Abstract
Study was conducted to use underutilized freshwater mussel (Lamellidens marginalis) for the recovery of proteins using pH shift method and to study the functionality and characteristics of the recovered isolates. From the pH range tested (pH 2.0–13.0), maximum protein yields were obtained during solubilization at pH 2.0 and pH 13.0 (p < 0.05). During the protein recovery process, pH 13.0 was found to have minimal effect on proteins resulting in higher protein yields compared to pH 2.0. Isolates obtained by both acidic and alkaline solubilization processes had low stability and poor gel network. Total lipid content, total myoglobin, and pigment contents were reduced significantly (p < 0.05) during pH shift processing, resulting in whiter protein isolates and protein gels. All the essential amino acids were present in the isolates recovered by acid and alkaline solubilization, indicating the complete recovery of amino acids. No microbial counts were observed in any of the isolates prepared using acid and alkaline-aided processing. Acid and alkaline solubilization (pH shift) process was found to be promising for the recovery of proteins from underutilized freshwater mussel thus by reducing the supply demand gap.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last 50 years, the daily intake of protein particularly from meat, eggs, milk, and dairy products rose from 39 g per capita in 1961 to 52 g in 2011, a 33% increase. Between 1961 and 2011, the daily per capita availability of protein from animal products rose from 9 to 20 g, an increase of 116%, which is projected to reach 22 g by 25 g by 2050 (FAO 2017). This increasing demand for animal protein will create a greater pressure on conventional animal protein resources, causing their quick depletion. Globally, fish contribute around 18% of the total animal protein intake (FAO 2009) and it will be a major target source for future protein needs. In order to withstand the pressure on aquatic resources, they must be managed before and after the catch. In this way, low-cost fish and by-products should be used for human edible purposes as they contain valuable nutrients, which is otherwise a serious loss to human kind (Surasani 2018). In order to serve the increasing demand for protein and protein foods, there is an urgent need to search for low-cost unconventional protein resources and freshwater mussels can be a good source with great future potential. The freshwater mussel Lamellidens marginalis is widely distributed in ponds and water bodies of India and is a very good source of many nutrients (Yusufzi et al. 2010). Despite of the huge resources and availability in India, these mussels are not used for human edible purposes, which is a huge loss to mankind. In view of reducing the gap between supply and demand for nutritious food, valuable nutrients from the freshwater mussels should be recovered and used for human edible purpose.
Preliminary work done by a few scientists (Niki et al. 1985; Huang et al. 1997, etc.) on the recovery of nutritional components from surimi wash water was not found satisfactory due to poor protein yields caused by the loss of water soluble components (Table 1). In the later stages, Hultin and Kelleher (1999, 2000) patented acid and alkaline solubilization process for the isolation of fish muscle proteins with better stability, functionality, and recovery yields. Thereafter, many researcher used this process for the isolation of proteins from different low-cost fish, fish by-products, and fish processing waste (Cortes-Ruis et al. 2001; Kristinsson and Demir 2003; Kristinsson and Ingadottir 2006; Fatin et al. 2015; Surasani et al. 2017a, b; Surasani et al. 2018). Protein recovery yields and functionality depends on the method used for the protein isolation and the source of raw material.
Utilization of processing wastes and low-cost fish and shellfish for human consumption will reduce pollution problems as well as demand supply gap and product cost (Surasani 2017). Due to the greater abundance of freshwater mussels in India and its rich nutritional profile, the objectives of the study were set to isolate proteins from freshwater mussels meat using pH shift method (acid and alkaline solubilization) method. Characteristics and functionality of the proteins isolated through pH shift processing of freshwater mussels were also studied.
Materials and methods
Raw material
Live mussels were procured from local water bodies (Ludhiana, India) and transported to the laboratory in live condition. The shells of the mussels were opened followed by shucking the meat and grinding using a laboratory mincer to a fine and thick paste. This paste was used as raw material for the isolation of proteins. All the preparatory and processing steps including raw material processing were performed at a temperature < 5 °C.
Proximate composition
The proximate composition (%), i.e., moisture, protein, ash, and fat content of the raw freshwater mussel meat, was determined by the method of AOAC (2000) (AOAC 950.46; AOAC 981.10; AOAC 920.153; AOAC 948.15). Protein content (%) of the sample was calculated by multiplying the obtained nitrogen value with factor of 6.25.
Preparation of homogenate
The meat paste obtained from the freshwater mussel was added with 6 volumes of cold deionized water and homogenized at 12,000 rpm for 60 s (2 × 30 s) using Ultra-Turrax homogenizer (Germany), equipped with T 25 element. After the homogenization, the homogenate was transferred to beakers for pH adjustment using 2 M HCl or 2 M NaOH under slow stirring condition (Surasani et al. 2017a, b). Diluted acid or alkali was used to keep the pH of the homogenate constant throughout the process.
