolysate, on mild hypertensive subjects. J Hum Hypertens 14(8):519–523
Kelleher SD, Hultin HO (1994) Functional chicken muscle protein isolates prepared using low ionic strength, acid solubilization/precipitation. 53rd Annual Reciprocal Meat Conference: 76–81
Kim YS, Park JW, Choi YJ (2003) New approaches for the effective recovery of fish proteins and their physicochemical characteristics. Fish Sci 69:1231–1239
Kristinsson HG (2002) Acid-induced unfolding of flounder haemoglobin: evidence for a molten globular state with enhanced pro-oxidative activity. J Agric Food Chem 50(26):7669–7676
Kristinsson H, Demir N (2003) Functional fish protein ingredients from fish species of warm and temperate waters: comparison of acid- and alkali-aided processing vs. conventional surimi processing. In: Advances in Seafood Byproducts 2002 Conference proceedings. Anchorage, P.J. Bechtel (Edt.): Alaska Sea Grant College Program, University of Alaska: 277–295
Kristinsson HG, Hultin HO (2002) Changes in trout hemoglobin conformations and solubility after exposure to acid and alkali pH. J Agric Food Chem 52:3633–3643
Kristinsson HG, Hultin HO (2003) Changes in conformation and subunit assembly of cod myosin at low and high pH and after subsequent refolding. J Agric Food Chem 51:7187–7196
Kristinsson HG, Ingadottir B (2006) Recovery and properties of muscle proteins extracted from tilapia (Oreochromis niloticus) light muscle by pH shift processing. J Food Sci 71:E132–E141
Kristinsson HG, Liang Y (2006) Effect of pH-shift processing and surimi processing on Atlantic croaker (Micropogonias undulates). J Food Sci 71(5):304–312
Kristinsson H, Theodore AE, Demir N, Ingadottir B (2005) A comparative study between acid- and alkali-aided processing and surimi processing for the recovery of proteins from Channel catfish muscle. J Food Sci 70(4):C298–C306
Lanier T, Carvajal P, Yongsawatdigul J (2005) Surimi gelation chemistry. In: Park JW (ed) Surimi and surimi seafood. CRC, Boca Raton, pp 435–477
Lavigne C, Tremblay F, Asselin G, Jacques H, Marette A (2001) Prevention of skeletal muscle insulin resistance by dietary cod protein in high fat-fed rats. Am J Physiol Endocrinol Metab 281(1):E62–E71
Lavigne C, Marette A, Jacques H (2003) Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metab 278(3):E491–E500
Lee HJ, Lee GW, Yoon IS, Park SH, Park SY, Kim JS, Heu MS (2016) Preparation and characterization of protein isolate from yellowfin tuna (Thunnus albacores) roe by isoelectric solubilization/precipitation process. Fish Aquat Sci 19(14):1–10
León JM, Mariscal AG, TéllezSill V (1990) Preparation of fish protein isolate and hydrolyzate (Mugil cephalus) and their incorporation into Mexican foods. Arch Latinoam Nutr 40(1):55–68
Lone DA, Wani NA, Wani IA, Masoodi FA (2015) Physico-chemical and functional properties of rainbow trout fish protein isolate. Int Food Res J 22(3):1112–1116
Lopez-Enriquez RL, Ocano-Higuera VM, Torres-Arreola W, Ezquerra-Brauer JM, Marquez-Rios E (2015) Chemical and functional characterization of sarcoplasmic proteins from giant squid (Dosidicus gigas) mantle. J Chem 538721:1–10
Marmon S (2012) Protein isolation from herring (Clupea harengus) using the pH-shift process. Thesis for the Degree of Doctor of Philosophy, Department of Chemical and Biological Engineering, Chalmers University of Technology, Göteborg, Sweden
Martins VG, Palezi SC, Costa JAV, Prentice C (2014) Hydrolysis of insoluble fish protein residue from whitemouth croaker (Micropogonias furnieri) by fungi. Braz Arch Biol Technol 57(1):96–102
Ninomiya K, Ookawa T, Tsuchiya T, Matsumoto JJ (1990) Concentration of fish waste soluble protein and its gelation properties. Nippon Suisan Gakkaishi 56:1641–1645
Nolsøe H, Undeland I (2009) The acid and alkaline solubilization process for the isolation of muscle proteins: state of art. Food Bioprocess Technol 2:1–27
Nolsøe H, Imer S, Hultin HO (2007) Study of phase separation by filtration instead of centrifugation affects protein yield and gel quality during an alkaline solubilization process—different surimi processing methods. Int J Food Sci Technol 42:139–147
Nolsøe H, Marmon SK, Undeland I (2011) Application of filtration to recover solubilized proteins during pH-shift processing of blue whiting (Micromesistius poutassou); effects on protein yield and qualities of protein isolates. Open Food Sci J 5:1–9
Palafox H, JH C’r-M, Navarrete del Toro MA, FL G’a-C˜o (2009) Protein isolates from jumbo squid (Dosidicus gigas) by pH-shift processing. Process Biochem 44:584–587