Protein solubility
Solubility curve
pH of the homogenate, which was divided into different lots, was adjusted to pH 2.0 to 13.0 with 1.0 unit interval using 2 M HCl and 2 M NaOH. The solubility of proteins at each pH was calculated and used to draw the solubility curve. After the pH adjustment, 30 g of homogenate was taken at each pH and was transferred to 50-ml centrifuge tubes followed by centrifugation at 5000×g for 20 min using Sorvall ST 16R refrigerated centrifuge (Thermo Fisher Scientific, Germany). At each solubilization pH, 2 g of sample was taken before and after centrifugation to calculate total protein content using Biuret method (Robinson and Hogden 1940). Sample was diluted with cold de-ionized water (pH 11.0) to analyze the protein content, wherever is necessary.
Solubility after isoelectric precipitation
After constructing the protein solubility curve, pH with maximum solubility (pH 2.0 in acidic range and pH 13.0 in alkaline range) was selected and the homogenate was adjusted to these pH using 2 M HCl and 2 M NaOH under continuous stirring condition. After the pH adjustment, the homogenate was kept for 60 min at 4 °C to aid protein solubilization (Surasani et al. 2017, b). This was followed by centrifugation at 5000×g for 20 min for separating the soluble fraction from insoluble matter. Supernatant was collected after screening through the two layered cheese cloth and was readjusted to precipitation pH of 5.5. pH adjusted samples were kept for 30 min at 4 °C to aid precipitation followed by centrifugation for 20 min at 5000×g. Two grams of sample was taken before and after the centrifugation to analyze the protein content using Biuret method.
Protein measurements
Total protein of homogenate was determined by taking a 2-g sample at each pH before centrifugation and diluting it 10 times with cold DI water at pH 11.0 to aid in solubilization of the proteins. The samples were homogenized with a hand held Tissue Tearor for 20 s and then analyzed for protein content using the Biuret method (Kristinsson and Ingadottir 2006). Supernatants were diluted 10 times with cold DI water at pH 11.0, followed by analyzing for protein content using the Biuret method. Ten percent deoxycholic acid was used to reduce the cloudiness formed by the lipids in the solution. T90+ UV/VIS spectrometer (PG Instruments Ltd., India) was used to read the absorbance at 540 nm. A standard curve with bovine serum albumin (BSA) (1–10-mg/ml concentration) was plotted and used for calculating protein concentrations based on the absorbance.
Protein recovery
Protein recovery at every step was expressed as recovery (%) and was calculated using the formula:
-
$$ \mathrm{Protein}\ \mathrm{recovery}\ \mathrm{after}\ 1\mathrm{st}\ \mathrm{centrifugation}:\% Protein=\frac{\left(B\times {W}_B\right)}{\left(A\times {W}_A\right)}\times 100 $$
-
$$ \mathrm{Protein}\ \mathrm{recovery}\ \mathrm{after}\ 2\mathrm{nd}\ \mathrm{centrifugation}:\% Protein=100\hbox{--} \left\{\frac{\left(C\times {W}_C\right)}{\left(B\times {W}_B\right)}\times 100\right\} $$
-
$$ \mathrm{Total}\ \mathrm{process}\ \mathrm{recovery}:\% Protein=\left(\frac{\left(B\times {W}_B\right)-\left(C\times {W}_C\right)}{A\times {W}_A}\right)\times 100 $$
where A: homogenate protein content, WA: homogenate weight, B: protein content of supernatant obtained in 1st centrifugation, WB: weight of the supernatant obtained in 1st centrifugation, C: protein content of the supernatant obtained in 2nd centrifugation, WC: weight of the supernatant obtained in 2nd centrifugation.
Lipid content and its removal
Total lipids in the homogenate as well as protein isolates were estimated using soxhlet apparatus (AOAC 920.39B 2000). Lipid content (dry weight basis) was calculated using the formula:
-
$$ \% Fat\ \left( dry\ weight\right)=\frac{g\ of\ fat\ in\ sample}{g\ of\ dried\ sample}\times 100 $$
The reduction in lipid content was calculated using the formula:
-
$$ Lipid\ reduction\ \left(\%\right)=\left\{1-\left(\frac{Lipid\ content\ of\ isolate}{Lipid\ content\ of\ homogenate}\right)\right\}\times 100 $$
Total myoglobin content and its removal
Total myoglobin content in the homogenate as well as protein was estimated using the spectrometric method (Chaijan et al. 2006). Two grams of sample was added with 20 ml of 40 mM, pH 6.8 phosphate buffer and homogenized using a homogenizer at 3000×g for 30 min at 4 °C. The homogenate was filtered through Whatman no. 1 paper and the obtained supernatant/permeate was added with 0.2 ml of 1% (w/v) sodium dithionite and mixed well. The absorbance of the solution was measured at 555 nm using phosphate buffer (pH 6.8) as blank.
Myoglobin content was calculated using the formula:
-
$$ Myoglobin\ content\ \left( mg/g\right)=\left(A\times 16.111\times F\times S\times WS\times 7.6\right)\times 100 $$
where A = absorbance, F = dilution factor, WS = sample weight in g, 7.6 = millimolar extinction coefficient, 16.111 = molecular weight.
Myoglobin removal (%) was calculated as:
-
$$ Myoglobin\ removal\ \left(\%\right)=\left\{1-\left(\frac{myoglobin\ content\ in\ isolate}{myoglobin\ content\ in\ homogenate}\right)\right\}\times 100 $$
Total pigment content and its removal
Total pigments in the raw material as well as protein isolates were measured using the method of Rawdkuen et al. (2009). One-gram sample was added with 9 ml acetone acid (2% HCl, 90% acetone, and 8% deionized water) and incubated for 1 h. It was followed by filtration using Whatman no. 1 paper and the absorbance of the filtrate was measured at 640 nm with acetone as a blank. The concentration of pigments (hematin) was calculated by using the ratio of 680 and weight of the sample.
-
$$ Total\ pigment\ content\ (ppm)=A640\times 680 $$
(A640 is the absorbance at 640 nm and 680 is the conversion factor for converting the total pigments into parts per million of hematin)
Total pigment removal (%) was calculated using the formula:
-
$$ Pigment\ removal\ \left(\%\right)=\left\{1-\left(\frac{pigment\ content\ in\ isolate}{pigment\ content\ in\ homogenate}\right)\right\}\times 100 $$
Color analysis
Color was measured using HunterLab (Virginia, U.S.) as described by Lubana et al. (2016). Whiteness of the samples was calculated by using L (lightness), a (Red/Greenness) and b (Yellow/Blueness) values, obtained from the instrumental analysis.
-
$$ Whiteness=100-{\left\{{\left(100-{L}^{\ast}\right)}^2+{a}^{\ast 2}+{b}^{\ast 2}\right\}}^{1/2} $$
Foaming properties
Foaming capacity (%) and foaming stability (%) of protein isolates obtained by acid and alkaline solubilization were measured by using the methodology of Sathe et al. (1982). One gram of protein was dissolved in 50 ml distilled water by heating at 60 °C. The solution was homogenized at 9000 rpm for 5 min to prepare the foam. Homogenized solution was gently poured along the side wall into a measuring cylinder and the volume of foam was noted. The volume of foam was noted again after 30. Foaming capacity (%) was calculated as the ratio of volume of foam to the volume of original liquid. The foam stability (%) was calculated as the ratio of the initial volume of foam to the volume of foam after 30 min.
SDS-PAGE analysis
SDS-PAGE analysis was performed as per the method described by Laemmli (1970). Methodology of Harlow and Lane (1988) was used to prepare samples, reagents with slight modifications. Samples were added with 5× sample loading buffer (SDFCL, India) and incubated at 95 °C for 5 min and loaded into 5% stacking gel. Twelve percent gel was used to resolve proteins at a constant current of 20 mA for 3–4 h. Four-microgram broad range marker (SDFCL, India) was loaded on each gel. Gel was stained overnight with Coomassie Brilliant Blue R250 followed by destaining and observation of protein bands.
Amino acid analysis
Amino acid composition was analyzed using reverse phase HPLC on Water Picotag TM amino acids analysis system. Analysis was done after hydrolyzing the protein in the samples with 6 N HCl to yield free amino acid (Bidlingmeyer et al. 1984). The amino acid composition of protein isolates and raw material was expressed as g/100 g protein.
Microbial quality analysis
Microbial count of the raw material and protein isolates was determined by estimating the total plate count (AOAC 2000) and was expressed as CFU/ml or CFU/g.
Gel preparation
Protein isolate from mussel meat obtained through pH shift processing was cut into small pieces (1 cm thick) followed by mincing using a laboratory mincer. Salt (2.5%) was added to the isolates after adjusting the moisture of isolates to 80% (w/w). This mixture was finely mixed and chopped followed by stuffing into polyvinylidine chloride (PVC) casings with 2.5 cm diameter. Both ends of the stuffed casings were sealed tightly and kept for incubation for 30 min at 40 °C followed by heating for 20 min at 90 °C (Kristinsson and Ingadottir 2006). Heat set gels were kept in chilled water for 30 min and stored over night at 4 °C prior to analysis.
Gel quality analysis
Folding test
Folding test of protein gel was performed as per the method described by Kudo et al. (1973). Protein gel was cut into 3 mm slice and folded with hand at room temperature. The ability of gels to withstand the folding was assessed using a five point system. 5: No crack was observed after folding the gel into four, 4: no crack was observed when the gel was folded into two but there was a crack after folding it into four, 3: no crack was observed when the gel was folded into two but the gel was broken into two after folding it into four, 2: gel was cracked when it was folded into two, 1: gel was completely broken into two when it was folded into two.
Shear test
Shear/cutting test of the protein gels was performed using a texture analyzer as per the method used by Reddy (2016), with slight modification. Protein gels were cut using a blade set (HDP/BSW) with a test speed of 2 mm/s to 10 mm distance at 30 °C and 20 g force. Pre- and post-test speed of 2 mm/s was used. A total of six measurements were made for each lot and the average value was reported. A force-time graph was generated with a data acquisition rate of 200 pps and taxt-plus software was used to calculate the test results.