Panpipat W, Chaijan M (2016) Biochemical and physicochemical characteristics of protein isolates from bigeye snapper (Priacanthus tayenus) head by-product using pH shift method. Turkish J Fish Aquat Sci 16:41–50
Park JD, Jung CH, Kim JS, Cho DM, Cho MS, Choi YJ (2003) Surimi processing using acid and alkali solubilization of fish muscle protein. J Korean Soc Food Sci Nutr 32(3):400–405
Pattaravivat J, Morioka K, Shirosaki M, Itoh Y (2008) Effect of washing conditions on the removal of lipid from the fatty fish escolar (Lepidocybium flavobrunneum) meat. J Biol Sci 8(1):34–42
Perez-Alvarez JA, Fernandez-Lopez J (2006) Chemistry and biochemistry of colour in muscle foods. In: Hui YH, Nip WK, Nollet LML, Paliyath G, Simpson BK (eds) Food biochemistry and food processing. Blackwell Publishing, Iowa, pp 337–350
Perez-Mateos M, Lanier T (2006) Comparison of Atlantic menhaden gels from surimi processed by acid or alkaline solubilization. J Food Chem 101:1223–1229
Pérez-Mateos M, Amato P, Lanier TC (2004) Gelling properties of Atlantic croaker surimi processed by acid or alkaline solubilization. J Food Sci 6:FCT328–FCT333
Petty HT (2007) Effect of low and high pH on lipid oxidation in Spanish mackerel. Ph.D. Thesis. The Graduate School of the University of Florida, pp. 163
Petty HT, Kristinsson HG (2004) Impact of antioxidants on lipid oxidation during acid and alkali processing of Spanish mackerel. IFT Annual Meeting, Las Vegas, NV, Abstract 49B-7
Picot L, Bordenave S, Didelot S, Fruitier-Arnaudin I, Sannier F, Thorkelsson G, Berge JP, Guerard F, Chabeaud A, Piot JM (2006) Antiproliferative activity of fish protein hydrolysates on human breast cancer cells. Process Biochem 41(5):1217–1222
Raghavan S, Kristinsson HG, Leeuwenburgh C (2008) Radical scavenging and reducing ability of tilapia (Oreochromis niloticus) protein hydrolysates. J Agric Food Chem 56:10359–10367
Rawdkuen S, Sai-Ut S, Khamsorn S, Chaijan M, Benjakul S (2009) Biochemical and gelling properties of tilapia surimi and protein recovered using an acid-alkaline process. Food Chem 112:112–119
Shabanpour B, Etemadian Y (2016) Chemical changes and shelf-life of conventional surimi and proteins recovered using pH change method from common carp (Cyprinus carpio) muscle during 5 months storage at −18°C. Iran J Fish Sci 15(1):311–332
Shabanpour B, Etemadian Y, Alami M (2015a) Comparative study on some biochemical characteristics of surimi from common carp and silver carp and proteins recovered using an acid-alkaline process. Iran J Fish Sci 14(3):583–597
Shabanpour B, Etemadian Y, Taghipour B (2015b) Physicochemical and rheological parameters changes for determining the quality of surimi and kamaboko produced by conventional, acid and alkaline solubilization process methods from common kilka (Clupeonella cultriventris caspia). Iran J Fish Sci 14(4):826–845
Shaviklo GR (2006) Quality assessment of fish protein isolates using surimi standard methods. Project Report, UNU-Fisheries Training Programme: 1–34
Shaviklo GR (2008) Evaluation and utilization of fish protein isolate products. Master Thesis in Food Science, Department of Food Science and Human Nutrition, University of Iceland
Shaviklo GR, Arason S, Thorkelsson G, Martinsdottir E (2010) Sensory attributes of haddock balls affected by added fish protein isolate and frozen storage. J Sensory Stud 25(3):316–331
Shaviklo GR, Thorkelsson G, Arason S, Sveinsdottir K (2012) Characteristics of freeze-dried fish protein isolated from saithe (Pollachius virens). J Food Sci Technol 49(3):309–318
Storro I, Mozuraityte R (2013) Sensory shelf life for protein products made from salmon backbones; effect of added antioxidants on the shelf life of taste neutral proteins. SINTEF Fisheries and Aquaculture Project No. PT 6020534, Report no. A 23776: 1–15
Surasani VKR, Khatkar SK, Singh S (2017a) Effect of process variables on solubility and recovery yields of proteins from pangas (Pangasius pangasius) frames obtained by alkaline solubilization method: characteristics of isolates. Food Bioprod Process 106:137–146
Surasani VKR, Tyagi A, Kudre T (2017b) Recovery of proteins from rohu processing waste using ph shift method: characterization of isolates. J Aquat Food Prod Technol 26(3):356–365
Surasani VKR, Kudre T, Ballari RV (2018) Recovery and characterization of proteins from pangs (Pangasius pangasius) processing waste obtained through pH shift processing. Environ Sci Pollut Res 25:11987–11998. https://doi.org/10.1007/s11356-018-1456-x