Expressible moisture
Expressible moisture content of the protein gels was measured using the method described by Feng and Hultin (2001). Protein gel was cut into 3-mm-thick pieces and was placed between five layers of Whatman filter paper. A standard metal weight of 3000 g was placed on the filter paper for 1 min. The difference in the weight of gel before and after pressing was used to calculate the expressible moisture content;
-
$$ Expressible\ moisture\ \left(\%\right)=\frac{\left( Pre\ pressed\ wei. of\ gel- after\ pressed\ wei. of\ gel\right)}{Pre\ pressed\ wei. of\ gel}\times 100 $$
Statistics analysis
Results are expressed as mean ± SD. Significant difference (P < 0.05) between the treatment attributes was analyzed using analysis of variance (ANOVA) and t test between treatments. SPSS Version 20 software was used for the analysis.
Results and discussion
Proximate composition
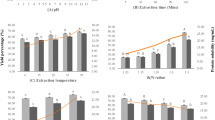
Protein recovery yields and their quality depend on the raw material composition and quality. Moisture, protein, fat, and ash content of the mussel meat used for the protein isolation was 79.88 ± 0.34, 8.40 ± 0.22, 1.04 ± 0.07, and 2.63 ± 0.11, respectively. Haldar et al. (2014) during their studies on mussel found the moderate amount of carbohydrate (8.01 ± 0.38%), protein (8.30 ± 0.67%) and less amount of crude fiber (0.01 ± .001%) and fat (1.02 ± 0.20%) in the mussel meat. Results obtained in this study were supported by previous reports.
Effect of pH on mussel protein solubility and recovery
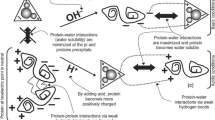
Solubility and associated recoveries of the mussel proteins at each pH from pH 1.0 to 13.0 are given in Table 2. Maximum protein solubility was observed at pH 2.0 in the acidic pH range and at pH 13.0 in the alkaline pH range (p < 0.05). Maximum mussel protein was recovered at pH 13.0 (90.91%), while minimum protein recovery was observed at pH 4.0 and 5.0 (8.78 and 8.89%), respectively. A sudden increase in protein solubility was found when the pH was shifted from pH 3.0 to 2.0 and pH 10.0 to 11.0. Minimum protein solubility at pH 4.0–5.0 might be due to the low solubility of myosin, the major protein fraction with its isoelectric pH nearer to 5.0. When the pH is shifted either above or below of this pH range (pH 4.0–5.0), electrostatic repulsions between the proteins will be increased due to the increase in protein surface charges, causing more solubilization (Hamm 1994; Kelleher and Hultin 2000). The sudden increase in protein solubility was attributed to more ionizable groups, having pKa values between those pH values (Undeland et al. 2002). Similar results were also reported by Chen et al. (2007) and Surasani et al. (2017a, b).
Process yields are very much important in any extraction process, on which the viability of the process depends (Surasani et al. 2018). Recovery yields obtained during mussel protein isolation through pH shift processing are given in Table 3. High protein yields were obtained by alkaline-aided processing than the acid-aided processing (p < 0.05). The total process recoveries obtained during acid- and alkaline-aided processing of mussel proteins were 63.08 and 85.03%, respectively. Similar results were reported by Vareltzis and Undeland (2012) during their studies on blue mussel. They reported that acid-aided (pH 2.6) and alkaline-aided (pH 12.0) processing of blue mussel gave recovery yields of 43.0% and 58.0%, respectively. Solubilization of common carp proteins at pH 12.5 and pH 2.5 gave protein yields of 87.6% and 76.3%, respectively (Tian et al. 2016). Acid- and alkaline-aided solubilization of sardine proteins resulted in protein yields of 73.0 and 77.0%, respectively. Acid solubilization of common carp and silver carp gave protein yields of 70.20% and 75.18%, while alkaline solubilization resulted in the protein yields of 64.5% and 80.89%, respectively (Shabanpour et al. 2015). Similar findings were also reported by Surasani et al. (2017a, 2018) during acid and alkaline processing of pangas proteins.
Characteristics of isolates
Color
Color values of mussel protein isolates and protein gels made of isolates recovered by pH shift process are given in Table 4. Protein gels were found to have highest L (lightness) and whiteness values followed by isolates and raw material (p < 0.05). None of the color values showed significant differences between the isolates and their gels. Raw material was found to have low values of b (yellowness) and high values of a (redness) than protein isolates and gels indicating the significant removal of the pigments during acid and alkaline solubilization process. pH shift processing caused an increase in L (lightness) values and decrease in a (rednesss) values, resulting in higher whiteness of isolates (Panpipat and Chaijan 2016). Color of isolates and their gels in the present study followed the same trend as reported by earlier studies. Nolsφe et al. (2011) reported that the whiteness values were maximum for blue whiting surimi gels, followed by protein isolates and starting mince. Similar findings were observed in the present study. The lower whiteness values for protein isolates compared to their gels might be due to differences in light reflection from the protein-protein aggregations.
Total lipid, myoglobin, and pigment content
Total lipids, pigments, and myoglobin content in the raw material as well as the protein isolates were reduced significantly by acid- and alkaline-aided solubilization (Table 6). Acid-aided process could cause greater removal of total lipids (88.48%), compared to alkaline-aided process (83.91%). During the alkaline solubilization, there might be a reaction between lipids and the added alkali that caused the soap formation and associated co-precipitation of lipids during the isoelectrcic precipitation process (Panpipat and Chaijan 2016). Surasani et al. (2018) found that the acidic and alkaline processing caused lipid reductions of 97.11 and 90.33% in pangas protein isolates. Rawdkuen et al. (2009) and Kristinsson et al. (2005) observed lipid reductions of 85.2% and 85.4% during the pH shift processing of tilapia and catfish muscle concentrates, respectively. Lipid reductions obtained through pH shift processing of mussel protein isolates were supported by the earlier findings (Batista et al. 2007; Freitas et al. 2015; Surasani et al. 2018).
pH shift processing resulted in significant reductions (p < 0.05) of myoglobin content in the mussel protein isolates (89.11 and 89.47%). There was no significant difference found between the removal percentage of these two processes. Similar observations were made by Surasani et al. (2018) during protein isolation from pangas waste. When compared to surimi processing, pH shift process caused significant reductions in the myoglobin content of the protein isolates (Chaijan et al. 2006; Rawdkuen et al. 2009). Factors that influence the efficiency to extract myoglobin include type of fish, variety and composition of raw material used, washing process, and the time of storage (Chaijan and Benjakul 2006).
Pigment content was also reduced significantly during acid and alkaline solubilization process (p < 0.05). Acid- and alkali-aided solubilization caused a pigment reduction of 45.35% and 38.85%, respectively. Similar results were observed by by Panpipat and Chaijan (2016) and Surasani et al. (2018) during their studies on protein isolation from big eye snapper and pangas. Extreme acidic pH might have caused the degradation of heme pigments resulting in high pigment removal, compared to alkaline pH (Panpipat and Chaijan 2016). Another reason for this might be the proton-catalyzed displacement process and a protoporphyrin IX ring destruction at high acidic pH that promoted haem loss (Chaijan and Undeland 2015). Similar observations were also made by Jafarpour et al. (2013) and Shabanpour et al. (2015).
Foaming capacity and foaming stability
Foaming properties of mussel proteins recovered by pH shift process are presented in Table 5. Proteins obtained by acid-aided solubilization were found to have high foaming capacity, than the proteins obtained by alkali-aided solubilization (p < 0.05). These differences might be due to the difference in hydrophobic residues of proteins (Surasani et al. 2017a, b). Similar observations were made by Surasani et al. (2018) during their studies on pangas protein isolates. Information on foaming properties of pH shift processed proteins is scanty. Improvement in foaming capacity can be done by reducing the surface tension and by increasing the exposure of hydrophobic residues at air-water interface (Mutilangi et al. 1996). The additional hydrophobic residues at air-water interface can form a large hydrophobic sphere on surface that will result in excess foaming (Jongjareonrak et al. 2010).
Foam stability is the measure of protein-protein interaction in the matrix (Mutilangi et al. 1996; Jongjareonrak et al. 2010). In this study, proteins obtained by alkali-aided solubilization were found to have high foam stability, than the proteins obtained by acid-aided solubilization (p < 0.05). These differences in foam stability might be because of protein aggregation that interferes the protein water interactions needed for the formation of foam (Kinsella 1976). Similar observations were made by Surasani et al. (2018) during their studies on pangas proteins.
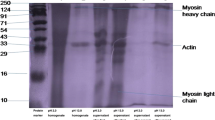
SDS-PAGE pattern
SDS-PAGE analysis of mussel proteins showed that the protein bands ranging from 6–10 to 200 kDa (Fig. 1). Protein in the supernatants obtained using acid- and alkali-aided solubilization had a band of 124 kDa, which might be the dissociated fraction of heavy meromyosin. All the protein samples showed a band at approximately 43 kDa, which might be actin. Acid-solubilized protein showed more number of bands compared to alkaline-processed protein, including bands at approximately 6–10 kDa which might be due to protein denaturation at extreme acidic pH and associated proteolysis. Similar observations were recorded by Vareltzis and Undeland (2012) durimg protein recovery from blue mussel. During the pH shift process, proteolytic enzymes will be activated, causing hydrolysis of heavy meromyosin chain (Kelleher and Hultin 2000). Maximum number of protein bands were disappeared in supernatants obtained after 2nd centrifugation indicating the maximum protein precipitation and recovery. Similar findings were reported by Kristinsson and Liang (2006), Vareltzis and Undeland (2012), and Surasani et al. (2018).