Tahergorabi R, Beamer SK, Matak KE, Jaczynski J (2012) Functional food products made from fish protein isolate recovered with isoelectric solubilization/precipitation. LWT - Food Sci Technol 48(1): 89–95
Tahergorabi R, Matak KE, Jaczynski J (2015) Fish protein isolate: development of functional foods with nutraceutical ingredients. J Functional Foods 18:746–756
Takeda H, Seki N (1996) Enzyme-catalyzed crossing-linking and degradation of myosin heavy chain in walleye Pollock surimi paste during setting. Fish Sci 62:462–467
Taskaya L, Jaczynski J (2009) Flocculation-enhanced protein recovery from fish processing by-products by isoelectric solubilization/precipitation. LWT-Food Sci Technol 42:570–575
Thawornchinsombut S, Park JW (2006) Frozen stability of fish protein isolate under various storage conditions. J Food Sci 71(3):C227–C232
Theodore AE, Kristinsson HG (2007) Angiotensin converting enzyme inhibition of fish protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J Sci Food Agric 87:2353–2357
Theodore AN, Raghavan S, Kristinsson HG (2008) Antioxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J Agric Food Chem 56:7459–7466
Thorkelsson G, Sigurgisladottir S, Geirsdottir M, Jóhannsson R, Guérard F, Chabeaud A, Bourseau P, Vandanjon L, Jaouen P, Chaplain-Derouiniot M, Fouchereau-Peron M, Martinez-Alvarez O, Le Gal Y, Ravallec-Ple R, Picot L, Berge JP, Delannoy C, Jakobsen G, Johansson I, Batista I, Pires C (2008) Mild processing techniques and development of functional marine protein and peptide ingredients. In Børresen, Improving seafood products for the consumer, Woodhead Publishing Limited
Tian J, Wang Y, Zhu Z, Zeng Q, Xin M (2015) Recovery of tilapia protein isolate by high-intensity ultrasound-aided alkaline isoelectric solubilization/precipitation process. Food Bioprocess Technol 8:758–769
Tian Y, Wang W, Yuan C, Zhang L, Liu J, Liu J (2016) Nutritional and digestive properties of protein isolates extracted from the muscle of the common carp using ph-shift processing. J Food Process Prserv. https://doi.org/10.1111/jfpp
Tongnuanchan P, Benjakul S, Prodpran T, Songtipya P (2013) Properties and stability of protein-based films from red tilapia (Oreochromis niloticus) protein isolate incorporated with antioxidant during storage. Food Bioprocess Technol 6(5):1113–1126
Undeland I, Kelleher SD, Hultin HO (2002) Recovery of functional proteins from herring (Clupea harengus) light muscle by an acid or alkaline solubilization process. J Agric Food Chem 50(25):7371–7379
Undeland I, Kelleher SD, Hultin HO, Mcclements J, Thongraung C (2003) Consistency and solubility changes in herring (Clupea harengus) light muscle homogenates as a function of pH. J Agric Food Chem 51(14):3992–3998
Undeland I, Hall G, Wendin K, Gangby I, Rutgersson A (2005) Preventing lipid oxidation during recovery of functional proteins from herring (Clupea harengus) fillets by an acid solubilization process. J Agric Food Chem 4:5625–5634
Vareltzis PK, Evaggelia P, Ntoumas D, Adamopoulos KG (2012) Process characteristics and functionality of sardine (Sardina pilchardus) muscle proteins extracted by a ph-shift method. Annals Food Sci Technol 13(2):132–143
Wu HC, Chen HM, Shiau CY (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res Int 36(9–10):949–957
Yasothai R, Giriprasad R (2015) Acid/alkaline solubilization method of processing protein. Int J Sci Environ Technol 4(1):96–100
Yeung CJ, Jinx-Soo K (2005) Fish protein recovered using ph shifting method and its physicochemical properties. J Ocean Univ China 4(3):224–228
Yongsawatdigul J, Park JW (2004) Effects of alkali and acid solubilization on gelation characteristics of rockfish muscle proteins. J Food Sci 69(7):499–505
Zayas JF (1997) Functionality of proteins in food. Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-59116-7
Acknowledgements
Authors wish to express their sincere thanks to Dr. C.V. Raju, Head and Professor and Dr. I.P. Lakshmisha, Scientist, Department of Fish Processing Technology, College of Fisheries, KVAFSU, Mangalore, Karnataka, India for their constant support and technical help in writing this paper. The authors also wish to express their sincere thanks to Mrs. Manvinder Kaur, M.A. M.Ed., for her technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Surasani, V.K.R. Acid and alkaline solubilization (pH shift) process: a better approach for the utilization of fish processing waste and by-products. Environ Sci Pollut Res 25, 18345–18363 (2018). https://doi.org/10.1007/s11356-018-2319-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2319-1