SDS-PAGE profile of the mussel proteins and the protein isolates recovered at pH 2.0 and pH 13.0, M. protein marker; 1. pH 2.0 homogenate; 2. pH 13.0 homogenate, 3. Supernatant after 1st centrifugation obtained at pH 2.0; 4. Supernatant after 1st centrifugation obtained at pH 13.0; 5. Supernatant after 2nd centrifugation obtained at pH 2.0; 6. Supernatant after 2nd centrifugation at pH 13
Amino acid profile
Amino acid profile of mussel meat homogenate and protein isolates obtained through pH shift processing is given in Table 6. In the present study, the effect of processing on amino acids was minimum and all the amino acids that were present in the homogenate were recovered into isolates. The total number of essential amino acids that were present in the homogenate were also found in the protein isolates, indicating their complete recovery. Álvarez et al. (2017) during their studies on ultrasound-assisted protein recovery from mackerel stated that pH shift processing could recover all the amino acids that were present in the homogenate. Similar observations were noted by Surasani et al. (2017a, 2018) during their studies on protein isolates recovered from pangas waste. They found that the pH shift processing recovered all the amino acids that were present in the homogenate. Findings in the present study are in agreement with the earlier findings. Some recent studies also reported that pH shift process caused enrichment of essential amino acids in the protein isolates (Surasani et al. 2017a and 2018). However, the final amino acid profile of the protein isolates obtained through pH shift processing depends on the quality of raw material used and the pH at which the protein was solubilized.
Microbial stability
During food production and storage, one of the important parameters to measure is its microbial quality (Nolsøe and Undeland 2009). Immediately after harvesting, live fish or fresh fish are dominated by psychrotrophic Gram negative bacteria (Huss 1983). Acid- or alkali-aided processing results in extreme food pH that has a killing effect on microbes (Jay 1986). In the present study, raw mussel meat had a total plate count of 4.56 × 102, while isolates showed no microbial counts. Similar reports were published by Surasani et al. (2017a, b) and Surasani et al. (2018) during their studies on protein isolates recovered from pangas and rohu processing waste.
Gel quality
Gel texture
Shear test results of the protein gels made of mussel protein isolates are given in Table 5. Gels from mussel proteins obtained by acid-aided processing had low shear strength and work of shear values than the protein gels from alkali-aided processing (p < 0.05). Lower shear test values of protein gels from acid process indicate poor gel network resulting poor gel strength, which is also supported by the high expressible moisture content (p < 0.05). Surasani et al. (2018) and Kristinsson and Liang (2006) observed that the protein gels obtained from the acid-aided processing of pangas and tilapia had better gel strength compared to protein gels from alkali-aided processing. Similar findings were reported by Hultin and Kelleher (1999) during protein recovery from mackerel and cod. Differences in gel forming ability are due to the effect of processing on protein integrity and bonding (Chaijan et al. 2006). Factors that influence protein gelling ability include type of fish or species, extraction method used, and the time of extraction (Freitas et al. 2015).
Expressible moisture content
Expressible moisture is the measure of integrity of the gel to hold water. Expressible moisture content (%) of gels from proteins obtained by acid-aided processing was high than the protein gels from alkali-aided processing (p < 0.05), indicating the poor gel network of acid-processed proteins (Table 5). During the studies on proteins recovered from pangas processing waste, Surasani et al. (2018) found that proteins from acid-aided processing had gels with low expressible moisture content than gels made of alkali-processed proteins. Similar observations were made by Freitas et al. (2011) and Fatin et al. (2015) during their studies on Argentine anchovy residue and Japanese scad proteins. In the present study, a reverse trend was observed, which might be due to protein denaturation at extreme acidic pH than alkaline pH.
Folding test
Gels made of protein isolates obtained by acid- and alkali-aided solubilization had an average folding test score of 1, indicating the poor gelling ability of these proteins. Gels made of herring protein isolates had very strong gels that had folding test score of 5 (Marmon and Undeland 2010). Surasani et al. (2018) reported that the gels made of acid- and alkaline-processed isolates of pangas had folding test scores of 5 and 1, indicating the strong gel network in gels made of acid-processed proteins, compared to alkaline-processed isolate gel. In the present study, gels made of both acid- and alkali-processed isolates had poor gel network which might be due to protein denaturation at extreme pH values during the extraction process.
Conclusions
Proteins from freshwater mussel meat can be recovered using pH shift processing with good yields and functionality. Alkali-aided process was found to be more satisfactory for recovering proteins from mussel proteins, as acid-aided processing resulted in low protein yields and weaker protein gels. Despite of the higher yields, proteins obtained by alkali-aided solubilization had poor functionality, which might be due to protein denaturation at high alkaline pH. Further studies are needed to find out the optimum alkaline pH that can result in minimum protein denaturation and maximum yields. pH shift processing found to be promising in the utilization of freshwater mussels for the recovery of functional proteins thus by reducing the supply demand gap as well pollution problems.
References
Álvarez C, Lélu P, Lynch S, Tiwari BK (2017) Optimised protein recovery from mackerel whole fish by using sequential acid/alkaline isoelectric solubilization precipitation (ISP) extraction assisted by ultrasound. LWT Food Sci Technol 88:210–216. https://doi.org/10.1016/j.lwt.2017.09.045
AOAC (2000) Association of official analytical chemists, 16th edn. Washington, DC
Batista I, Pires C, Nelhas R (2007) Extraction of sardine proteins by acidic and alkaline solubilisation. Food Sci Technol Int 13(3):189–194
Bidlingmeyer BA, Cohen SA, Tarvin TL (1984) Rapid analysis of amino acids using precolumn derivatisation. J Chromatogr 336:93–104
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2006) Physicochemical properties, gel forming ability and myoglobin content of sardine (Sardinnella gibbosa) and mackerel (Rastrelliger kanagurta) surimi produced by conventional method and alkaline solubilization process. Eur Food Res Technol 222:58–63
Chaijan M, Undeland I (2015) Development of a new method for determination of total haem protein in fish muscle. Food Chem 173:1133–1141
Chen YC, Tou JC, Jaczynski J (2007) Protein recovery from rainbow trout (Oncorhynchus mykiss) processing byproducts via isoelectric solubilization/precipitation and its gelation properties as affected by functional additives. J Agric Food Chem 55(22):9079–9088
Cortes-Ruis J, Pachero-Aguilar R, Garcia-Sanchez G, Lugo-Sanches ME (2001) Functional characterization of a protein concentrate from bristly sardine made under acidic conditions. J Aquat Food Prod Technol 10:5–23
FAO (2009) Global agriculture towards 2050. High Level Expert Forum Rome, 12–13 October 2009
FAO (2017) The future of food and agriculture; trends and challenges. Rome
Fatin NS, Huda N, David W (2015) Physicochemical properties of Japanese scad (Decapterus Maruadsi) surimi prepared using the acid and alkaline solubilization methods. Int J Sci Eng Res 6(4):141–147
Feng YM, Hultin HO (2001) Effect of pH on the rheological and structural properties of gels of water-washed chicken-breast muscle at physiological ionic strength. J Agric Food Chem 49:3927–3935
Freitas IR, Cortez-Wega WR, Prentice C (2015) Evaluation of properties of protein recovered from fish muscle by acid solubilization process. Int Food Res J 22(3):1067–1073
Freitas IR, Gauterio GV, Rios DG, Prentice C (2011) Functionality of protein isolates from argentine anchovy (Engraulis anchoita) residue using pH shift processing. J Food Sci Eng 1:374–378
Haldar A, Dey TK, Dhar P, Chakarabarti J (2014) Exploring the nutritive values of the fresh water mussel Lamellidens marginalis as potential functional food. IOSR J Env Sci Toxicol Food Technol 8(8):1–7
Hamm R (1994) The influence of pH on the protein net charge in the myofibrillar system. Rec Meat Conf Proc 47:5–9
Harlow E, Lane D (1988) Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Huang L, Chen Y, Morrissey MT (1997) Coagulation of fish proteins from frozen fish mince wash water by ohmic heating. J Food Process Eng 20:285–300
Hultin HO, Kelleher SD (1999) Process of isolating a protein composition from a muscle source and protein composition. Patent US6005073
Hultin HO, Kelleher SD (2000) High efficiency alkaline protein extraction. Patent US6136959
Huss HH (1983) Fresk Fisk K valitet Og Holdbarhed. Fiskeriministeriets Forsφgslaboratorium
Jafarpour SA, Shabanpour B, Filabadi SS (2013) Biochemical properties of fish protein isolate (FPI) from silver carp (Hypophthalmychthis molitrix) by application of acid-alkali process compared to traditional prepared surimi. Ecopersia 1(3):315–327
Jay JM (1986) Modern food microbiology. Van Nostrand Reinhold Company, New York
Jongjareonrak A, Rawdkuen S, Chaijan M, Benjakul S, Osako K, Tanaka M (2010) Chemical compositions and characterization of skin gelatin from farmed giant catfish (Pangasianodon gigas). LWT Food Sci Technol 43:161–165
Kelleher SD, Hultin HO (2000) Functional chicken muscle protein isolates prepared using low ionic strength, acid solubilisation/precipitation. Rec Meat conf Proc 3:76–81
Kinsella JE (1976) Functional properties of proteins in foods, a survey. CRC Crit Rev Food Sci Nutr 7:219–280
Kristinsson H, Demir N (2003) Functional fish protein ingredients from fish species of warm and temperate waters: comparison of acid- and alkali-aided processing vs. conventional surimi processing. In: Betchel P (ed) Advances in seafood byproducts 2002 conference proceedings. Alaska Sea Grant College program University of Alaska, pp. 277–295
Kristinsson H, Ingadottir B (2006) Recovery and properties of muscle proteins extracted from tilapia (Oreochromis niloticus) light muscle by pH shift processing. J Food Sci 1(3):E132–E141
Kristinsson HG, Liang Y (2006) Effect of pH-shift processing and surimi processing on Atlantic croaker (Micropogonias undulates) muscle proteins. J Food Sci 71:C304–C312
Kristinsson H, Theodore AE, Demir N, Ingadottir B (2005) A comparative study between acid- and alkali-aided processing and surimi processing for the recovery of proteins from channel catfish muscle. J Food Sci 70(4):C298–C306
Kudo G, Okada M, Miyauchi D (1973) Gel-forming capacity of washed and unwashed flesh of some Pacific coast species of fish. Marine Fish Rev 32:10–15
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Lubana GK, Kaur B, Surasani VKR (2016) Quality changes in fresh rohu (Labeo rohita) cutlets added with fibers from ragi, oat and jowar. Nutr Food Sci 46(4):571–582
Marmon SK, Undeland I (2010) Protein isolation from gutted herring (Clupea harengus) using pH-shift processes. J Agric Food Chem 58:10480–10486
Mutilangi WAM, Panyam D, Kilara A (1996) Functional properties of properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J Food Sci 61(2):270–274
Niki H, Kato T, Deya E, Igarashi S (1985) Recovery of protein from effluent of fish meat in producing surimi and utilization of recovered protein. Nippon Suisan Gakkaishi 51(6):959–964
Nolsøe H, Undeland I (2009) The acid and alkaline solubilization process for the isolation of muscle proteins: state of art. Food Bioprocess Technol 2:1–27
Nolsφe H, Marmon SK, Undeland I (2011) Application of filtration to recover solubilized proteins during ph-shift processing of blue whiting (Micromesistius poutassou); effects on protein yield and qualities of protein isolates. Open Food Sci J 5:1–9
Panpipat W, Chaijan M (2016) Biochemical and physicochemical characteristics of protein isolates from bigeye snapper (Priacanthus Tayenus) head by-product using pH shift method. Turk J Fish Aquat Sci 16:41–50
Rawdkuen S, Sai-Ut S, Khamsorn S, Chaijan M, Benjakul S (2009) Biochemical and gelling properties of tilapia surimi and protein recovered using an acid-alkaline process. Food Chem 112:112–119
Reddy SVK (2016) Effect of formulation and processing methods on the quality and acceptability of cutlets made from minced meat of pangas (Pangasius pangasius). SAARC J Agric 14(1):25–36
Robinson HW, Hogden CG (1940) The biuret reaction in the determination of serum proteins. J Biol Chem 135:707–725
Sathe SK, Deshpande SS, Salunkhe DK (1982) Functional properties of lupin seed (Supinus mutabilis) proteins and protein concentrates. J Food Sci 7:191–197
Shabanpour B, Etemadian Y, Taghipour B (2015) Physicochemical and rheological parameters changes for determining the quality of surimi and kamaboko produced by conventional, acid and alkaline solubilization process methods from common kilka (Clupeonella cultriventris caspia). Iran J Fish Sci 14(4):826–845
Surasani VKR (2017) Influence of rohu (Labeo rohita) deboning by-product on composition, physical properties and sensorial acceptability of rohu cutlets. Nutr Food Sci 47(3):398–408
Surasani VKR (2018) Acid and alkaline solubilization (pH shift) process: a better approach for the utilization of fish processing waste and by-products. Env Sci Pollut Res 25:18345–18363. https://doi.org/10.1007/s11356-018-2319-1
Surasani VKR, Khatkar SK, Singh S (2017a) Effect of process variables on solubility andrecovery yields of proteins from pangas (Pangasius pangasius) frames obtained by alkalinesolubilization method: characteristics of isolates. Food Bioprod Process 106:137–146
Surasani VKR, Tyagi A, Kudre T (2017b) Recovery of proteins from rohu processing waste using ph shift method: characterization of isolates. J Aquat Food Prod Technol 26(3):356–365
Surasani VKR, Kudre T, Ballari RV (2018) Recovery and characterization of proteins from pangas (Pangasius pangasius) processing waste obtained through pH shift processing. Env Sci Pollut Res 25:11987–11998. https://doi.org/10.1007/s11356-018-1456-x
Tian Y, Wang W, Yuan C, Zhang L, Liu J, Liu J (2016) Nutritional and digestive properties of protein isolates extracted from the muscle of the common carp using pH shift processing. J Food Process Preserv 41:e12847. https://doi.org/10.1111/jfpp.12847
Undeland I, Kelleher SD, Hultin HO (2002) Recovery of functional proteins from herring (Clupea harengus) light muscle by an acid or alkaline solubilization process. J Agric Food Chem 50(25):7371–7379
Vareltzis PK, Undeland I (2012) Protein isolation from blue mussels (Mytilus edulis) using an acid and alkaline solubilisation technique—process characteristics and functionality of the isolates. J Sci Food Agric 92(15):3055–3064
Yusufzi SI, Singh H, Shirdhankar MM (2010) An evaluation of different methods for transportation of the freshwater mussel Lamellidens corrianus (Lea 1834). Int J Aqua 18(4):676–692
Acknowledgements
Authors wish to express their sincere thanks to the Dean, College of Fisheries, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India, for the support and facilities provided during the work period. Authors wish to express their sincere thanks to Mrs. Manvinder Kaur for her technical help during the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Surasani, V.K.R., Mandal, A. & Pandey, A. Utilization of freshwater mussel (Lamellidens marginalis) for the isolation of proteins through pH shift processing: characterization of isolates. Environ Sci Pollut Res 25, 31497–31507 (2018). https://doi.org/10.1007/s11356-018-3154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3154-0